Effects of Three Modification Methods on the In Vitro Gastrointestinal Digestion and Colonic Fermentation of Dietary Fiber from Lotus Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lotus Leaves Modification Treatment and IDF Preparation
2.3. In Vitro Gastrointestinal Digestion
2.4. Extraction of Bound Polyphenols
2.5. Determination of TPC and Flavonoid Composition
2.6. Determination of Antioxidant Capacity
2.7. In Vitro Colonic Fermentation
2.8. SCFA Analysis
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results and Discussion
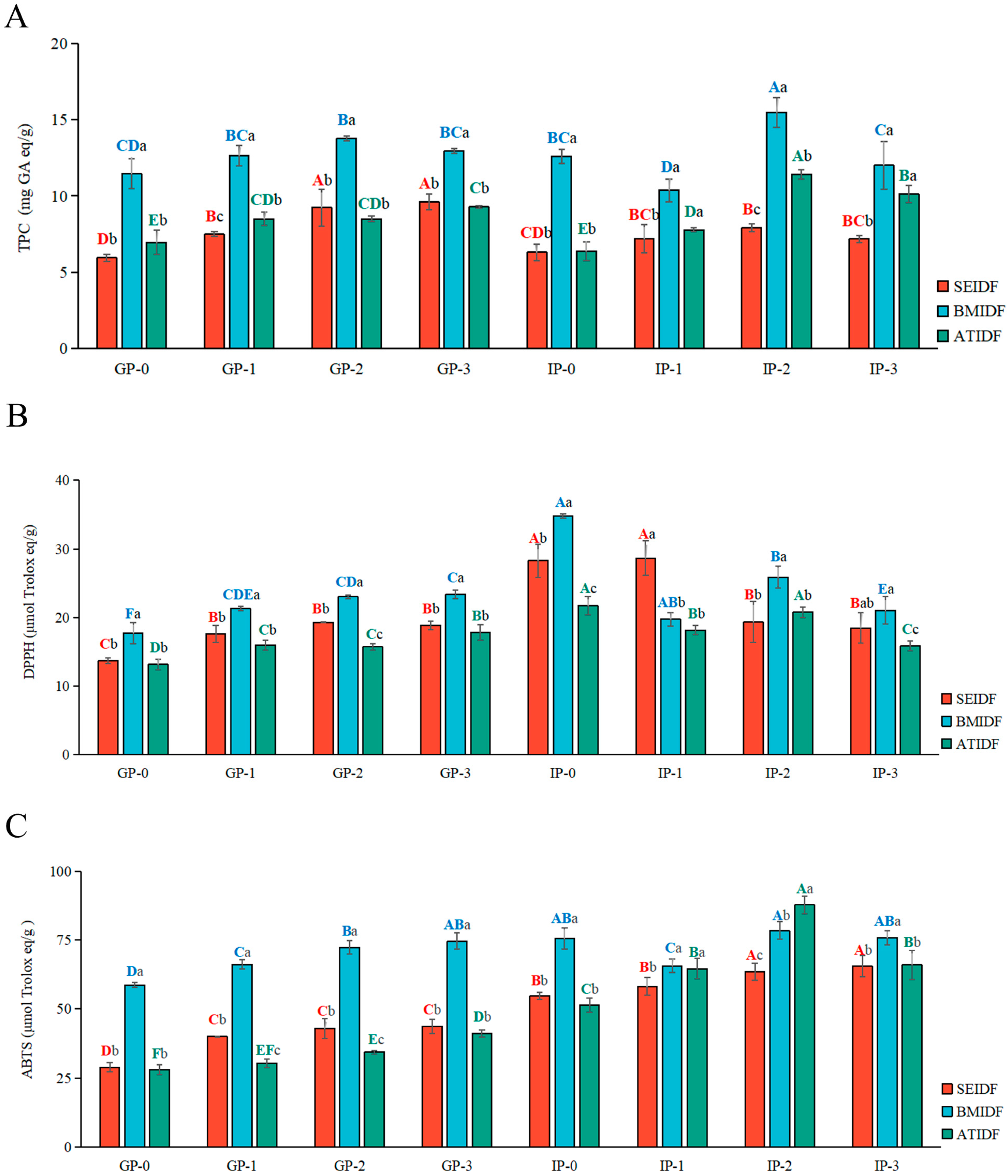
3.1. TPC During In Vitro Gastrointestinal Digestion
3.2. Flavonoid Composition During In Vitro Gastrointestinal Digestion
3.3. Antioxidant Capacity During In Vitro Gastrointestinal Digestion
3.4. TPC and Antioxidant Capacity of Bound Polyphenols After In Vitro Gastrointestinal Digestion
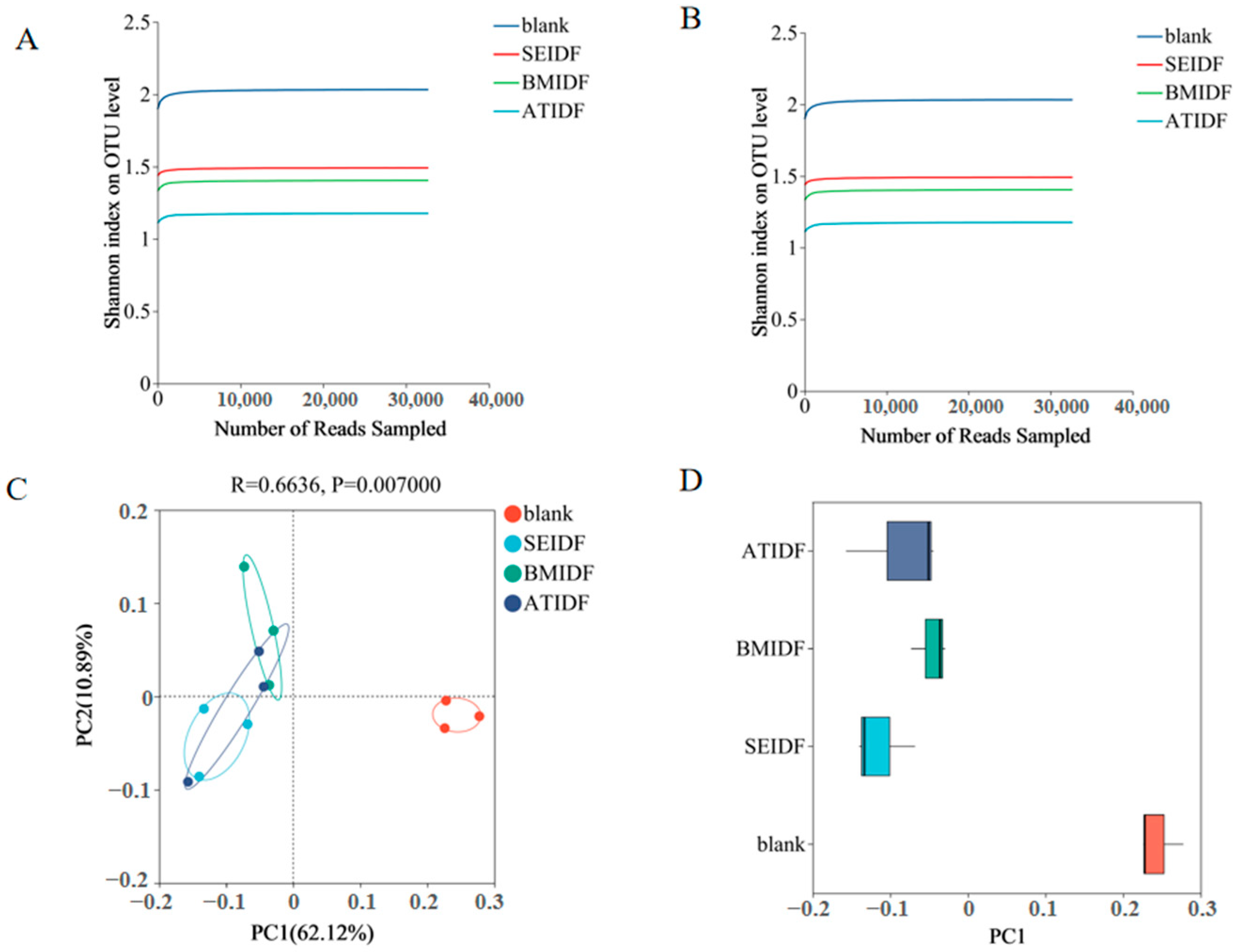
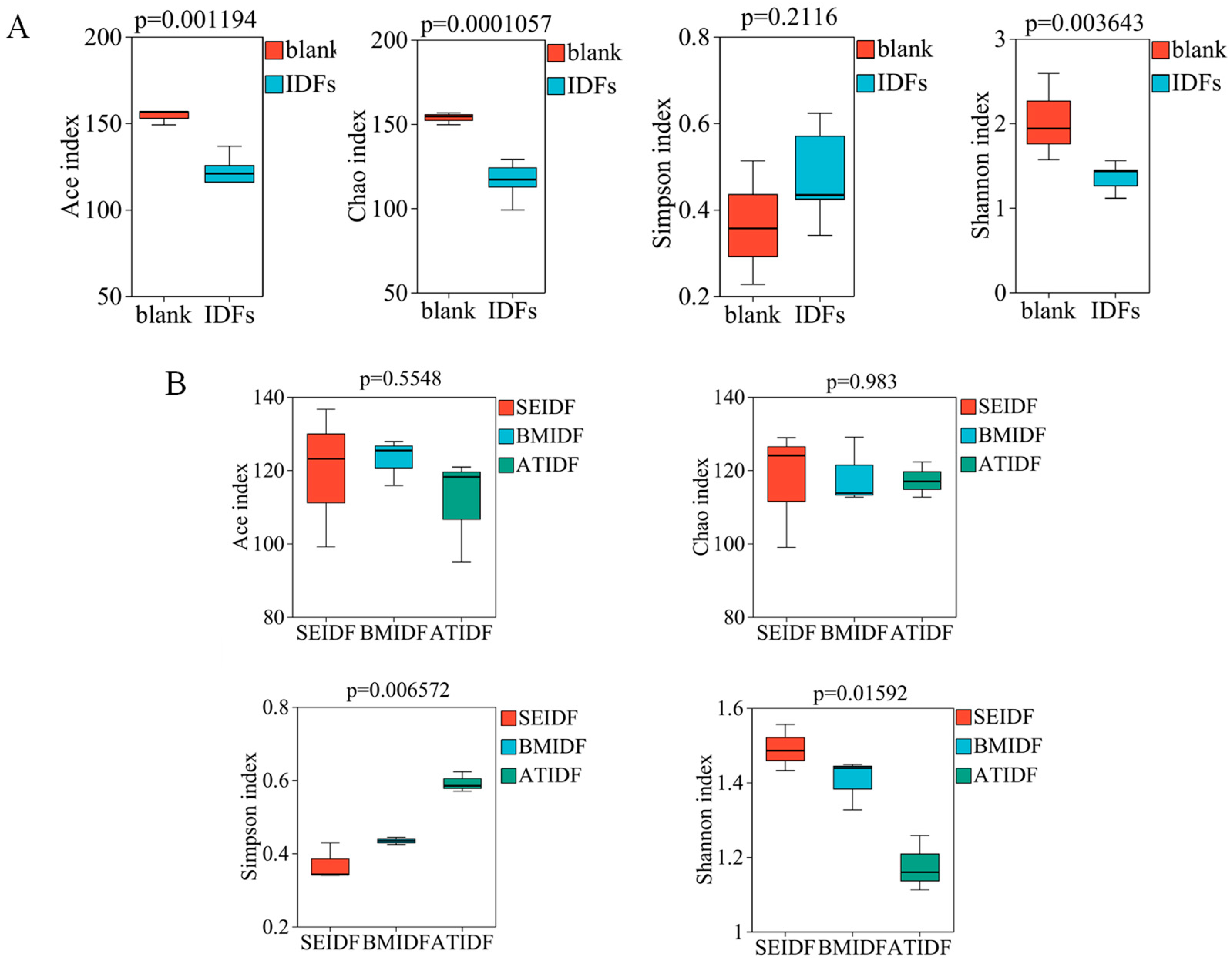
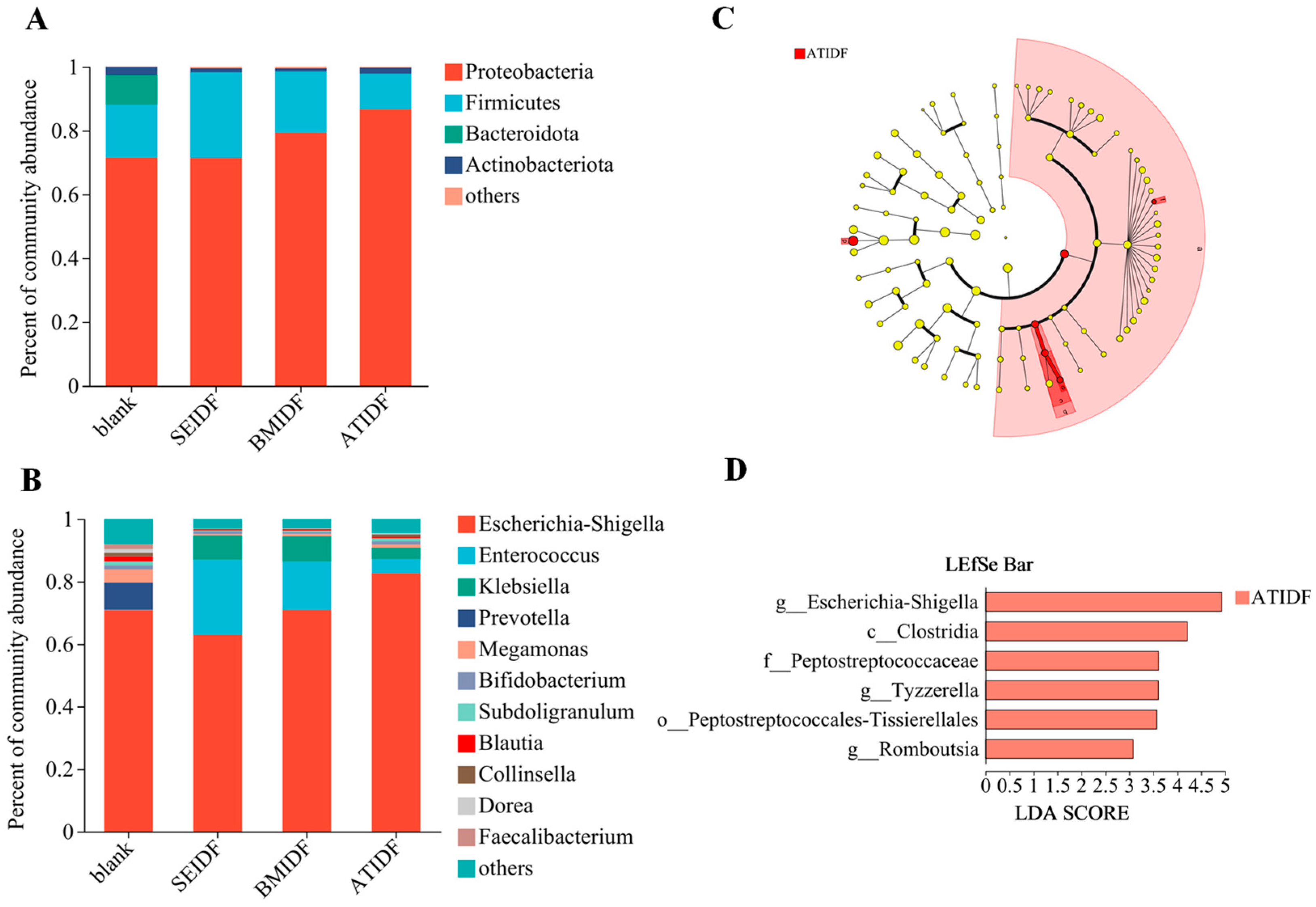
3.5. Gut Microbiota During In Vitro Colonic Fermentation
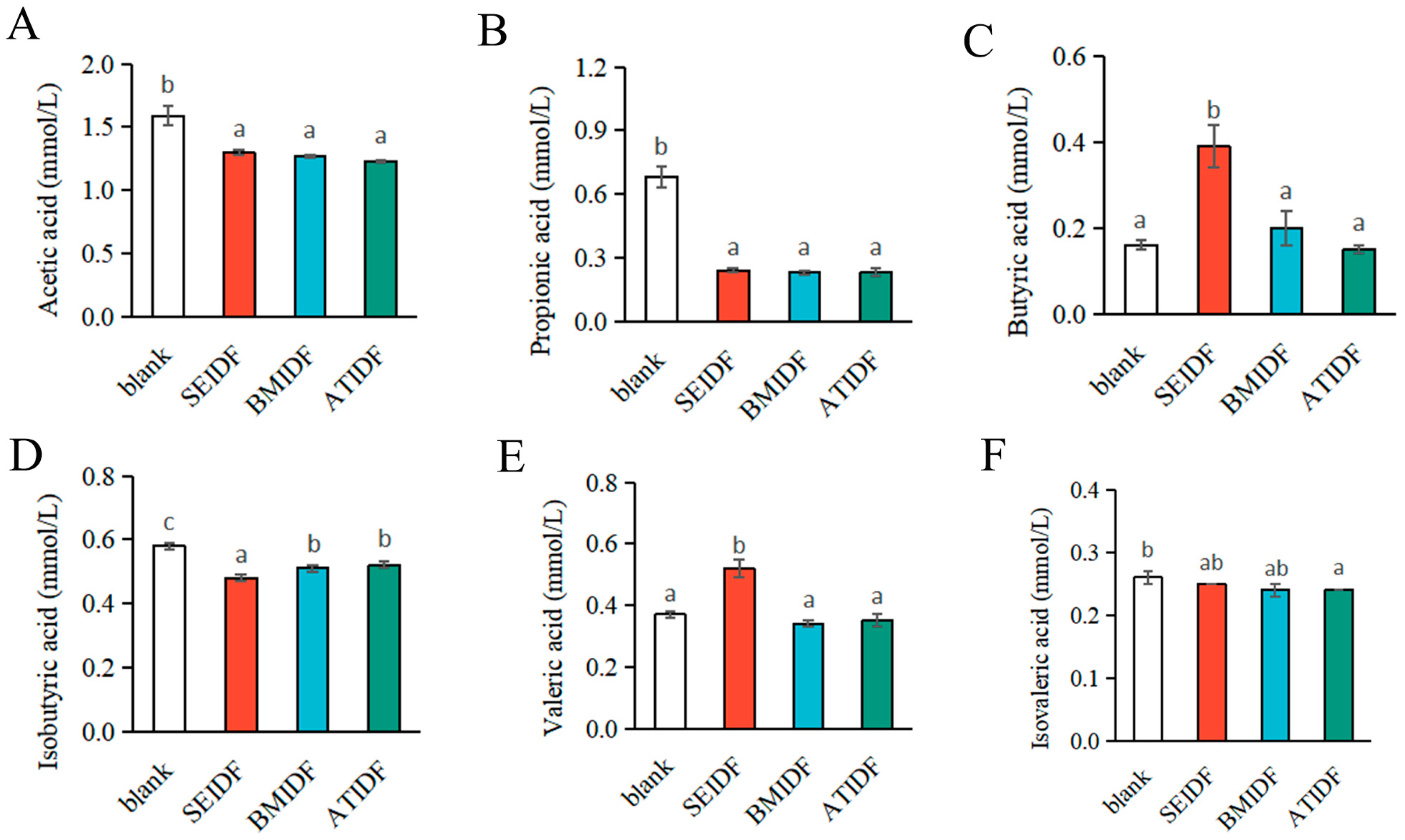
3.6. SCFA Concentrations After In Vitro Colonic Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J. Gut fermentation of dietary fibres: Physico–chemistry of plant cell walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Qin, B.; Dawson, H.D.; Schoene, N.W.; Polansky, M.M.; Anderson, R.A. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition 2012, 28, 1172–1179. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2018, 83, 235–247. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef]
