Physiological–Biochemical Characteristics and a Transcriptomic Profiling Analysis Reveal the Postharvest Wound Healing Mechanisms of Sweet Potatoes under Ascorbic Acid Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sweet Potato Roots
2.2. Artificial Wounding
2.3. AA Treatment and Wound Healing
2.4. Sampling
2.5. Determination of Weight Loss Rate
2.6. Determination of Disease Index
2.7. Determination of Firmness
2.8. Staining and Microscopy
2.9. Determination of Total Phenol, Total Flavonoid, and Lignin Contents
2.10. Determination of Individual Phenolic Acid and Lignin Monomer Contents
2.11. Determination of the Malondialdehyde (MDA) Content
2.12. Determination of the Permeability of Cell Membranes
2.13. Determination of PAL, C4H, 4CL, Cinnamyl Alcohol Dehydrogenase (CAD), and POD Activities
2.14. RNA Sequencing
2.15. Quantitative Real-Time PCR (qRT-PCR)
2.16. Statistical Analysis
3. Results
3.1. Weight Loss Rate, Disease Index, Firmness, and Appearance Changes
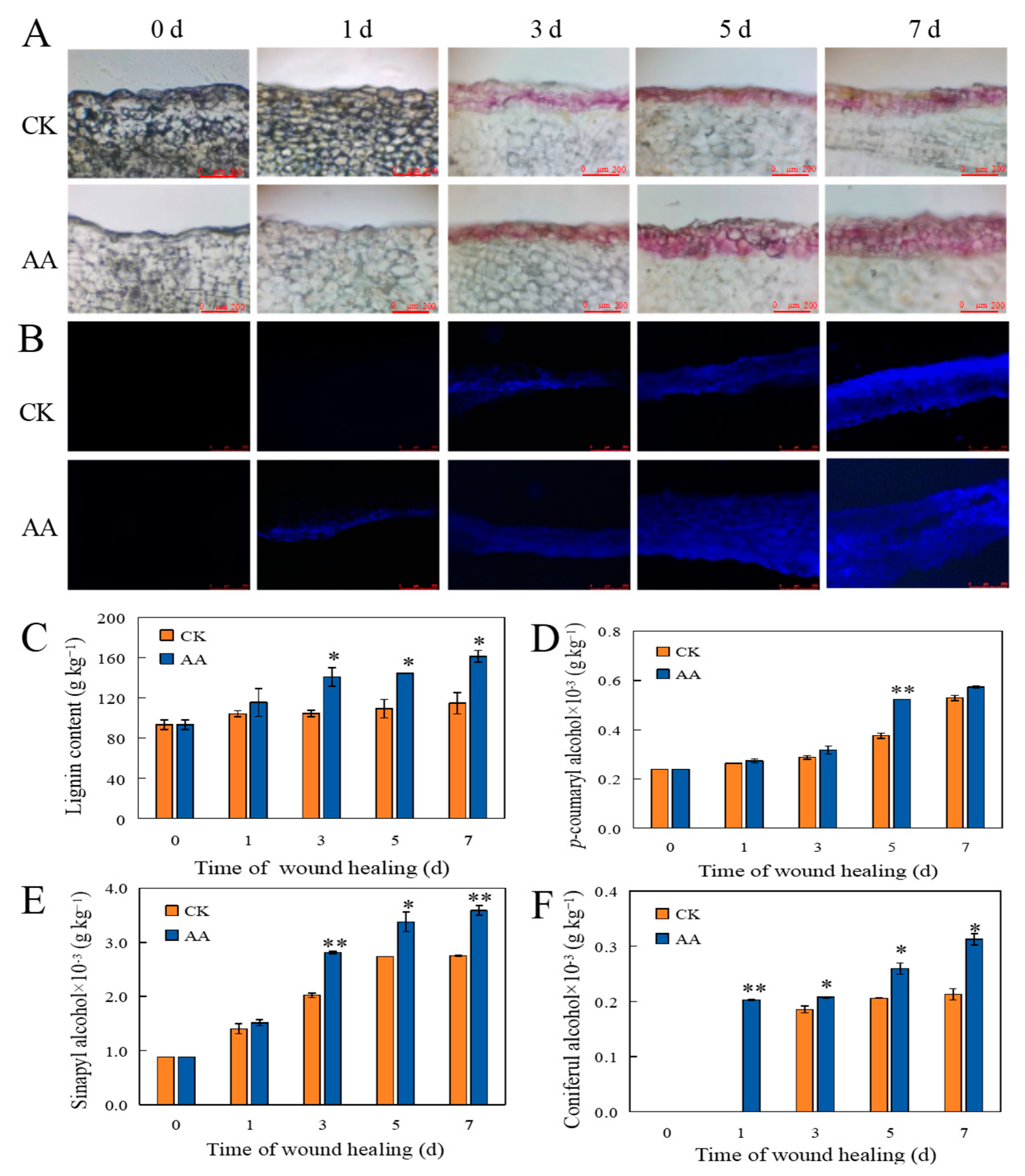
3.2. The Accumulation of Lignin and SPP
3.3. Total Lignin, Phenol, and Flavonoid Contents
