Virtual Cold Chain Method with Comprehensive Evaluation to Reveal the Effects of Temperature Abuse on Blueberry Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Simulated Cold Chain Design
2.3. Quality Evaluation
2.3.1. Commercial Attributes
2.3.2. Biochemical Attributes
2.3.3. Physiological Attributes
2.3.4. Antioxidant Activity and Enzyme Activity Attributes
2.4. Statistical Analysis
3. Results and Discussion
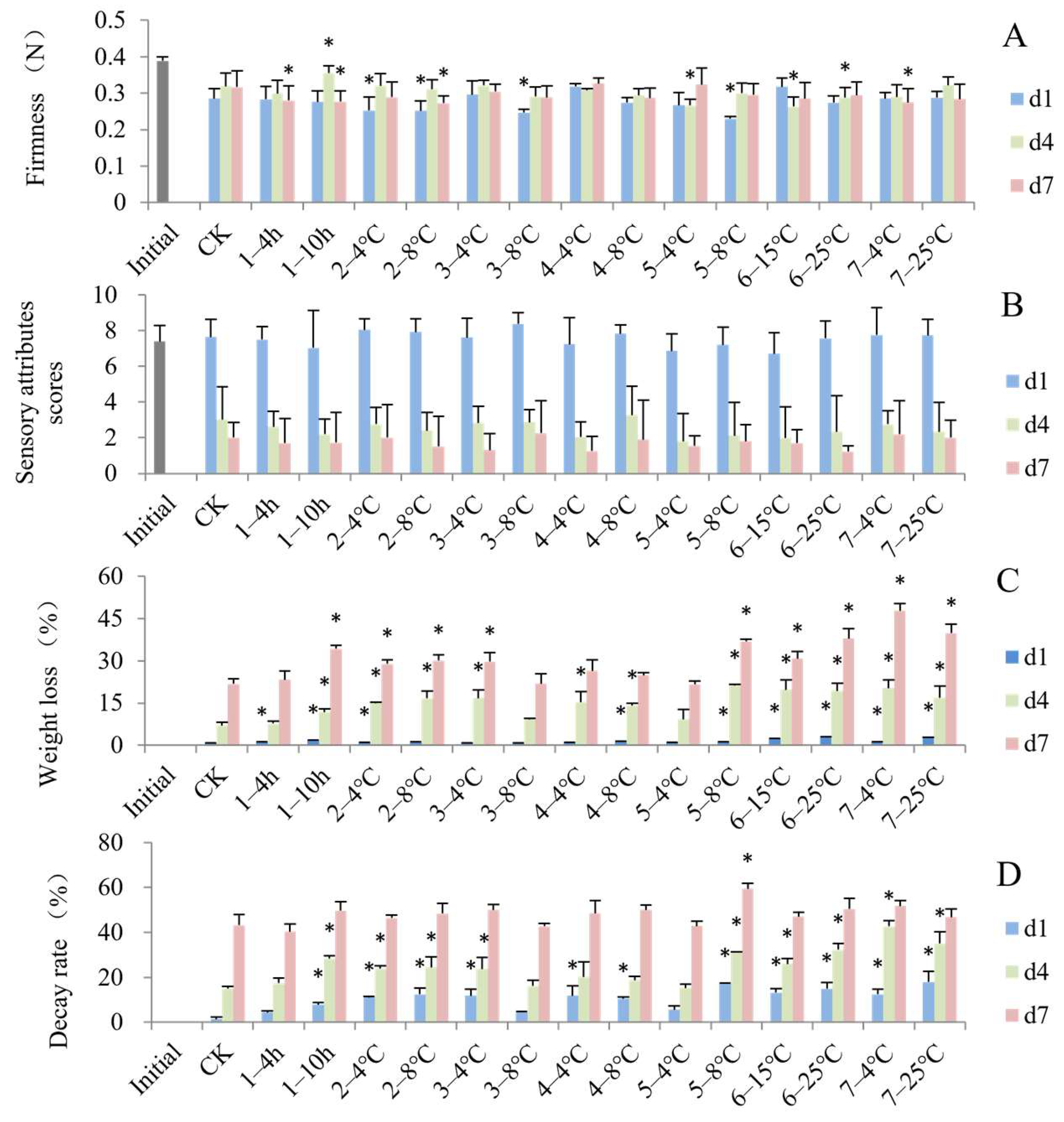
3.1. Impact of Simulated Cold Chain Steps on Blueberry Commercial Attributes
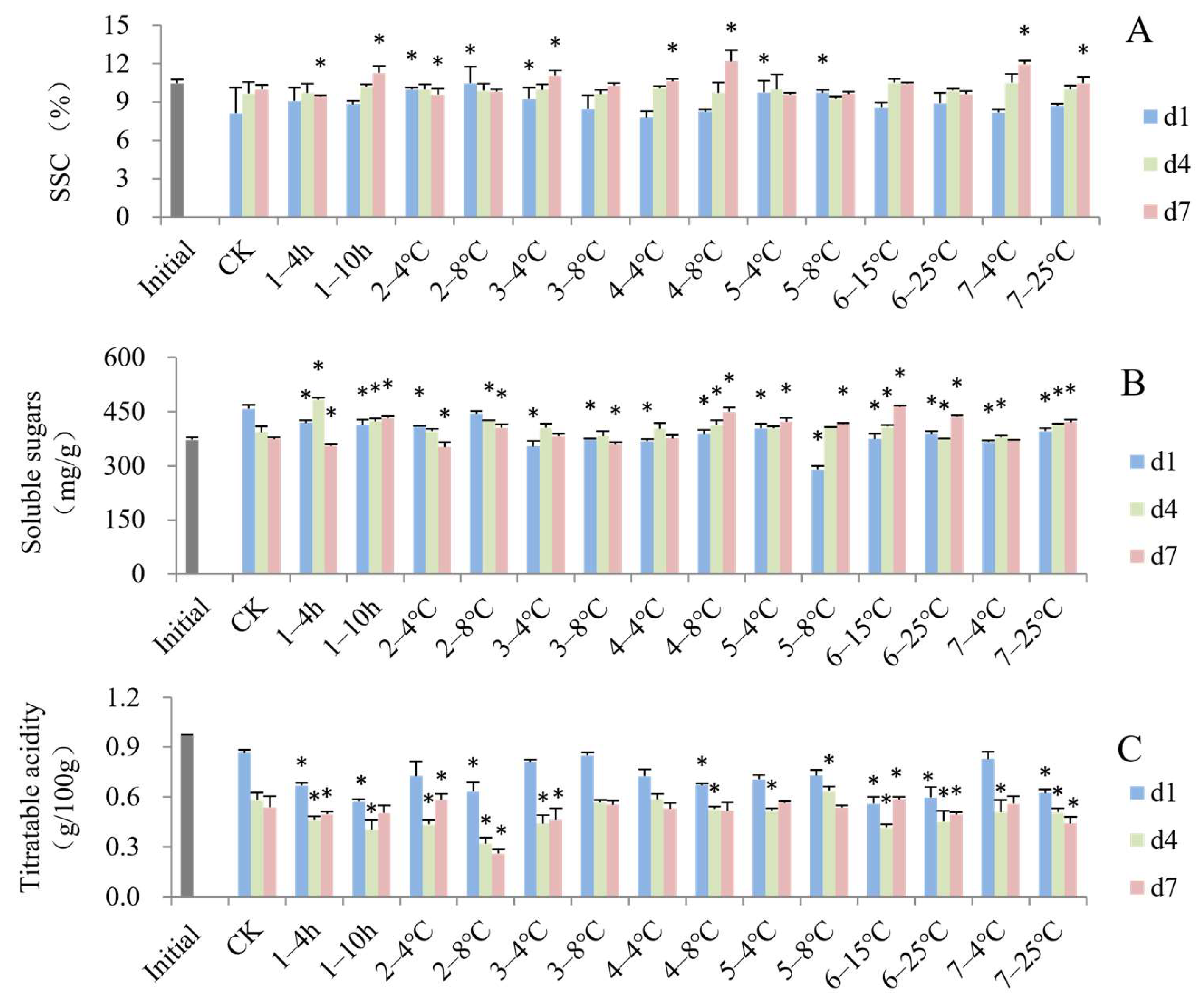
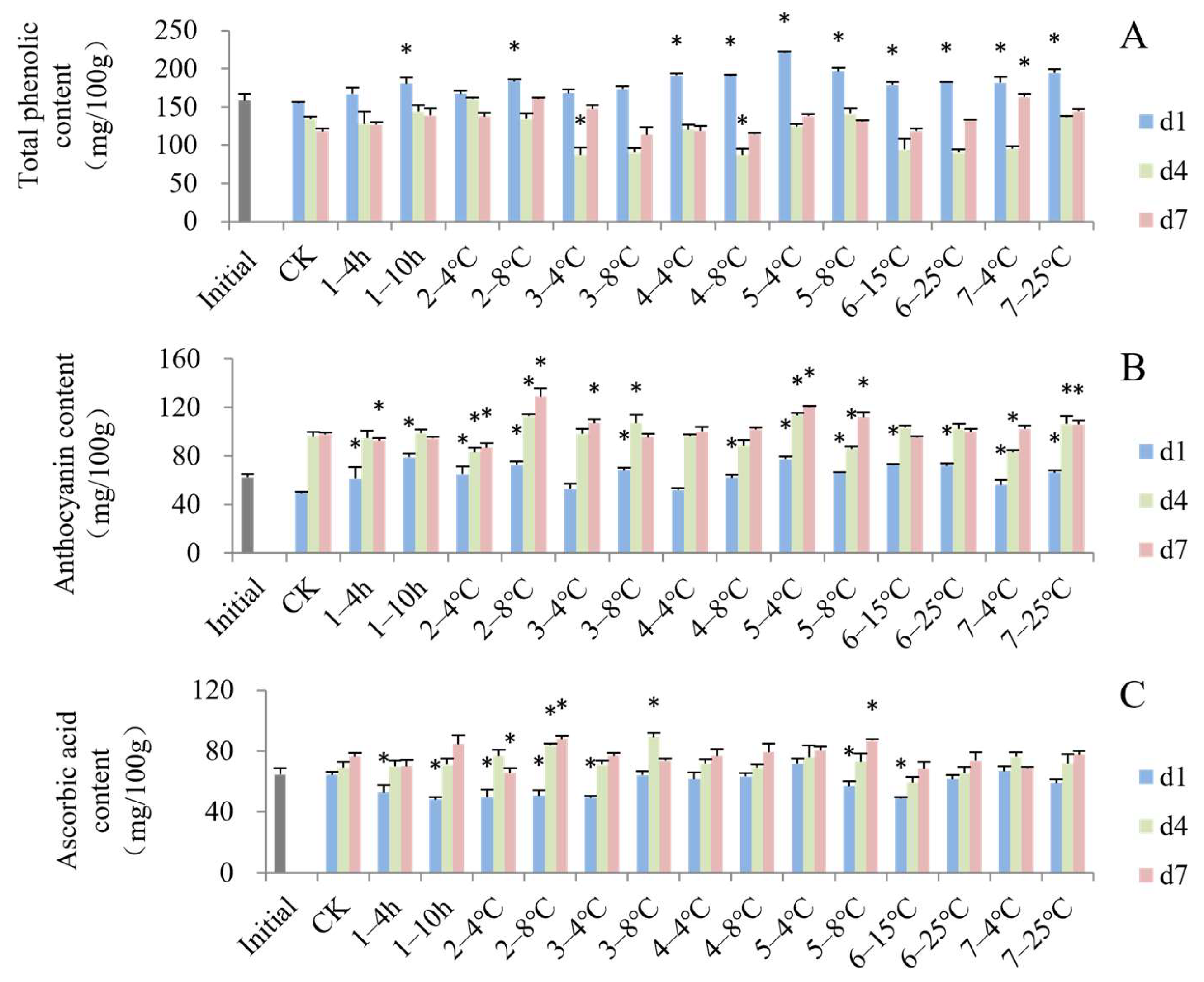
3.2. Impact of Simulated Cold Chain Steps on Blueberry Biochemical Attributes
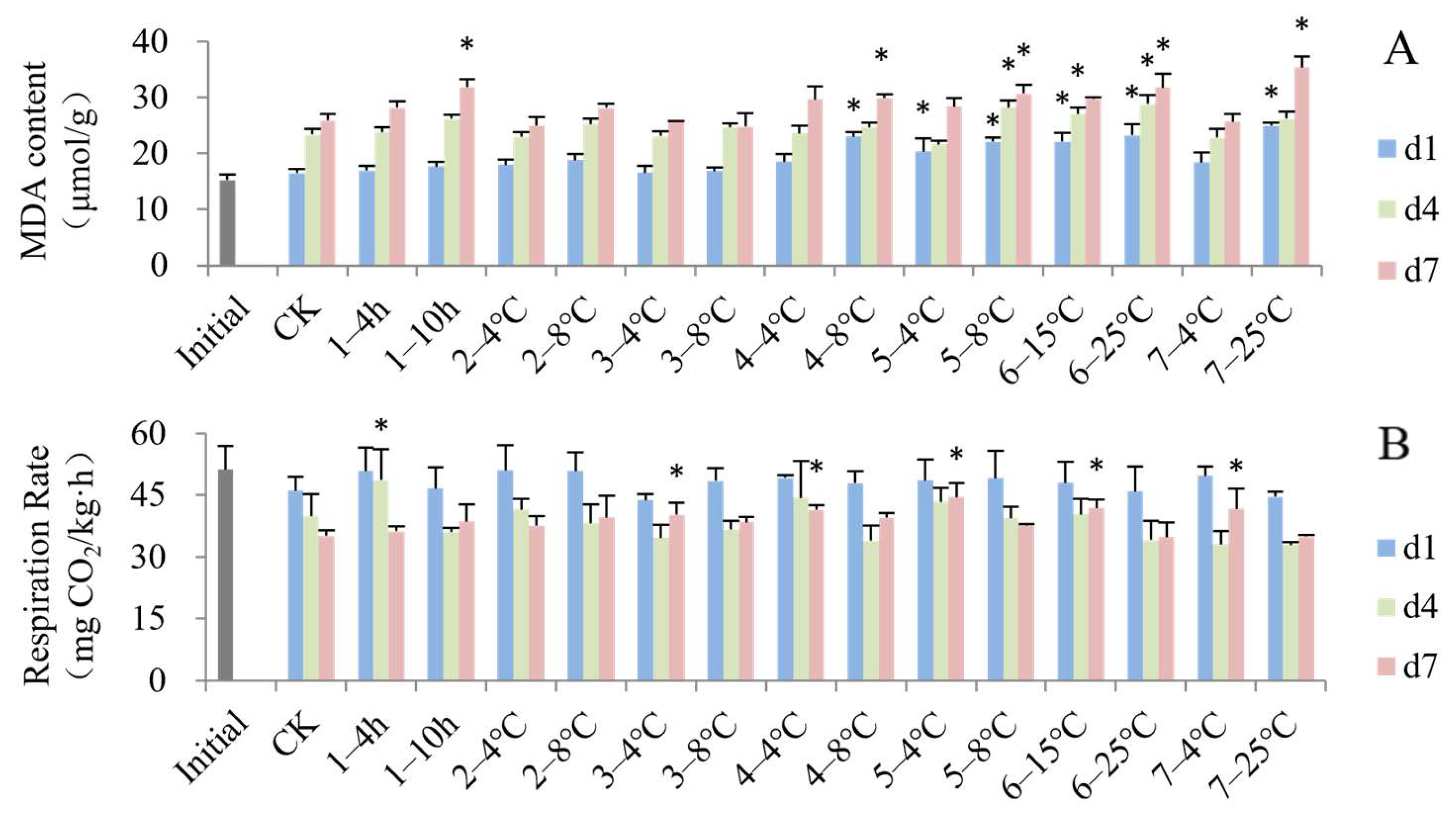
3.3. Impact of Simulated Cold Chain Steps on Blueberry Physiological Attributes
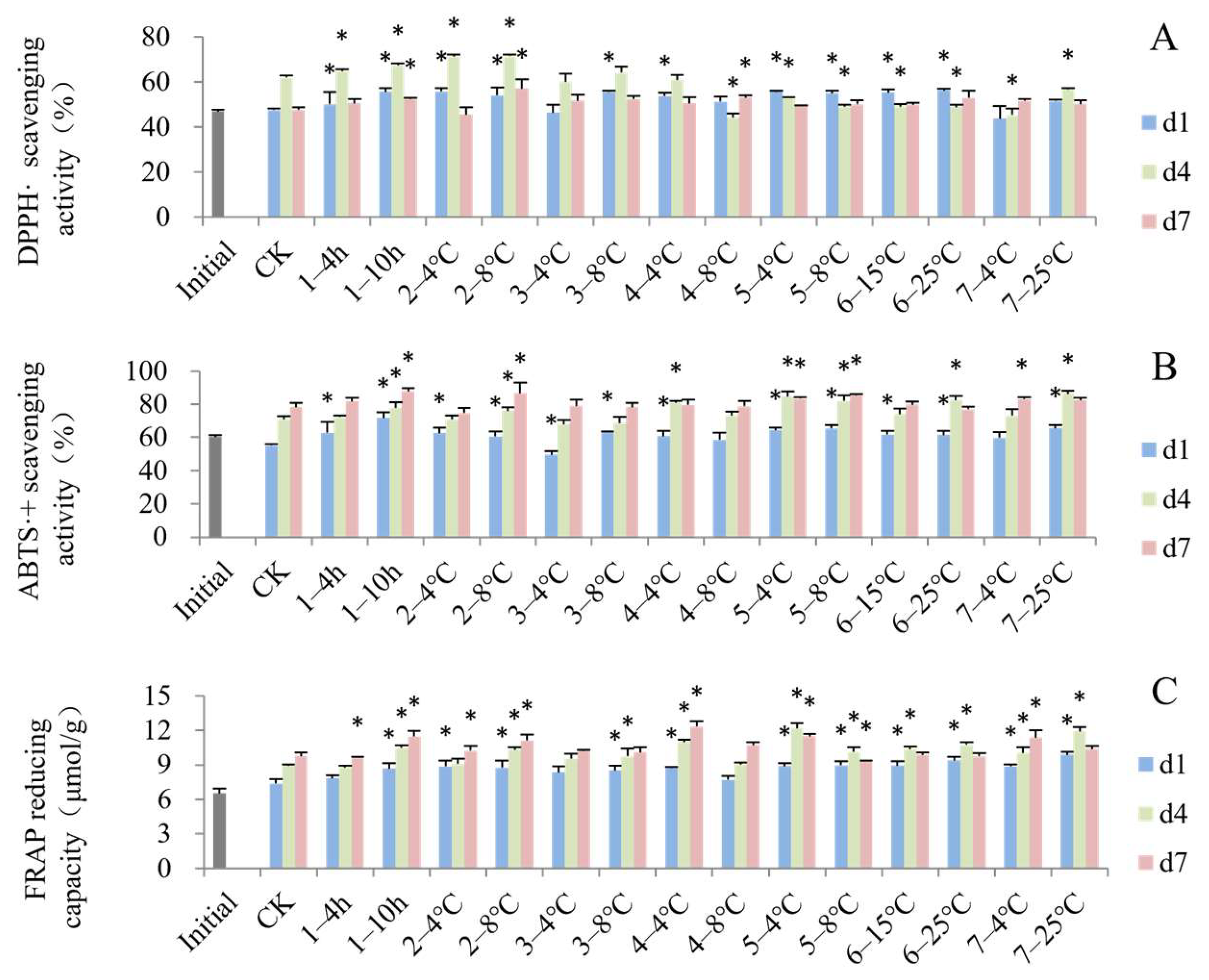
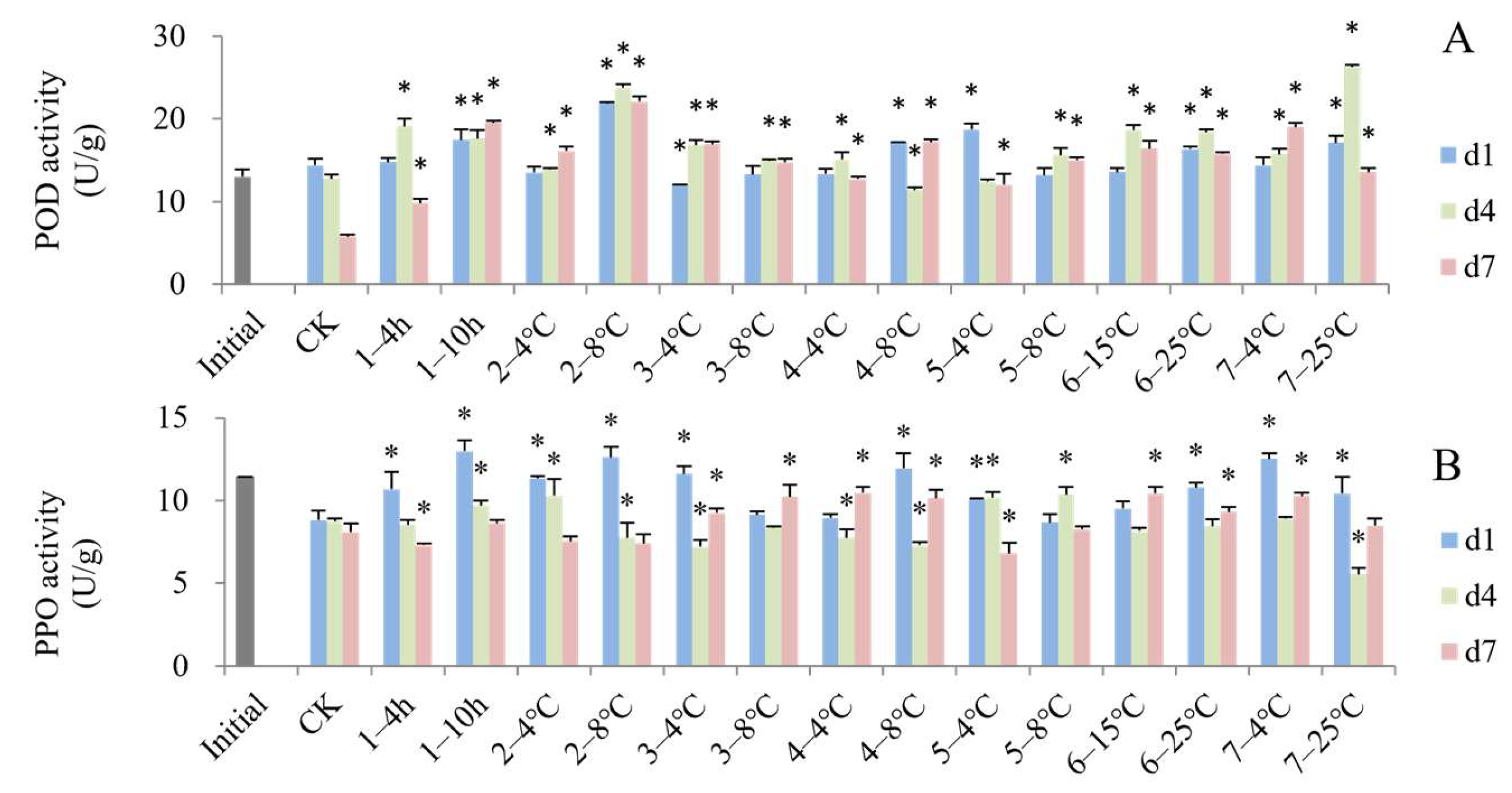
3.4. Impact of Simulated Cold Chain Steps on Blueberry Antioxidant Activity and Enzyme Activity
3.5. Critical Qualities in Response to Cold Chain Temperature
3.6. Critical Temperature Control Steps Along Simulated Cold Chains
3.6.1. Numbers of Qualities Affected by Different Simulated Cold Chain Steps
3.6.2. Evaluation of the Critical Steps by Comprehensive Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravichandran, K.S.; Krishnaswamy, K. Sustainable Food Processing of Selected North American Native Berries to Support Agroforestry. Food Sci. Nutr. 2023, 63, 4235–4260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.; Bian, S.; Hong, S.; Xu, K.; Zang, Y.; Zheng, W. Melatonin Improves the Storage Quality of Rabbiteye Blueberry (Vaccinium ashei) by Affecting Cuticular Wax Profile. Food Chem. X 2024, 21, 101106. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of Edible Coatings on the Quality of Fresh Blueberries (Duke and Elliott) under Commercial Storage Conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent Advances in Postharvest Technologies to Extend the Shelf Life of Blueberries (Vaccinium sp.), Raspberries (Rubus idaeus L.) and Blackberries (Rubus sp.). J. Berry Res. 2019, 9, 687–707. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.