Structure Elucidation and Immunostimulatory Activity Evaluation of a Galactoglucan from Alpinia officinarum Hance
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification
2.2. Molecular Weight Analysis
2.3. FT-IR Analysis
2.4. Monosaccharide Composition Analysis
2.5. Methylation Analysis
2.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.7. Cell Culture
2.8. Cell Viability
2.9. Neutral Red Phagocytosis Analysis
2.10. Nitric Oxide (NO) Assay
2.11. RNA Sequencing Analysis
2.12. Statistical Analysis
3. Results and Discussion
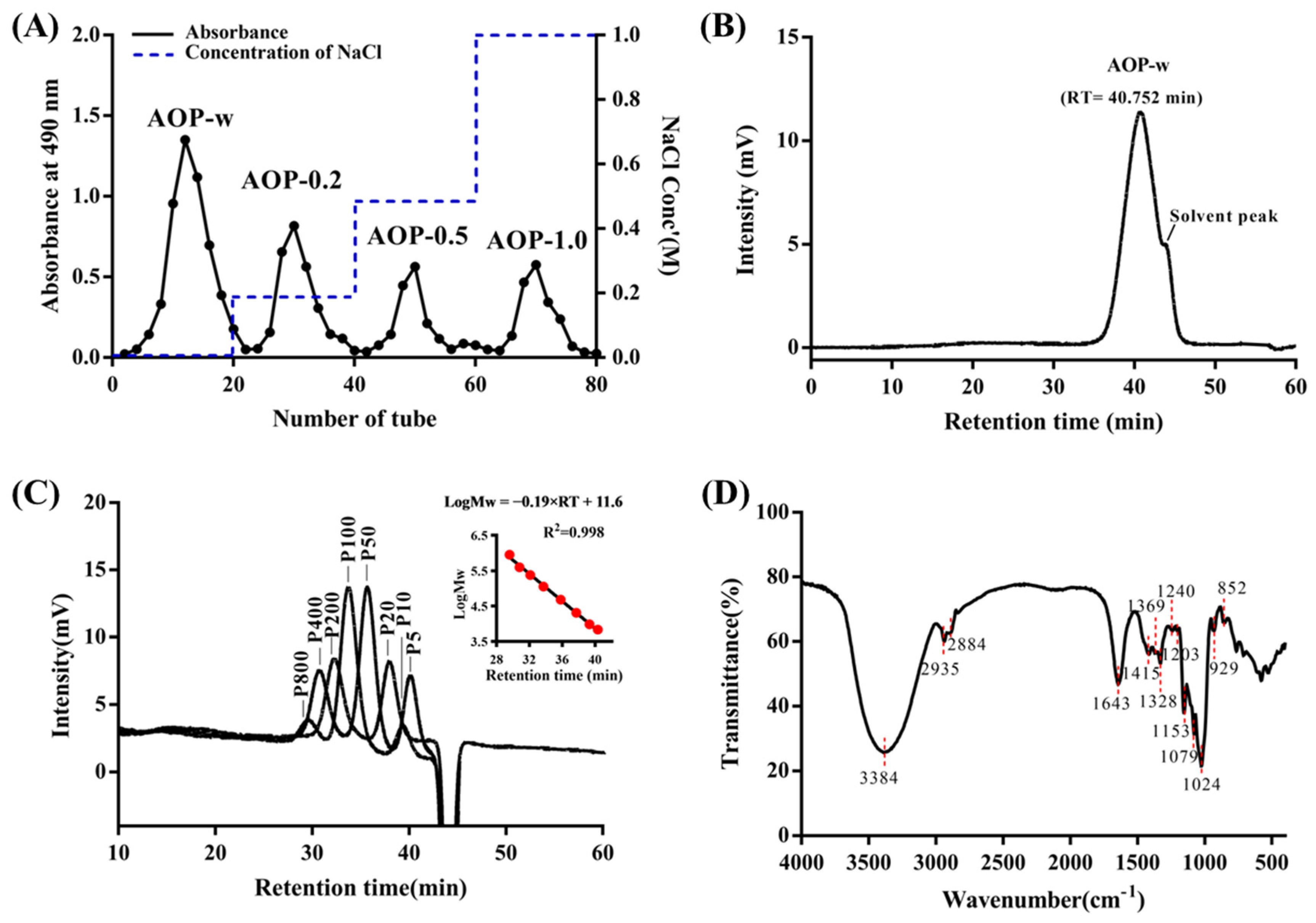
3.1. Homogenous Polysaccharide Obtained from A. officinarum
3.2. Functional Groups of AOP-w
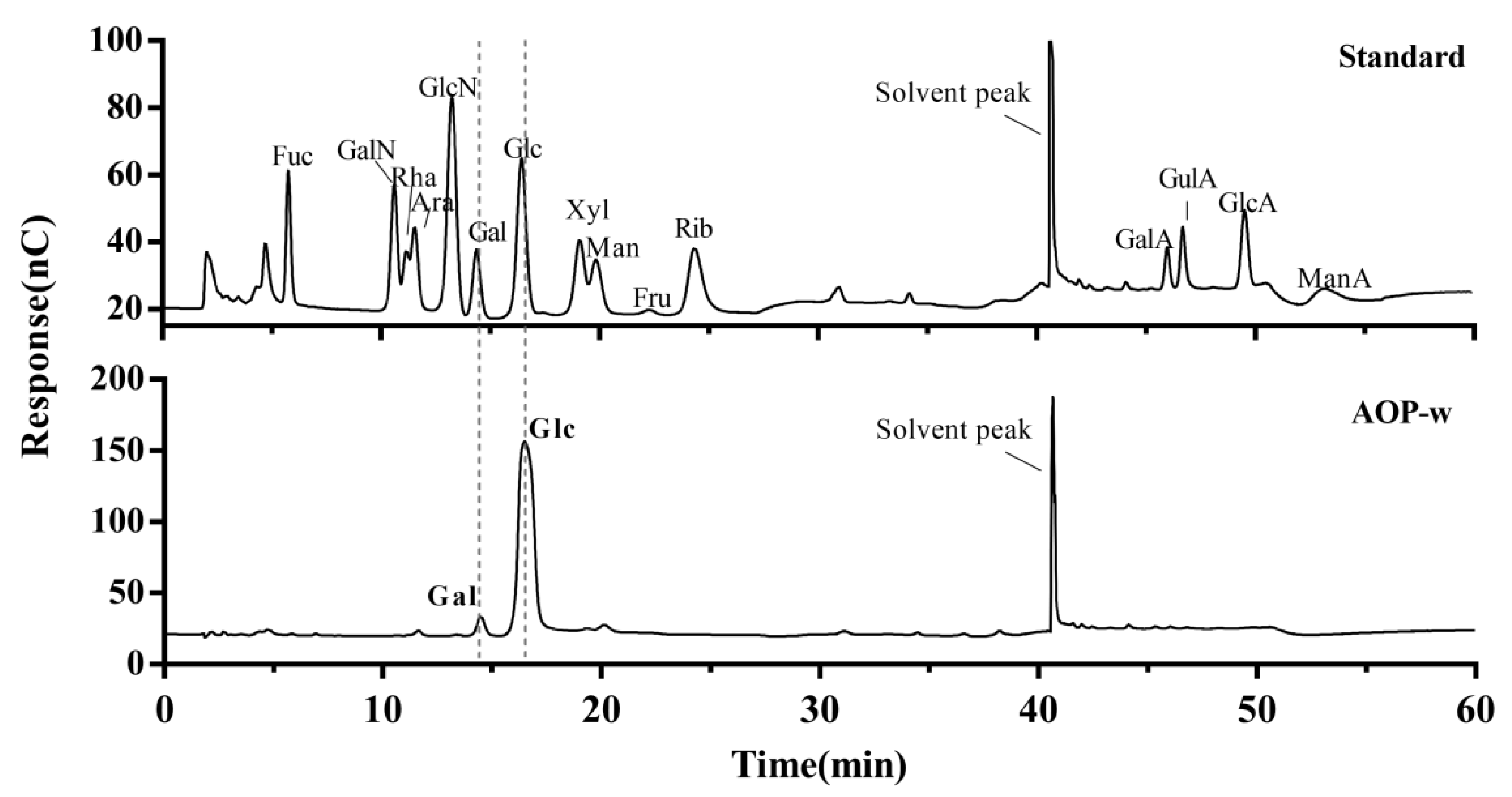
3.3. Monosaccharide Composition of AOP-w
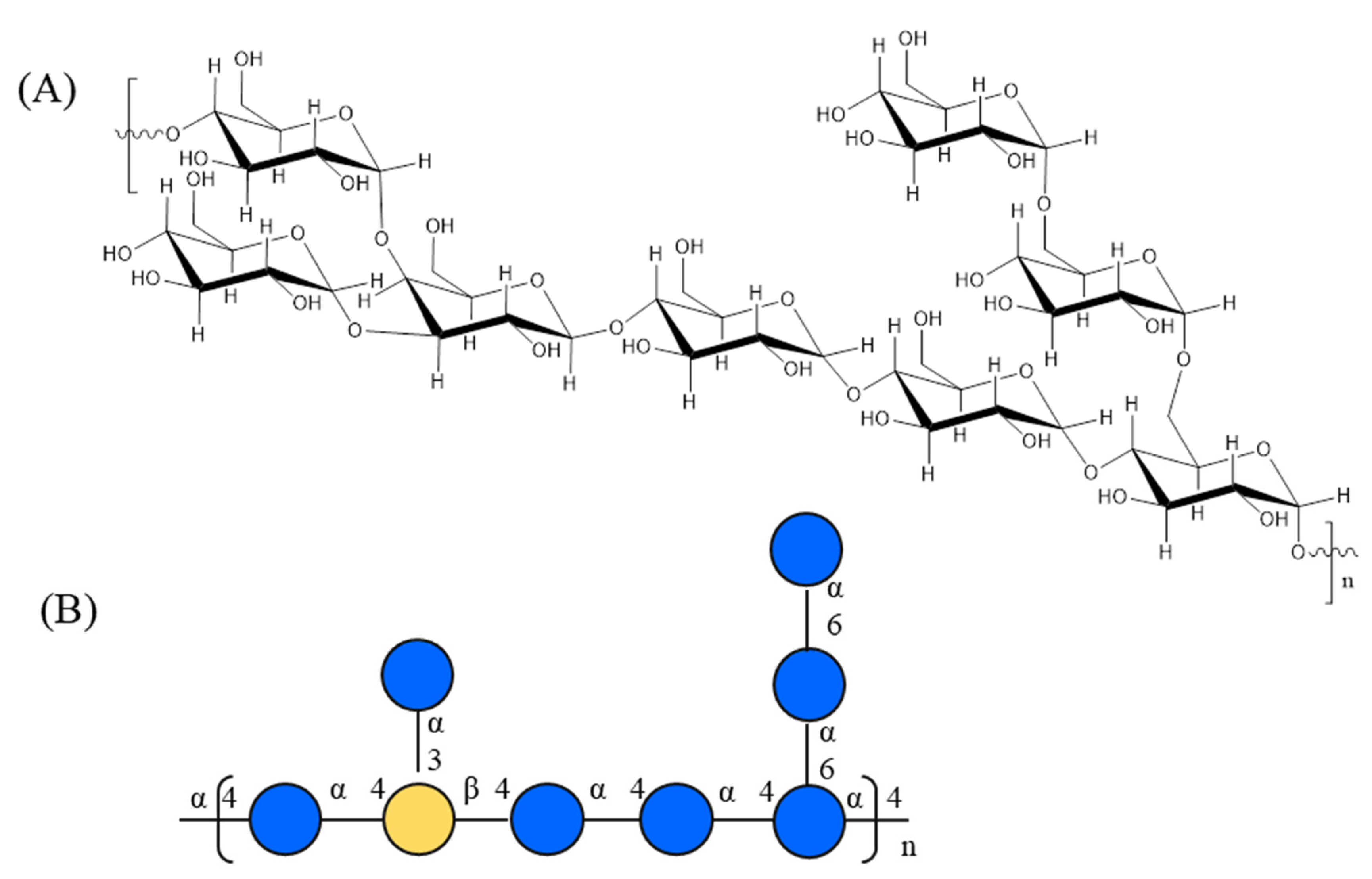
3.4. Glycosidic Linkage Analysis of AOP-w
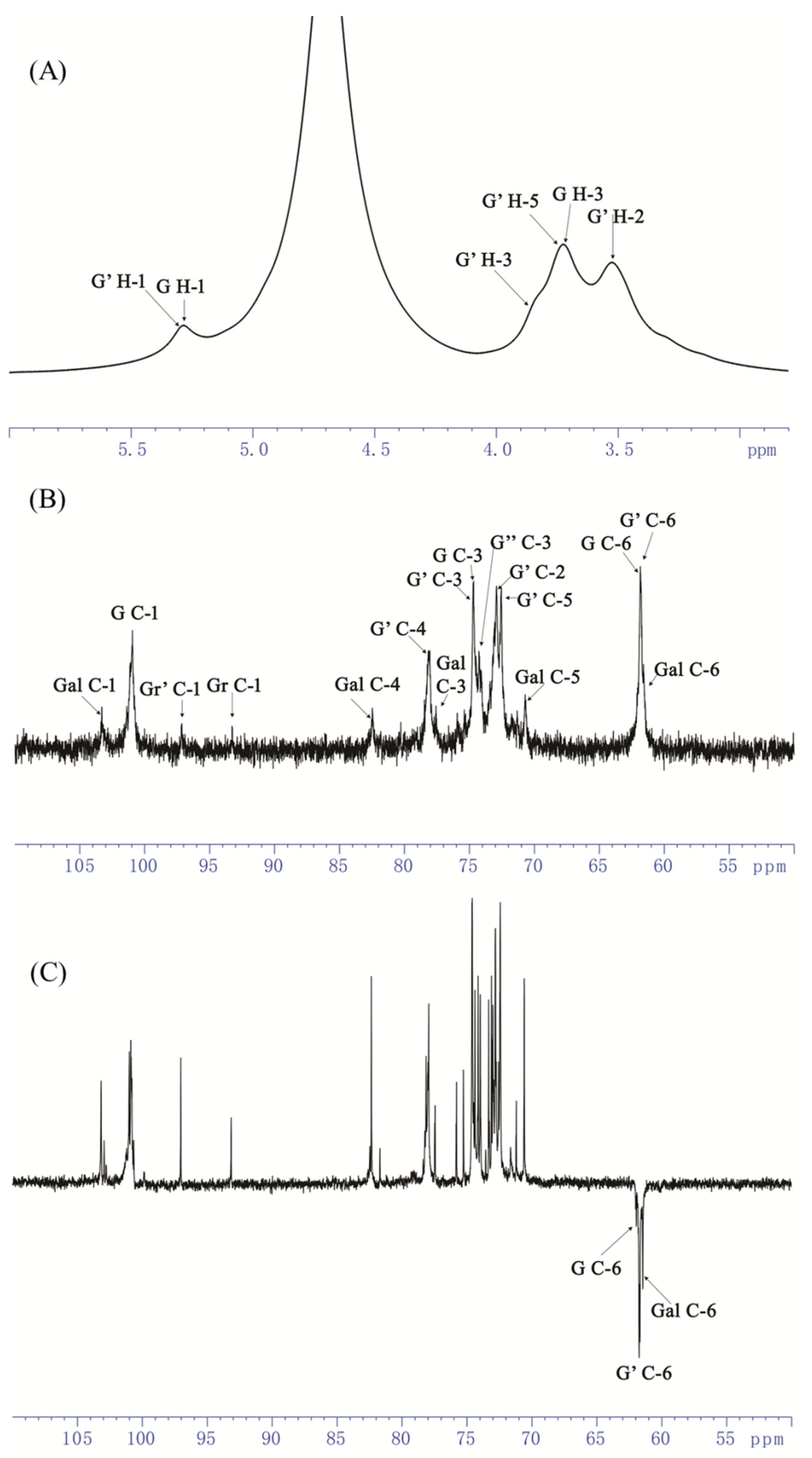
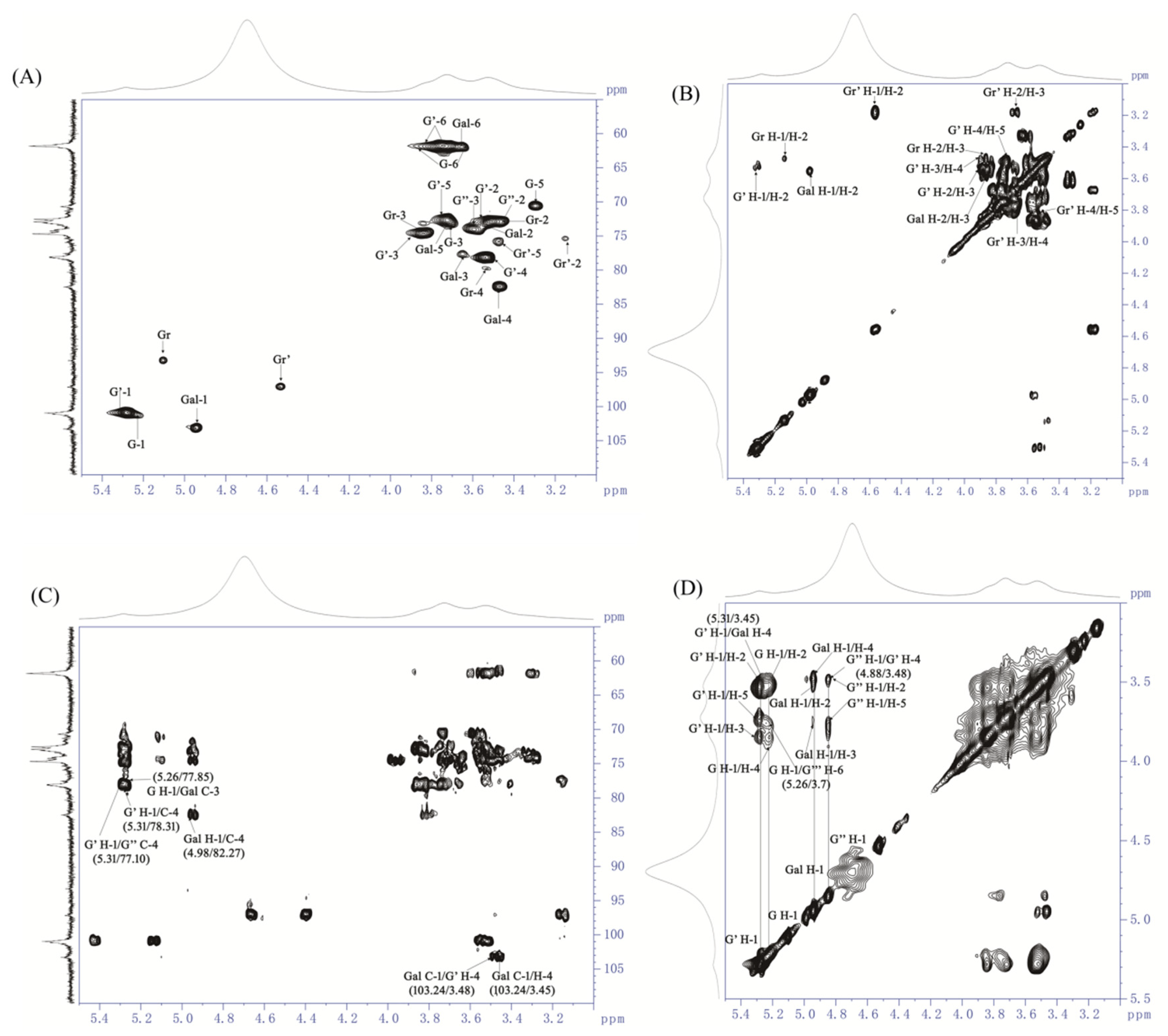
3.5. NMR Analysis of AOP-w
3.6. Immunoregulatory Activity of AOP-w
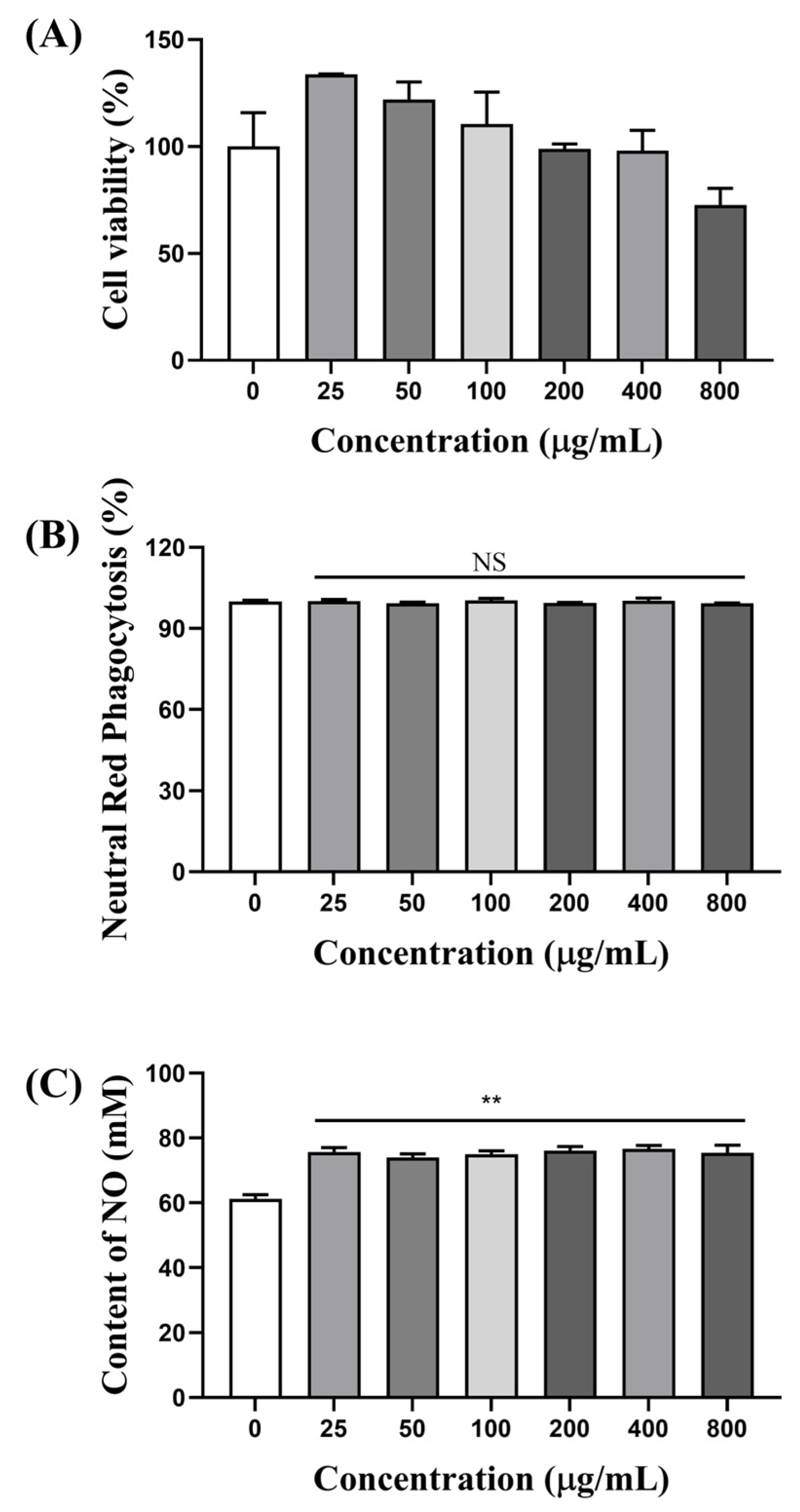
3.6.1. AOP-w Increased the Cell Viability of RAW 264.7 Cells
3.6.2. Effects of AOP-w on Phagocytosis
3.6.3. AOP-w Promoted NO Secretion
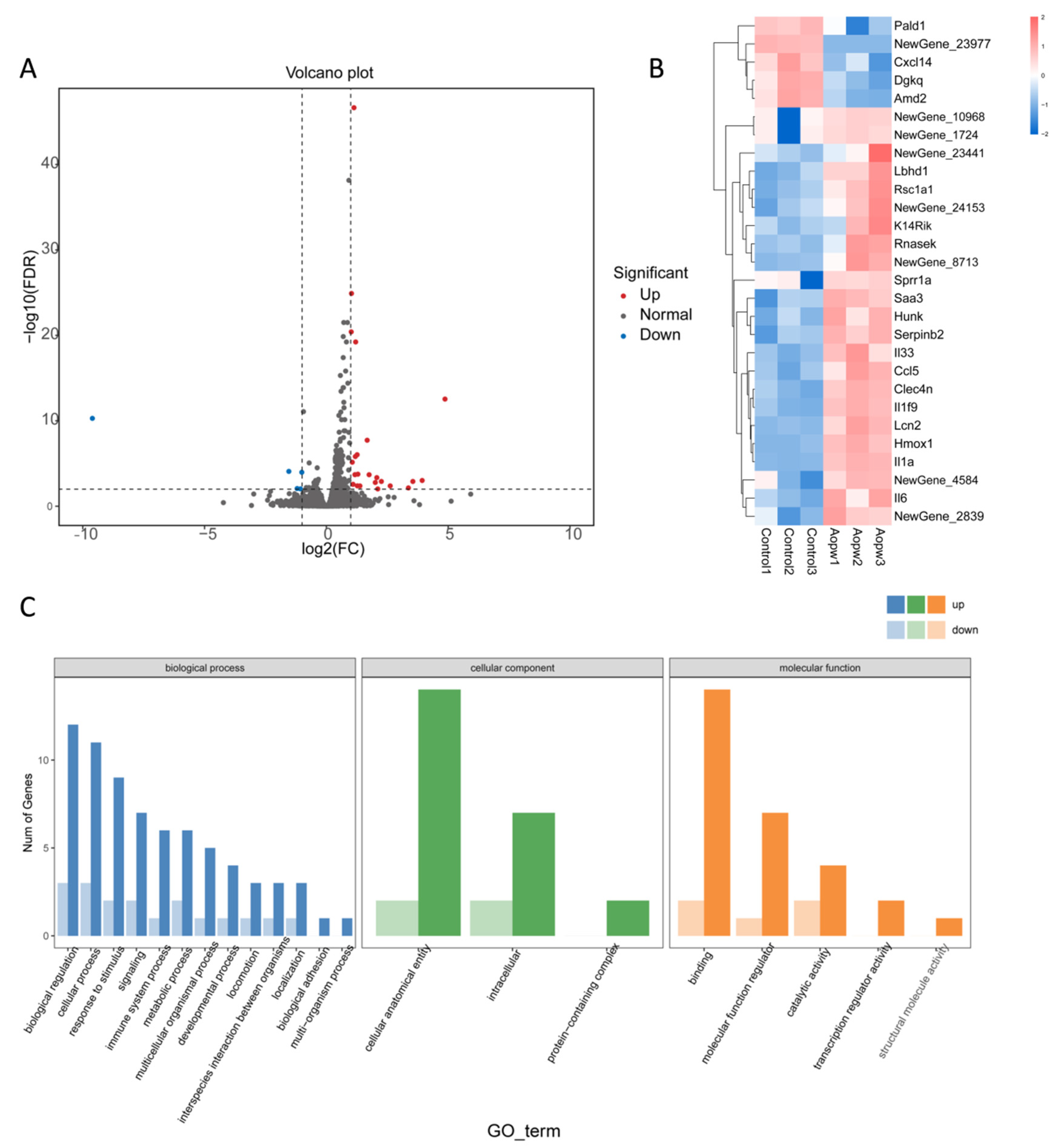
3.6.4. Transcriptome Analysis Using RNA-Seq
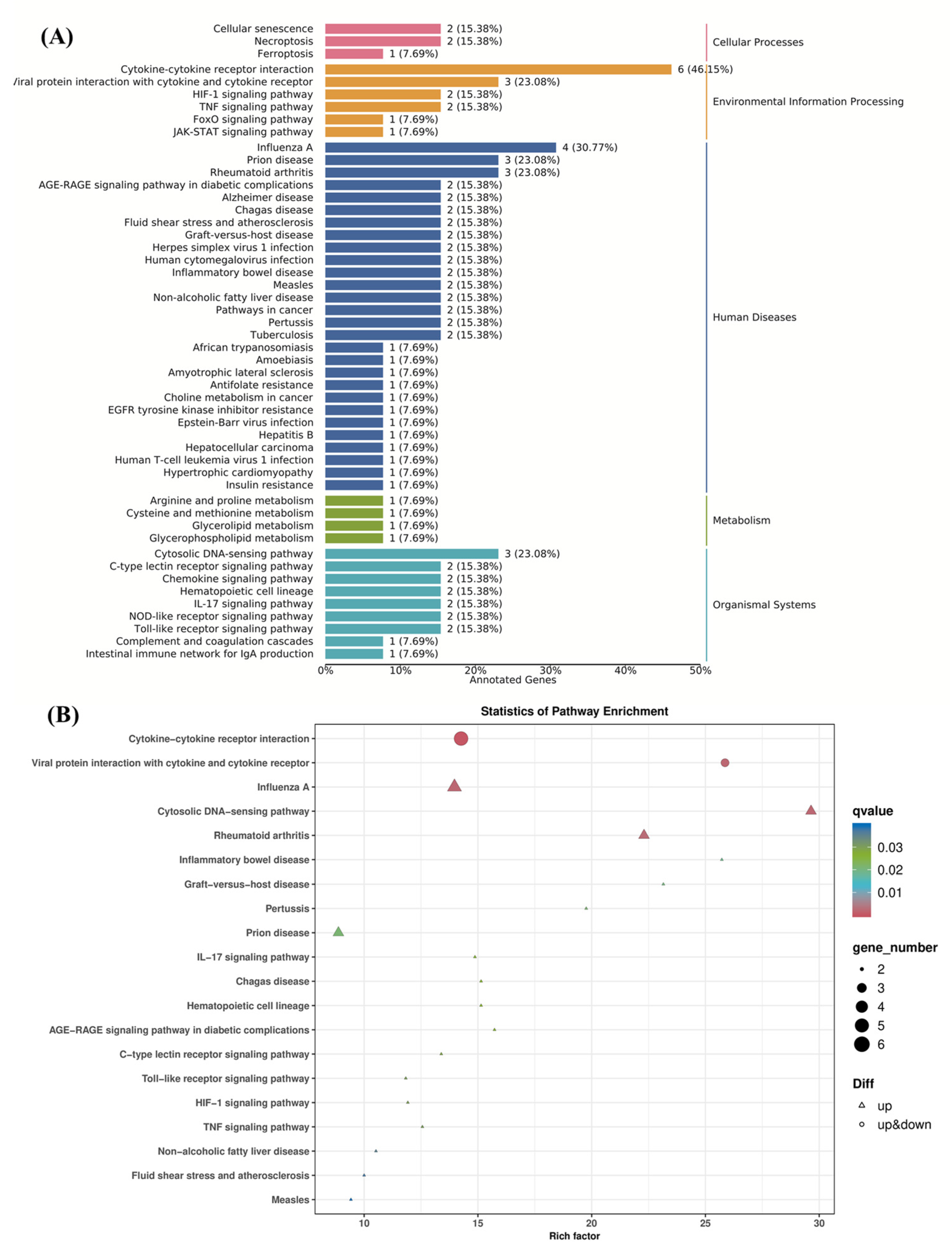