3.4. The Contents of Individual Phenolic Acids and Lignin Monomers
3.5. The Contents of MDA and the Permeability of Cell Membranes
3.6. The Activities of PAL, C4H, C4L, CAD, and POD
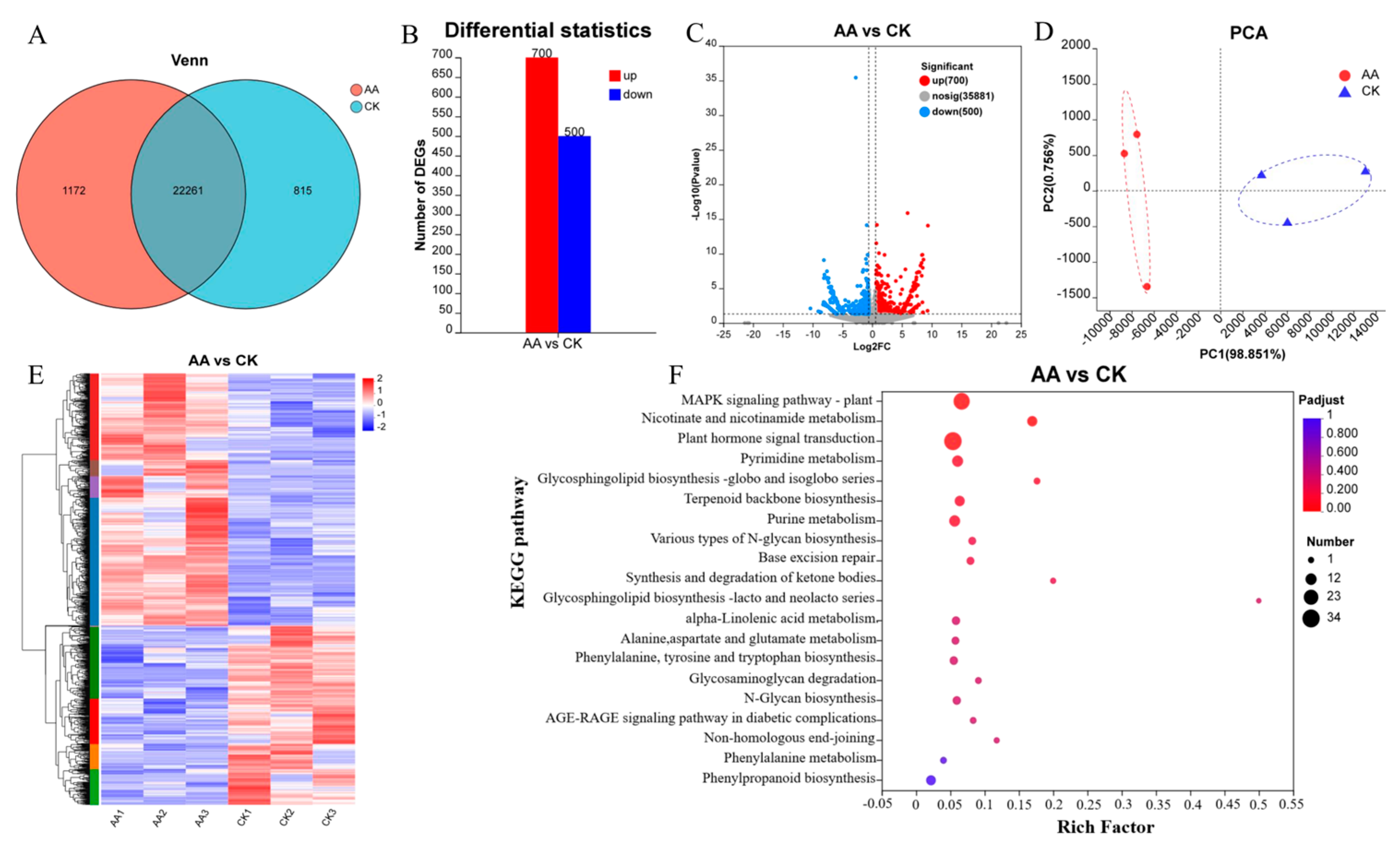
3.7. Analysis of Transcriptomic Data from Sweet Potato Wound Sites
3.7.1. Overview of the RNA Sequencing Data
3.7.2. Related Genes That Play a Role in Healing after AA Treatment
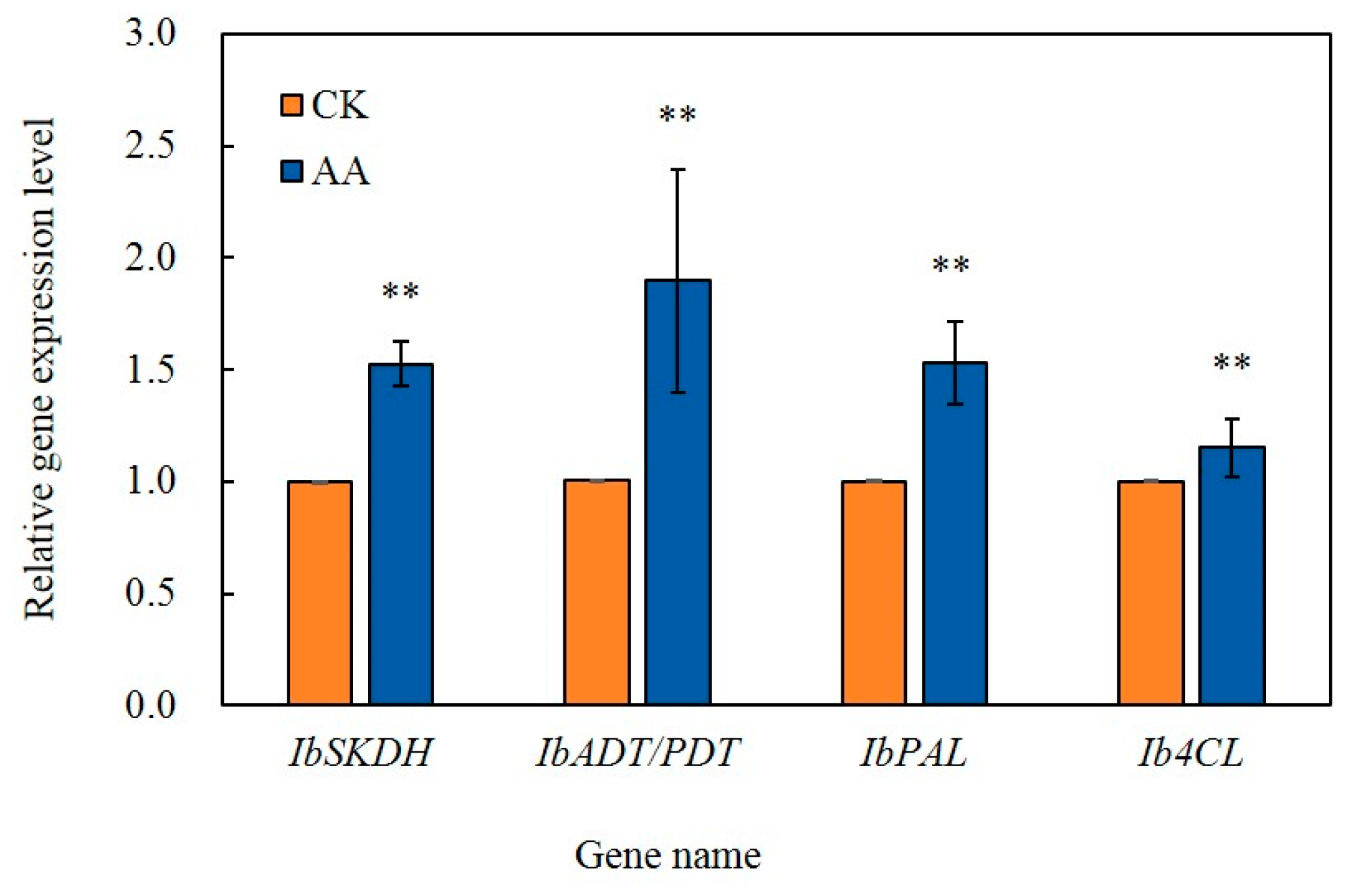
3.8. Analysis by qRT-PCR
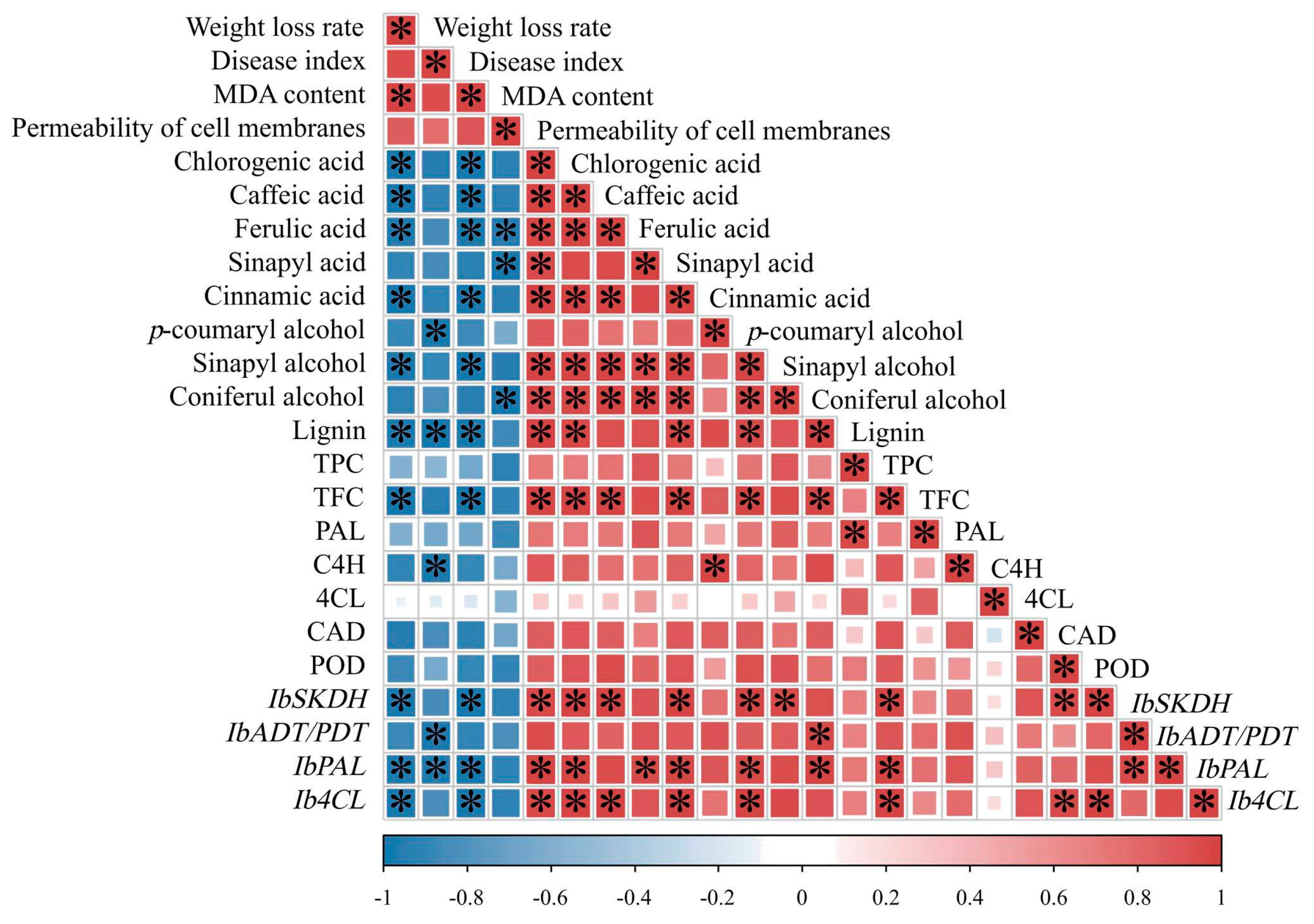
3.9. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vithu, P.; Dash, S.K.; Rayaguru, K. Post-harvest processing and utilization of sweet potato: A review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar] [CrossRef]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Kirchner, S.M.; Sturm, B.; Hensel, O. Pre-harvest curing: Effects on skin adhesion, chemical composition and shelf-life of sweetpotato roots under tropical conditions. East Afr. Agri. For. J. 2017, 82, 130–143. [Google Scholar] [CrossRef]