-P.; Brecht, J.K. Quality Curves for Highbush Blueberries as a Function of the Storage Temperature. Small Fruits Rev. 2004, 3, 423–440. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Y. A Novel Approach to Enhance Blueberry Quality During Storage Using Cold Plasma at Atmospheric Air Pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Jiang, X.; Ding, J.; Guo, L.; Chu, X.; Zhao, Y.; Chen, M.; Yan, J.; Dai, J.; Qin, W.; Liu, Y. Effects of H.Uvarum Combined with KGM on Postharvest Diseases of Blueberry. Food Biosci. 2023, 53, 102730. [Google Scholar] [CrossRef]
- Mercier, S.; Villeneuve, S.; Mondor, M.; Uysal, I. Time–Temperature Management Along the Food Cold Chain: A Review of Recent Developments. Compr. Rev. Food Sci. Food Saf. 2017, 16, 647–667. [Google Scholar] [CrossRef]
- Liu, G. Food Losses and Food Waste in China: A First Estimate; OECD Food, Agriculture and Fisheries Papers; OECD Publishing: Paris, France, 2014; Volume 66. [Google Scholar]
- Göransson, M.; Nilsson, F.; Jevinger, Å. Temperature Performance and Food Shelf-Life Accuracy in Cold Food Supply Chains–Insights from Multiple Field Studies. Food Control 2018, 86, 332–341. [Google Scholar] [CrossRef]
- Kelly, K.; Madden, R.; Emond, J.P.; Do Nascimento Nunes, M.C. A Novel Approach to Determine the Impact Level of Each Step along the Supply Chain on Strawberry Quality. Postharvest Biol. Technol. 2019, 147, 78–88. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Donnell, C.P.; Emond, J.P.; Do Nascimento Nunes, M.C. Blueberry Supply Chain: Critical Steps Impacting Fruit Quality and Application of a Boosted Regression Tree Model to Predict Weight Loss. Postharvest Biol. Technol. 2021, 179, 111590. [Google Scholar] [CrossRef]
- Rediers, H.; Claes, M.; Peeters, L.; Willems, K.A. Evaluation of the Cold Chain of Fresh-Cut Endive from Farmer to Plate. Postharvest Biol. Technol. 2009, 51, 257–262. [Google Scholar] [CrossRef]
- Kelly, K.; Yagiz, Y.; Li, Z.; Mahnken, G.; Borejsza-Wysocki, W.; Marshall, M.; Sims, C.A.; Peres, N. Sensory and Physicochemical Quality, Residual Fungicide Levels and Microbial Load in ‘Florida Radiance’ Strawberries from Different Disease Control Treatments Exposed to Simulated Supply Chain Conditions. Foods 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zuo, M.; Zhu, W.; Zuo, J.; Lü, E.; Yang, X. A Comprehensive Review of Cold Chain Logistics for Fresh Agricultural Products: Current Status, Challenges, and Future Trends. Trends Food Sci. Technol. 2021, 109, 536–551. [Google Scholar] [CrossRef]