3.6.5. KEGG Pathway Enrichment Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abubakar, I.B.; Malami, I.; Yahaya, Y.; Sule, S.M. A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J. Ethnopharmacol. 2018, 224, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Vishwas, S.; Khursheed, R.; Paudel, K.R.; Gupta, S.; Porwal, O.; Alshahrani, S.M.; Jha, N.K.; et al. Journey of Alpinia galanga from kitchen spice to nutraceutical to folk medicine to nanomedicine. J. Ethnopharmacol. 2022, 291, 115144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Tan, Y.; Li, Y.; Xue, Q.; Li, H.; Zhang, X.; Pan, Y.; Xu, J.; Zhang, J. Alpinia officinarum Hance extract ameliorates diabetic gastroparesis by regulating SCF/c-kit signaling pathway and rebalancing gut microbiota. Fitoterapia 2024, 172, 105730. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; You, P.; Xu, Y.; Ye, X.; Tu, Y.; Liu, Y.; Yang, M.; Liu, D. Anti-Helicobacter pylori-associated gastritis effect of the ethyl acetate extract of Alpinia officinarum Hance through MAPK signaling pathway. J. Ethnopharmacol. 2020, 260, 113100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, L.; Zhu, J.; Feng, Y.; Wu, X. Anti-inflammatory activities and glycerophospholipids metabolism in KLA-stimulated RAW 264.7 macrophage cells by diarylheptanoids from the rhizomes of Alpinia officinarum. Biomed. Chromatogr. 2018, 32, e4094. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.A.; Jeong, S.; Lee, S.; Park, H.J.; Kim, N.J.; Lim, S. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2009, 126, 258–264. [Google Scholar] [CrossRef]