- Solari-Godiño, A.; Pérez-Jiménez, J.; Saura-Calixto, F.; Borderías, A.; Moreno, H. Anchovy mince (Engraulis ringens) enriched with polyphenol-rich grape pomace dietary fibre: In vitro polyphenols bioaccessibility, antioxidant and physico-chemical properties. Food Res. Int. 2017, 102, 639–646. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT-Food Sci. Technol. 2017, 87, 450–456. [Google Scholar] [CrossRef]
- Sauvaitre, T.; Etienne-Mesmin, L.; Sivignon, A.; Mosoni, P.; Courtin, C.M.; Van De Wiele, T.; Blanquet-Diot, S. Tripartite relationship between gut microbiota, intestinal mucus and dietary fibers: Towards preventive strategies against enteric infections. FEMS Microbiol. Rev. 2020, 45, fuaa052. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Cantu-Jungles, T.M. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct. 2021, 12, 10658–10666. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar]
- Wang, Z.; Cheng, Y.; Zeng, M.; Wang, Z.; Qin, F.; Wang, Y.; Chen, J.; He, Z. Lotus (Nelumbo nucifera Gaertn.) leaf: A narrative review of its phytoconstituents, health benefits and food industry applications-sciencedirect. Trends Food Sci. Technol. 2021, 112, 631–650. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zheng, T.; Zeng, Y.; Fu, L.; Zhou, T.; Jia, F.; Xu, Y.; He, K.; Yang, Y. Effects of shear emulsifying/ball milling/autoclave modification on structure, physicochemical properties, phenolic compounds, and antioxidant capacity of lotus (Nelumbo) leaves dietary fiber. Front. Nutr. 2023, 10, 1064662. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Zheng, Y.; Wang, D.; Liu, Z.; Deng, Y.; Zhao, Y. Structure, physicochemical and bioactive properties of dietary fibers from Akebia trifoliata (Thunb.) Koidz. seeds using ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction. Food Res. Int. 2020, 136, 109348. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Wojdyo, A.; Figiel, A.; Viiard, E. Fiber modification of brewers’ spent grain by autoclave treatment to improve its properties as a functional food ingredient. LWT-Food Sci. Technol. 2021, 149, 111877. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T.; Sun, H.; Zhang, M.; Chen, J.; Yan, Z. Optimization of extraction efficiency by shear emulsifying assisted enzymatic hydrolysis and functional properties of dietary fiber from deoiled cumin (Cuminum cyminum L.). Food Chem. 2015, 179, 270–277. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zeng, Y.; Zheng, T.; Jia, F.; Xu, P.; Xu, Y.; Cao, Y.; He, K.; Yang, Y. Effects of Four Extraction Methods on Structure and In Vitro Fermentation Characteristics of Soluble Dietary Fiber from Rape Bee Pollen. Molecules 2023, 28, 4800. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2020, 339, 127879. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, Y.W.; Ke, Y.; Li, C.; Zhang, Z.Q.; Wu, Y.L.; Hu, B.; Liu, A.; Luo, Q.Y.; Wu, W.J. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr. Polym. 2021, 251, 117041. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In vitro fermentation of raffinose to unravel its potential as prebiotic ingredient. LWT Food Sci. Technol. 2020, 126, 109322. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Viuda-Martos, M. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, Z.; Tu, Z.; Wang, H.; Zhang, L.; Xie, X.; Liu, G. Influence of in vitro gastrointestinal digestion on the bioavailability and antioxidant activity of polyphenols from Ipomoea batatas leaves. Int. J. Food Sci. Technol. 2017, 52, 1131–1137. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2009, 120, 599–606. [Google Scholar] [CrossRef]