- Huang, C.L.; Liao, W.C.; Chan, C.F.; Lai, Y.C. Storage performance of Taiwanese sweet potato cultivars. J. Food Sci. Technol. 2014, 51, 4019–4025. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, L.; Peng, C.L.; Shang, X.Q.; Lv, X.L.; Sun, J.; Li, C.; Wei, L.; Liu, X.L. Postharvest benzothiazole treatment enhances healing in mechanically damaged sweet potato by activating the phenylpropanoid metabolism. J. Sci. Food Agr. 2020, 100, 3394–3400. [Google Scholar] [CrossRef]
- Yang, R.; Han, Y.; Han, Z.; Ackah, S.; Li, Z.; Bi, Y.; Yang, Q.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, B.; Sun, J.; Fan, X.; Li, X.; Jiang, L.; Hao, G.; Pei, H.; Zhou, X. Heat shock treatment promoted callus formation on postharvest sweet potato by adjusting active oxygen and phenylpropanoid metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Ji, C.Y.; Kim, Y.H.; Lee, C.J.; Su, U.P.; Lee, H.U.; Kwak, S.S.; Kim, H.S. Comparative transcriptome profiling of sweetpotato storage roots during curing-mediated wound healing. Gene 2022, 833, 146592. [Google Scholar] [CrossRef]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO·/O2−· radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Sakimin, S.Z.; Patrie, S.S.; Juraimi, A.S.; Alam, M.A.; Aslani, F. Application of ascorbic acid in maintenance of minimally processed product quality of jackfruit (Artocarpus heterophyllus Lam). Bangl. J. Bot. 2017, 46, 413–418. [Google Scholar] [CrossRef]