- Xia, D.Z.; Yu, X.F.; Wang, H.M.; Ren, Q.Y.; Chen, B.M. Anti-obesity and hypolipidemic effects of ethanolic extract from Alpinia officinarum Hance (Zingiberaceae) in rats fed high-fat diet. J. Med. Food 2010, 13, 785–791. [Google Scholar] [CrossRef]
- Atmaca, H.; Pulat, C.C.; Ilhan, S. Synthesis of silver nanoparticles using Alpinia officinarum rhizome extract induces apoptosis through down-regulating Bcl-2 in human cancer cells. Biol. Futur. 2022, 73, 327–334. [Google Scholar] [CrossRef]
- Elgazar, A.A.; Selim, N.M.; Abdel-Hamid, N.M.; El-Magd, M.A.; El Hefnawy, H.M. Isolates from Alpinia officinarum Hance attenuate LPS-induced inflammation in HepG2: Evidence from in silico and in vitro studies. Phytother. Res. 2018, 32, 1273–1288. [Google Scholar] [CrossRef]
- Ni, J.; Chen, H.; Zhang, C.; Luo, Q.; Qin, Y.; Yang, Y.; Chen, Y. Characterization of Alpinia officinarum Hance polysaccharide and its immune modulatory activity in mice. Food Funct. 2022, 13, 2228–2237. [Google Scholar] [CrossRef]
- Heidari, H.; Abdollahi, M.; Khani, S.; Nojavan, F.; Khani, S. Effect of Alpinia officinarum extract on reproductive damages in streptozotocin induced diabetic male rats. J. Diabetes Metab. Disord. 