- GB/T 22918-2008; Technical Requirements for Temperature-Controlled Transportation of Perishable Food. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 24616-2019; Packaging, Labeling, Transport and Storage for Chilled and Frozen Foods in Logistics. Standardization Administration of the People’s Republic of China: Beijing, China, 2019.
- Falagán, N.; Miclo, T.; Terry, L.A. Graduated Controlled Atmosphere: A Novel Approach to Increase “Duke” Blueberry Storage Life. Front. Plant Sci. 2020, 11, 221. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Han, Z.; He, X.; Herrera-Balandrano, D.D.; Xiang, J. Comprehensive Evaluation on Phenolic Derivatives and Antioxidant Activities of Diverse Yellow Maize Varieties. Food Chem. 2025, 464, 141602. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Zhang, C.; Cao, F.; Li, W. Methyl Jasmonate and Salicylic Acid Treatment Changes the Nutritional Quality, Antioxidant Profile and Gene Expression of Postharvest Blackberry Fruit. Postharvest Biol. Technol. 2025, 219, 113205. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, K.; Zhang, B.; Sun, C.; Cai, C.; Zhou, C.; Xu, W.; Zhang, W.; Ferguson, I.B. Postharvest Responses of Chinese Bayberry Fruit. Postharvest Biol. Technol. 2005, 37, 241–251. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, L.; Ai, J.; Ji, R.; He, L.; Liu, C. Effect of Heat Shock and Potassium Sorbate Treatments on Gray Mold and Postharvest Quality of ‘XuXiang’ Kiwifruit. Food Chem. 2020, 324, 126891. [Google Scholar] [CrossRef]
- Hu, T.; Gavahian, M.; Pradhan, R.; Lu, S.; Chu, Y. Functional, Antioxidant, and Sensory Properties of Mixed-fruit (Pitaya, Watermelon, and Mint) and Pitaya Wines. Food Sci. Nutr. 2023, 11, 3442–3449. [Google Scholar] [CrossRef]