- Luo, M.; Hou, F.; Dong, L.; Huang, F.; Zhang, R.; Su, D. Comparison of microwave and high-pressure processing on bound phenolic composition and antioxidant activities of sorghum hull. Int. J. Food Sci. Technol. 2020, 55, 3190–3202. [Google Scholar] [CrossRef]
- Escobedo, A.; Loarca-Piña, G.; Gaytan-Martínez, M.; Orozco-Avila, I.; Mojica, L. Autoclaving and extrusion improve the functional properties and chemical composition of black bean carbohydrate extracts. J. Food Sci. 2020, 85, 2783–2791. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Z.; Fang, J.B.; Liu, Y.L.; Li, S.H. Flavonoids in lotus (Nelumbo) leaves evaluated by HPLC–MSn at the germplasm level. Food Res. Int. 2013, 54, 796–803. [Google Scholar] [CrossRef]
- Liao, L.; Chen, J.; Liu, L.; Xiao, A. Screening and binding analysis of flavonoids with alpha-amylase inhibitory activity from Lotus leaf. J. Brazil Chem. Soc. 2018, 29, 587–593. [Google Scholar] [CrossRef]
- Yang, X.; Lim, S.; Lin, J.; Wu, J.; Tang, H.; Zhao, F.; Liu, F.; Sun, C.; Shi, X.; Kuang, Y.; et al. Oxygen mediated oxidative couplings of flavones in alkaline water. Nat. Commun. 2022, 13, 6424. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Hu, X.; Chen, Y.; Xie, J.; Ao, T.; Wang, H.; Xie, J.; Yu, Q. Elucidation of the interaction effect between dietary fiber and bound polyphenol components on the anti-hyperglycemic activity of tea residue dietary fiber. Food Funct. 2022, 13, 2710–2728. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, J.; Li, Z.; Meng, Y.; Li, X. Digestion characteristics of jujube polysaccharide and its regulatory effect on intestinal microbiota and metabolites during in vitro fermentation. LWT 2024, 210, 116869. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Zhang, X.; Kazem, A.E. Microwave treatment enhances human gut microbiota fermentability of isolated insoluble dietary fibers. Food Res. Int. 2021, 143, 110293. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Whon, T.W.; Bae, J. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 63, 12073–12088. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Tian, X.; Wang, G.; Jin, K.; Ding, Y.; Cheng, D. Rice hull insoluble dietary fiber alleviated experimental colitis induced by low dose of dextran sulfate sodium in cadmium-exposed mice. Food Funct. 2022, 13, 7215–7225. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Ning, X.; Qin, Y.; Wang, Y.; Yu, Z.; Dong, R.; Zhang, Y.; Sun, S. Expansion of Escherichia-Shigella in gut is associated with the onset and response to immunosuppressive therapy of IgA nephropathy. J. Am. Soc. Nephrol. 2022, 33, 2276–2292. [Google Scholar] [CrossRef]
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Liu, H.; Xu, H.; Liu, F.; Song, H.; Zhao, X.; Li, H. Dysregulation of ruminococcaceae and megamonas could be predictive markers for rapid progression of mild cognitive impairment. Microb. Pathog. 2023, 183, 106272. [Google Scholar] [CrossRef]
- Kriegel, M.A. Subdoligranulum chews up joints: How a gut pathobiont can instigate arthritis. Trends Immunol. 2022, 44, 4–6. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Nitert, M.D. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2017, 9, 189–201. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, W.; Yang, X.; Zhu, L.; Wu, G.; Zhang, H. Methylglyoxal scavenging capacity of fiber-bound polyphenols from highland barley during colonic fermentation and its modulation on methylglyoxal-interfered gut microbiota. Food Chem. 2023, 434, 137409. [Google Scholar] [CrossRef]