- Zhao, H.; Nie, K.; Zhou, H.; Yan, X.; Zhan, Q.; Zheng, Y.; Song, C.P. ABI5 modulates seed germination via feedback regulation of the expression of the PYR/PYL/RCAR ABA receptor genes. New Phytol. 2020, 228, 596–608. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Lin, H.; Lin, M.; Fan, Z. Impacts of exogenous ROS scavenger ascorbic acid on the storability and quality attributes of fresh longan fruit. Food Chem. X 2021, 12, 100167. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, C.; Zhou, F.; Liu, C.; Tian, M.; Zeng, X.; Jiang, A. Vacuum packaging and ascorbic acid synergistically maintain the quality and flavor of fresh-cut potatoes. LWT Food. Sci. Technol. 2022, 162, 113356. [Google Scholar] [CrossRef]
- Antonova, G.F.; Chaplygina, I.A.; Varaksina, T.N.; Stasova, V.V. Ascorbic acid and xylem development in trunks of the Siberian larch trees. Russ. J. Plant Physiol. 2005, 52, 83–92. [Google Scholar] [CrossRef]
- Kärkönen, A.; Fry, S.C. Effect of ascorbate and its oxidation products on H2O2 production in cell-suspension cultures of Picea abies and in the absence of cells. J. Exp. Bot. 2006, 57, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Çoban, G.A.; Aras, S. Effects of ascorbic and oxalic acids on cucumber seedling growth and quality under mildly limey soil conditions. Gesunde Pflanz. 2023, 75, 1925–1932. [Google Scholar] [CrossRef]
- Xue, S.; Bi, Y.; Ackah, S.; Li, Z.; Li, B.; Wang, B.; Wang, Y.; Li, Y.; Prusky, D. Sodium silicate treatment accelerates biosynthesis and polymerization of suberin polyaliphatics monomers at wounds of muskmelon. Food Chem. 2023, 417, 135847. [Google Scholar] [CrossRef]
- Zhu, Y.; Zong, Y.; Liang, W.; Kong, R.; Gong, D.; Han, Y.; Li, Y.; Bi, Y.; Prusky, D. Sorbitol immersion accelerates the deposition of suberin polyphenolic and lignin at wounds of potato tubers by activating phenylpropanoid metabolism. Sci. Hortic. 2022, 297, 110971. [Google Scholar] [CrossRef]