- Huang, Q.; Wen, T.; Fang, T.; Lao, H.; Zhou, X.; Wei, T.; Luo, Y.; Xie, C.; Huang, Z.; Li, K. A Comparative Evaluation of the Composition and Antioxidant Activity of Free and Bound Polyphenols in Sugarcane Tips. Food Chem. 2025, 463, 14150. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Manda-Hakki, K.; Hassanpour, H. Effect of L-Glutathione Treatment on Biochemical Properties, Antioxidant Capacity and Antioxidant Enzymes Activity in Strawberry Fruits during Storage. Heliyon 2024, 10, e38046. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in Fruit Firmness, Cell Wall Composition and Cell Wall Degrading Enzymes in Postharvest Blueberries during Storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Mujumdar, A.S.; Jin, X.; Liu, Z.; Zhang, Y.; Xiao, H. Effects of Postharvest Ripening on Physicochemical Properties, Microstructure, Cell Wall Polysaccharides Contents (Pectin, Hemicellulose, Cellulose) and Nanostructure of Kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Z.; Yang, W.; Li, A.; Liu, J.; Zhang, Q.; Jiao, Z. Virtual Cold Chain Method to Evaluate the Effect of Rising Temperature on the Quality Evolution of Peach Fruit. Foods 2023, 12, 2403. [Google Scholar] [CrossRef]
- Xin, Y.; Jin, Z.; Chen, F.; Lai, S.; Yang, H. Effect of Chitosan Coatings on the Evolution of Sodium Carbonate-Soluble Pectin during Sweet Cherry Softening under Non-Isothermal Conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Law, C.L.; Ma, Y. Effect of Vibration and Broken Cold Chain on the Evolution of Cell Wall Polysaccharides during Fruit Cucumber (Cucumis sativus L.) Shriveling under Simulated Transportation. Food Packag. Shelf Life 2023, 38, 101126. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Mille, A.; Chiou, B.-S.; Takeoka, G.; Rubinsky, B.; Mchugh, T. Calcium Impregnation during Isochoric Cold Storage to Improve Postharvest Preservation of Fresh Blueberries. Postharvest Biol. Technol. 2024, 211, 112841. [Google Scholar] [CrossRef]
- Luo, K.; Li, Y.; Wang, C.; Kang, S.; Hu, X.; Zhang, L.; Li, X.; Al-Asmari, F.; Sameeh, M.Y.; Yang, B.; et al. Synergistic Bactericidal Effect of Ultrasound Combined with Oregano Essential Oil Nanoemulsion on Listeria Monocytogenes and Its Application in Blueberry Preservation. Food Control 2024, 165, 110619. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Wei, Z.W.; Yu, M.C.; Tsay, J.-S.; Hsu, M.