- Lee, C.K.; Oh, S.J.; Kim, H.J.; Kim, B.H.; Kim, B.K.; Park, Y.K.; Yang, B.G. P919 Gut microbial signature and phenotype-specific Indicators in fecal microbiota of Korean patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 17, i1028. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The role of Gut Microbiota in Various Neurological and Psychiatric Disorders—An Evidence Mapping based on Quantified evidence. Mediat. Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef]
- Ren, C.; Wen, Z.; Xu, Y.; Jiang, W.; Gu, Y. Clostridia: A flexible microbial platform for the production of alcohols. Curr. Opin. Chem. Biol. 2016, 35, 65–72. [Google Scholar] [CrossRef]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Thakkar, R.D.; Marcia, A.D.R. Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions. Sci. Rep. 2018, 8, 16655. [Google Scholar] [CrossRef] [PubMed]
- Robles Alonso, V.; Guarner, F. Linking the gut microbiota to human health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Cui, J.; Ye, C.; Zhao, J.; Yang, B.; Xu, Y.; Ji, S.; Wang, L.; Lv, X.; Ma, C.; et al. Depletion of butyrate-producing microbes of the Firmicutes predicts nonresponse to FMT therapy in patients with recurrent Clostridium difficile infection. Gut Microbes 2023, 15, 2236362. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, Y.; Chen, Q.; Fu, X.; Huang, Q.; Zhang, N.B.; Dong, H.; Li, C. Exploring the synergistic benefits of insoluble dietary fiber and bound phenolics: Unveiling the role of bound phenolics in enhancing bioactivities of insoluble dietary fiber. Trends Food Sci. Technol. 2024, 149, 104554. [Google Scholar] [CrossRef]
- Gio-Batta, M.; Sjöberg, F.; Jonsson, K. Fecal short chain fatty acids in children living on farms and a link between valeric acid and protection from eczema. Sci. Rep. 2020, 10, 22449. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Yang, P.; Liu, H.; Ye, W.; Xue, Z.; Peng, X.; Wang, X. Integrated genome-centric metagenomic and metaproteomic analyses unravel the responses of the microbial community to ammonia stress. Water Res. 2023, 242, 120239. [Google Scholar] [CrossRef]