2021, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Liu, A.X.; Zhang, Y.X.; Zhou, M.Y.; Li, X.Y.; Fu, M.H.; Pan, Y.P.; Xu, J.; Zhang, J.Q. A diarylheptanoid compound from Alpinia officinarum Hance ameliorates high glucose-induced insulin resistance by regulating PI3K/AKT-Nrf2-GSK3beta signaling pathways in HepG2 cells. J. Ethnopharmacol. 2022, 295, 115397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wang, Z.M.; Zhang, J.Z. Alpinia officinarum Hance (Gaoliangjiang, Galangal), Dietary Chinese Herbs Chemistry, Pharmacology and Clinical Evidence; Springer: New York, NY, USA, 2015; pp. 61–67. [Google Scholar]
- Bitari, A.; Oualdi, I.; Touzani, R.; Elachouri, M.; Legssyer, A. Alpinia officinarum Hance: A mini review. Mater. Today Proc. 2023, 72, 3869–3874. [Google Scholar] [CrossRef]
- Dong, G.-Z.; Lee, S.Y.; Zhao, H.-Y.; Lee, Y.-I.; Jeong, J.H.; Jeon, R.; Lee, H.J.; Ryu, J.-H. Diarylheptanoids from lesser galangal suppress human colon cancer cell growth through modulating Wnt/β-catenin pathway. J. Funct. Foods 2015, 18, 47–57. [Google Scholar] [CrossRef]
- Zhan, K.; Ji, X.; Luo, L. Recent progress in research on Momordica charantia polysaccharides: Extraction, purification, structural characteristics and bioactivities. Chem. Biol. Technol. Agric. 2023, 10, 58. [Google Scholar] [CrossRef]
- Liu, Z.H.; Niu, F.J.; Xie, Y.X.; Xie, S.M.; Liu, Y.N.; Yang, Y.Y.; Zhou, C.Z.; Wan, X.H. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Sun, J.; Qu, C.; Chen, X. Polysaccharides from traditional Asian food source and their antitumor activity. J. Food Biochem. 