-C.; Huang, P.H.; Liang, Y.S. Effects of Delayed Precooling on the Postharvest Quality and Anthracnose Incidence of “Irwin” Mangoes. J. Food Process. Preserv. 2024, 2024, 4004963. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An Overview of Current Status of Cold Chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Xu, S.; Chen, Y.; Wang, Y.; Li, X.; Sun, C. The Effects of Transportation Temperature on the Decay Rate and Quality of Postharvest Ponkan (Citrus reticulata Blanco) Fruit in Different Storage Periods. Sci. Hortic. 2019, 247, 42–48. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the Efficacy of Edible Coatings on Quality of Blueberry Fruits during Shelf-Life. LWT-Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Eum, H.L.; Hong, S.C.; Chun, C.; Shin, I.S.; Lee, B.Y.; Kim, H.K.; Hong, S.J. Influence of Temperature during Transport on Shelf-Life Quality of Highbush Blueberries (Vaccinium corymbosum L. Cvs. Bluetta, Duke). Hortic. Environ. Biotechnol. 2013, 54, 128–133. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Shelf-Life Extension of Highbush Blueberry Using 1-Methylcyclopropene Stored under Air and Controlled Atmosphere. Food Chem. 2011, 126, 1812–1816. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Zhang, F.; Ji, S. Ethylene Plays an Important Role in the Softening and Sucrose Metabolism of Blueberries Postharvest. Food Chem. 2020, 310, 125965. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.P. Quality of Strawberries after Storage in Constant or Fluctuating Temperatures. In Proceedings of the 20th International Congress of Refrigeration, Sydney, Australia, 19–24 September 1999. [Google Scholar]
- Ayala-Zavala, J.F.; Wang, S.; Wang, C.; González-Aguilar, G.A. Effect of Storage Temperatures on Antioxidant Capacity and Aroma Compounds in Strawberry Fruit. LWT-Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of Quality and Shelf-Life of Strawberries with Edible Coatings Enriched with Chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Causse, M. Fruit Load or Fruit Position Alters Response to Temperature and Subsequently Cherry Tomato Quality. J. Sci. Food Agric. 2005, 85, 1009–1016. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What Controls Fleshy Fruit Acidity? A Review of Malate and Citrate Accumulation in Fruit Cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M.; et al. Novel Roles for the Polyphenol Oxidase Enzyme in Secondary Metabolism and the Regulation of Cell Death in Walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; McDonald, J.E. Chemical Composition of Lowbush Blueberry Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 142–146. [Google Scholar] [CrossRef]
- Basiouny, F.M.; Chen, Y. Effects of Harvest Date, Maturity and Storage Intervals on Postharvest Quality of Rabbiteye Blueberries (Vaccinium ashei Reade). Process. Fla. State Hortic. Soc. 1988, 101, 281–284. [Google Scholar]
- Nunes, M.C.N.; Emond, J.P.; Brecht, J.K. Quality of strawberries as affected by temperature abuse during ground, in-flight and retail handling operations. Acta Hortic. 2003, 604, 239–246. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Nascimento, J.R.O.; Lajolo, F.M. Physico-Chemical Changes Related to Quality of Five Strawberry Fruit Cultivars during Cool-Storage. Food Chem. 2003, 83, 167–173. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Donnell, C.P.; Do Nascimento Nunes, M.C. Modelling the Biochemical and Sensory Changes of Strawberries during Storage under Diverse Relative Humidity Conditions. Postharvest Biol. Technol. 2019, 154, 148–158. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Controlling Temperature and Water Loss to Maintain Ascorbic Acid Levels in Strawberries during Postharvest Handling. J. Food Sci. 1998, 107, 1033–1036. [Google Scholar] [CrossRef]
- Loewus, F.A.; Loewus, M.W.; Seib, P.A. Biosynthesis and Metabolism of Ascorbic Acid in Plants. Crit. Rev. Plant Sci. 1987, 5, 101–119. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.; Luo, Z. Involvement of Energy Metabolism to Chilling Tolerance Induced by Hydrogen Sulfide in Cold-Stored Banana Fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef]
- Carvajal, F.; Palma, F.; Jamilena, M.; Garrido, D. Preconditioning Treatment Induces Chilling Tolerance in Zucchini Fruit Improving Different Physiological Mechanisms against Cold Injury. Ann Appl Biol 2015, 166, 340–354. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of Postharvest Temperature on Apoptosis-Related Enzyme Activity and Gene Expression in Peach Fruits (Prunus persica L. Cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, Y.; Pan, Y.; Li, X.; Liu, H.; Tao, S. Enhanced Cyanide-Resistant Respiration as the Predominant Respiratory Metabolic Pathway in Abnormal Chilling Injury Behavior of Postharvest Papaya. Postharvest Biol. Technol. 2023, 205, 112505. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Zhang, Q.; Wang, Y.; Dia, V.P.; Meng, X. Ripening Affects the Physicochemical Properties, Phytochemicals and Antioxidant Capacities of Two Blueberry Cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Ford, B.L.; Baldwin, E.A. Effect of Storage Temperature on Chilling Injury and Activity of Antioxidant Enzymes in Carambola “Arkin” Fruit. J. Food Process. Preserv. 2021, 45, e15178. [Google Scholar] [CrossRef]
- Wan, C.; Kahramanoğlu, İ.; Chen, J.; Gan, Z.; Chen, C. Effects of Hot Air Treatments on Postharvest Storage of Newhall Navel Orange. Plants 2020, 9, 170. [Google Scholar] [CrossRef]
- Liu, C.; Lin, K.; Kuo, Y. Application of Factor Analysis in the Assessment of Groundwater Quality in a Blackfoot Disease Area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Sargent, S.A.; Morais, A.M.M.B. Effects of Delays to Cooling and Wrapping on Strawberry Quality (Cv. Sweet Charlie). Food Control 1995, 6, 323–328. [Google Scholar] [CrossRef]
- Liu, J.; Lundqvist, J.; Weinberg, J.; Gustafsson, J. Food Losses and Waste in China and Their Implication for Water and Land. Environ. Sci. Technol. 2013, 47, 10137–10144. [Google Scholar] [CrossRef]

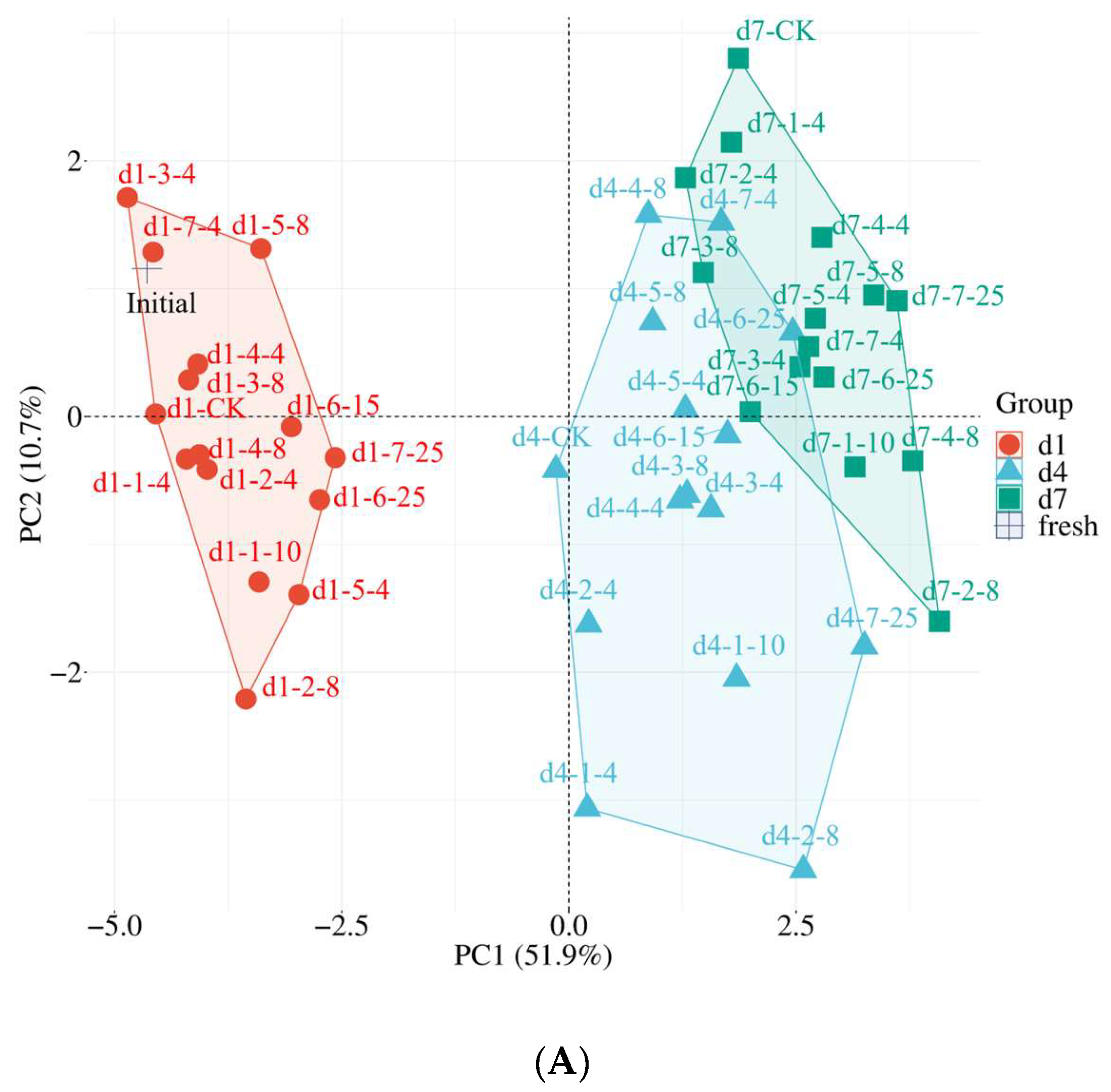
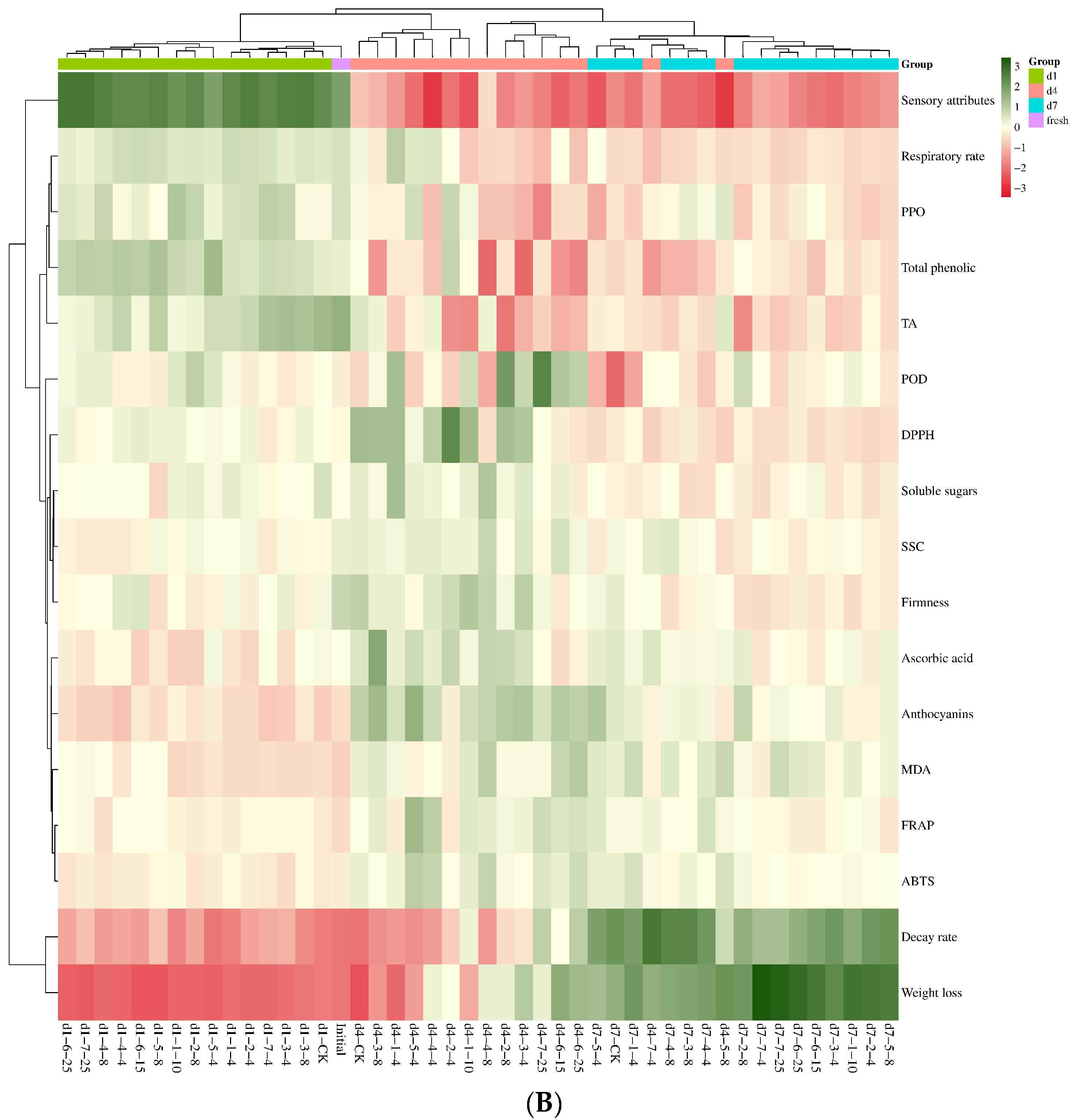
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalues | 8.820 | 1.811 | 1.447 |
| Contribution rate (%) | 51.882 | 10.654 | 8.510 |
| Cumulative contribution rate (%) | 51.882 | 62.535 | 71.045 |
| Sensory attributes | 0.963 | 0.057 | 0.113 |
| Anthocyanins | −0.922 | 0.156 | −0.025 |
| ABTS·+ | −0.916 | −0.019 | 0.129 |
| MDA | 0.872 | 0.089 | −0.162 |
| Weight loss | 0.868 | 0.222 | −0.256 |
| Decay rate | 0.860 | 0.268 | −0.262 |
| Respiratory rate | 0.816 | 0.181 | 0.228 |
| TA | 0.807 | −0.336 | 0.015 |
| FRAP | −0.798 | 0.017 | 0.220 |
| Ascorbic acid | −0.765 | 0.030 | −0.209 |