| Input Salt Type | SSF (0.4 L) | SGF (0.4 L) | SIF (0.4 L) |
|---|---|---|---|
| KCl (5 M) (mL) | 15.1 | 6.9 | 6.8 |
| KH2PO4 (0.5 M) (mL) | 3.7 | 0.9 | 0.8 |
| NaHCO4 (1 M) (mL) | 6.8 | 12.5 | 42.5 |
| NaCl (2 M) (mL) | - | 11.8 | 9.6 |
| MgCl2(H2O)6 (0.15 M) (mL) | 0.5 | 0.4 | 1.1 |
| (NH4)2CO4 (0.5 M) (mL) | 0.06 | 0.5 | - |
| HCl (6 M) (mL) | 0.09 | 1.3 | 0.7 |
| CaCl2(H2O)2 (0.3 M) (mL) | 0.025 | 0.005 | 0.04 |
| supplemented to 0.4 L with ultra-pure water | |||
| Gastrointestinal Digestion Phase | Rutin | Hyperoside | Isoquercitrin | Astragalin | Quercetin |
|---|---|---|---|---|---|
| SEIDF | |||||
| GP-0 | - | - | 2.44 ± 0.06 | - | - |
| GP-1 | - | - | 3.12 ± 0.28 | - | - |
| GP-2 | - | - | 4.64 ± 0.34 | - | - |
| GP-3 | - | - | 4.41 ± 0.83 | - | - |
| IP-0 | - | - | - | - | - |
| IP-1 | - | - | - | - | - |
| IP-2 | - | - | - | - | - |
| IP-3 | - | - | - | - | - |
| BMIDF | |||||
| GP-0 | - | 388.39 ± 11.55 | 389.05 ± 17.11 | 50.26 ± 3.02 | 6.86 ± 0.23 |
| GP-1 | - | 454.77 ± 24.48 | 442.20 ± 23.49 | 55.27 ± 2.29 | 21.34 ± 0.39 |
| GP-2 | - | 519.18 ± 25.44 | 498.69 ± 21.76 | 62.78 ± 4.05 | 34.99 ± 8.88 |
| GP-3 | - | 499.95 ± 5.01 | 489.01 ± 8.74 | 60.69 ± 1.62 | 26.10 ± 1.86 |
| IP-0 | - | - | - | - | - |
| IP-1 | - | - | - | - | - |
| IP-2 | - | - | - | - | - |
| IP-3 | - | - | - | - | - |
| ATIDF | |||||
| GP-0 | 12.49 ± 0.46 | 229.61 ± 35.95 | 195.56 ± 30.13 | 29.79 ± 4.83 | - |
| GP-1 | 15.49 ± 3.96 | 262.07 ± 89.79 | 223.50 ± 76.69 | 34.36 ± 11.96 | - |
| GP-2 | 20.43 ± 7.03 | 348.57 ± 133.94 | 298.57 ± 113.18 | 45.83 ± 16.10 | - |