2022, 46, e13927. [Google Scholar] [CrossRef]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, P.; Li, X.; Guo, L.; Gao, W. The potential roles of natural plant polysaccharides in inflammatory bowel disease: A review. Carbohydr. Polym. 2022, 277, 118821. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Ding, D.; Gao, J.; Hao, L.; Guo, X.; Liu, Y. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Yuandani, I.; Jantan, M.A.; Haque, A.S.; Rohani, S.E.; Nugraha, E.; Salim, A.W.; Septama, N.A.; Juwita, N.A.; Khairunnisa, H.R.; Nasution, D.S.; et al. Ibrahim, Immunomodulatory effects and mechanisms of the extracts and secondary compounds of Zingiber and Alpinia species: A review. Front. Pharmacol. 2023, 14, 1222195. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Kuang, Y.; Lian, X.; Li, H.; Zhou, M.; Tan, Y.; Zhang, X.; Pan, Y.; Zhang, J.; Xu, J. Physicochemical Characterization, Antioxidant and Anticancer Activity Evaluation of an Acidic Polysaccharide from Alpinia officinarum Hance. Molecules 2024, 29, 1810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yin, Y.; Xiao, S.J.; Chen, J.; Zhang, R.; Yang, T.; Zhou, T.Y.; Zhang, S.Y.; Hu, P.; Zhang, X. Extraction, structural characterization and immunoactivity of glucomannan type polysaccahrides from Lilium brownii var. viridulum Baker. Carbohydr. Res. 2024, 536, 109046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kim, S.Y.; Kim, E.A.; Lee, J.H.; Kim, Y.S.; Yu, S.K.; Chae, J.B.; Choe, I.H.; Cho, J.H.; Jeon, Y.J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Kac, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar]