| Total phenolics | 0.690 | 0.179 | 0.356 |
| SSC | −0.642 | 0.036 | 0.297 |
| PPO | 0.608 | 0.035 | 0.410 |
| DPPH· | −0.124 | 0.784 | −0.320 |
| POD | −0.217 | 0.728 | 0.349 |
| Soluble sugars | −0.233 | 0.573 | 0.044 |
| Firmness | −0.191 | 0.013 | −0.709 |
| Step | d1 | d4 | d7 | Total |
|---|---|---|---|---|
| 1—Delayed precooling for 4 h | 8 | 5 | 8 | 21 |
| 1—Delayed precooling for 10 h | 12 | 10 | 9 | 31 |
| 2—Stored in grower for 12 h at 4 °C | 11 | 7 | 8 | 26 |
| 2—Stored in grower for 12 h at 8 °C | 12 | 11 | 10 | 33 |
| 3—Transported to DC for 24 h, 4 °C | 7 | 6 | 7 | 20 |
| 3—Transported to DC for 24 h, 8 °C | 6 | 4 | 3 | 13 |
| 4—Stored at DC for 48 h, 4 °C | 6 | 5 | 5 | 16 |
| 4—Stored at DC for 48 h, 8 °C | 9 | 7 | 6 | 22 |
| 5—Transported to stores for 6 h, 4 °C | 10 | 7 | 7 | 24 |
| 5—Transported to stores for 6 h, 8 °C | 12 | 10 | 9 | 31 |
| 6—Displayed in stores for 48 h, 15 °C | 11 | 9 | 6 | 26 |
| 6—Displayed in stores 48 h, 25 °C | 12 | 10 | 6 | 28 |
| 7—Consumer storage for 24 h, 4 °C | 7 | 8 | 9 | 24 |
| 7—Consumer storage for 24 h, 25 °C | 11 | 10 | 7 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Liu, H.; Yang, W.; Zhang, Q.; Lv, Z.; Nawaz, M.; Jiao, Z.; Liu, J. Virtual Cold Chain Method with Comprehensive Evaluation to Reveal the Effects of Temperature Abuse on Blueberry Quality. Foods 2024, 13, 3731. https://doi.org/10.3390/foods13233731
Fu M, Liu H, Yang W, Zhang Q, Lv Z, Nawaz M, Jiao Z, Liu J. Virtual Cold Chain Method with Comprehensive Evaluation to Reveal the Effects of Temperature Abuse on Blueberry Quality. Foods. 2024; 13(23):3731. https://doi.org/10.3390/foods13233731
Chicago/Turabian StyleFu, Min, Hui Liu, Wenbo Yang, Qiang Zhang, Zhenzhen Lv, Muhammad Nawaz, Zhonggao Jiao, and Jiechao Liu. 2024. "Virtual Cold Chain Method with Comprehensive Evaluation to Reveal the Effects of Temperature Abuse on Blueberry Quality" Foods 13, no. 23: 3731. https://doi.org/10.3390/foods13233731
APA StyleFu, M., Liu, H., Yang, W., Zhang, Q., Lv, Z., Nawaz, M., Jiao, Z., & Liu, J. (2024). Virtual Cold Chain Method with Comprehensive Evaluation to Reveal the Effects of Temperature Abuse on Blueberry Quality. Foods, 13(23), 3731. https://doi.org/10.3390/foods13233731