| GP-3 | 21.31 ± 3.33 | 375.80 ± 54.28 | 325.02 ± 47.39 | 49.00 ± 7.23 | - |
| IP-0 | - | 16.90 ± 16.82 | 13.77 ± 13.58 | 12.52 ± 5.99 | - |
| IP-1 | - | - | - | - | - |
| IP-2 | - | - | - | - | - |
| IP-3 | - | - | - | - | - |
| Group | SEIDF | BMIDF | ATIDF |
|---|---|---|---|
| TPC of bound polyphenols (mg GA eq/g) | 3.21 ± 0.25 b | 6.51 ± 0.78 a | 0.66 ± 0.03 c |
| DPPH radical scavenging capacity (μmol Trolox/g) | 30.40 ± 2.25 a | 32.23 ± 2.43 a | 20.43 ± 1.04 b |
| ABTS radical scavenging capacity (μmol Trolox/g) | 29.87 ± 2.34 a | 33.51 ± 1.41 a | 10.38 ± 1.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Xu, Y.; Wu, Y.; Huangfu, X.; Chen, W.; He, K.; Yang, Y. Effects of Three Modification Methods on the In Vitro Gastrointestinal Digestion and Colonic Fermentation of Dietary Fiber from Lotus Leaves. Foods 2024, 13, 3768. https://doi.org/10.3390/foods13233768
Zheng H, Xu Y, Wu Y, Huangfu X, Chen W, He K, Yang Y. Effects of Three Modification Methods on the In Vitro Gastrointestinal Digestion and Colonic Fermentation of Dietary Fiber from Lotus Leaves. Foods. 2024; 13(23):3768. https://doi.org/10.3390/foods13233768
Chicago/Turabian StyleZheng, Hui, Yao Xu, Yuhang Wu, Xuantong Huangfu, Wenxiu Chen, Kai He, and Yong Yang. 2024. "Effects of Three Modification Methods on the In Vitro Gastrointestinal Digestion and Colonic Fermentation of Dietary Fiber from Lotus Leaves" Foods 13, no. 23: 3768. https://doi.org/10.3390/foods13233768
APA StyleZheng, H., Xu, Y., Wu, Y., Huangfu, X., Chen, W., He, K., & Yang, Y. (2024). Effects of Three Modification Methods on the In Vitro Gastrointestinal Digestion and Colonic Fermentation of Dietary Fiber from Lotus Leaves. Foods, 13(23), 3768. https://doi.org/10.3390/foods13233768