- Li, B.; Dobruchowska, J.M.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural investigation of water-soluble polysaccharides extracted from the fruit bodies of Coprinus comatus. Carbohydr. Polym. 2013, 91, 314–321. [Google Scholar] [CrossRef]
- Furevi, A.; Ruda, A.; Angles d’Ortoli, T.; Mobarak, H.; Ståhle, J.; Hamark, C.; Fontana, C.; Engström, O.; Apostolica, P.; Widmalm, G. Complete 1H and 13C NMR chemical shift assignments of mono-to tetrasaccharides as basis for NMR chemical shift predictions of oligo- and polysaccharides using the computer program CASPER. Carbohydr. Res. 2022, 513, 108528. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.R.; Park, J.; Mun, S.H.; Kwak, M.; Ko, H.J.; Baek, S.H. Structure and antiviral activity of a pectic polysaccharide from the root of Sanguisorba officinalis against enterovirus 71 in vitro/vivo. Carbohydr. Polym. 2022, 281, 119057. [Google Scholar] [CrossRef]
- Schilling, C.; Klau, L.J.; Aachmann, F.L.; Ruhmann, B.; Schmid, J.; Sieber, V. CRISPR-Cas9 driven structural elucidation of the heteroexopolysaccharides from Paenibacillus polymyxa DSM 365. Carbohydr. Polym. 2023, 312, 120763. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhu, Q.; Pang, D.; Liu, F.; Liao, S.; Zou, Y. Analysis of Lactobacillus rhamnosus GG in mulberry galacto-oligosaccharide medium by comparative transcriptomics and metabolomics. Front. Nutr. 2022, 9, 853271. [Google Scholar] [CrossRef] [PubMed]

| RT | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Linkage Type |
|---|---|---|---|---|
| 28.203 | 2,3,4,6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 0.103 | Glcp-(1→ |
| 43.028 | 2,3,6-Me3-Glcp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 0.684 | →4)-Glcp-(1→ |
| 46.793 | 2,3,4-Me3-Glcp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | 0.104 | →6)-Glcp-(1→ |
| 50.395 | 2,6-Me2-Galp | 43, 87, 97, 117, 129, 149 | 0.064 | →3,4)-Galp-(1→ |
| 55.388 | 2,3-Me2-Glcp | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201, 261 | 0.045 | →4,6)-Glcp-(1→ |
| Glycosyl Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a,b/C6 |
|---|---|---|---|---|---|---|
| α-D-Glcp-1→ | 5.26 | 3.6 | 3.7 | 3.95 | 3.17 | 3.6; 3.82 |
| G | 101.33 | 71.94 | 74.03 | 70.61 | 71.68 | 62.3 |
| →4)-α-D-Glcp-(1→ | 5.31 | 3.55 | 3.9 | 3.48 | 3.76 | 3.71; 3.81 |
| G′ | 100.89 | 72.91 | 74.56 | 78.31 | 72.53 | 61.89 |
| →4,6)-α-D-Glcp-(1→ | 4.88 | 3.49 | 3.67 | 3.36 | 3.57 | 3.78; 3.89 |
| G″ | 99.89 | 72.86 | 74.02 | 77.10 | 70.69 | 68.46 |
| →6)-α-D-Glcp-(1→ | 4.92 | 3.53 | 3.67 | 3.47 | 3.86 | 3.70 |
| G″’ | 99.10 | 72.73 | 74.63 | 70.87 | 71.47 | 66.80 |
| →4)-α-D-Glcp | 5.13 | 3.46 | 3.86 | 3.55 | 3.74 | 3.7; 3.79 |
| Gr | 93.33 | 72.8 | 74.67 | 79.52 | 72.11 | 61.67 |
| →4)-β-D-Glcp | 4.58 | 3.19 | 3.7 | 3.85 | 3.5 | 3.7; 3.75 |
| Gr′ | 97.01 | 75.56 | 77.5 | 78.39 | 75.13 | 61.67 |
| →3,4)-β-D-Galp-(1→ | 4.98 | 3.54 | 3.77 | 3.45 | 3.75 | 3.75; 3.85 |
| Gal | 103.24 | 73.55 | 77.85 | 82.27 | 73.04 | 61.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xiong, Y.; Hu, P.; Chen, L.; Wan, J.; Huang, C.; Liu, W. Structure Elucidation and Immunostimulatory Activity Evaluation of a Galactoglucan from Alpinia officinarum Hance. Foods 2024, 13, 4019. https://doi.org/10.3390/foods13244019
Xu Z, Xiong Y, Hu P, Chen L, Wan J, Huang C, Liu W. Structure Elucidation and Immunostimulatory Activity Evaluation of a Galactoglucan from Alpinia officinarum Hance. Foods. 2024; 13(24):4019. https://doi.org/10.3390/foods13244019
Chicago/Turabian StyleXu, Zhou, Yanxia Xiong, Pei Hu, Long Chen, Jianhua Wan, Chenggang Huang, and Wenjun Liu. 2024. "Structure Elucidation and Immunostimulatory Activity Evaluation of a Galactoglucan from Alpinia officinarum Hance" Foods 13, no. 24: 4019. https://doi.org/10.3390/foods13244019
APA StyleXu, Z., Xiong, Y., Hu, P., Chen, L., Wan, J., Huang, C., & Liu, W. (2024). Structure Elucidation and Immunostimulatory Activity Evaluation of a Galactoglucan from Alpinia officinarum Hance. Foods, 13(24), 4019. https://doi.org/10.3390/foods13244019