- Wu, J.; Pang, L.; Zhang, X.; Lu, X.; Yin, L.; Lu, G.; Cheng, J. Early discrimination and prediction of C. fimbriata-infected sweetpotatoes during the asymptomatic period using electronic nose. Foods 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, G.A.; Kyriacou, M.C.; Siomos, A.S.; Gerasopoulos, D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Zhang, T.T.; Wang, F.L.; Guan, W.Q.; Lin, Q.; Sun, X.L. The combination treatment of low voltage electrostatic field and Ultraviolet-C could accelerate the process of wound healing of potato tubers. LWT Food. Sci. Technol. 2024, 10, 116466. [Google Scholar] [CrossRef]
- Fugate, K.K.; Ribeiro, W.S.; Lulai, E.C.; Deckard, E.L.; Finger, F.L. Cold temperature delays wound healing in postharvest sugarbeet roots. Front. Plant Sci. 2016, 7, 499. [Google Scholar] [CrossRef]
- Torres, A.; Basurto, F.; Navarro-Ocana, A. Quantitative analysis of the biologically active compounds present in leaves of mexican sweet potato accessions: Phenols, flavonoids, anthocyanins, 3,4,5-tri-caffeoylquinic acid and 4-feruloyl-5-caffeoylquinic acid. Plant Food Hum. Nutr. 2019, 74, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Gruz, J.; Novak, O.; Strnad, M. Separation, characterization, and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) fruit by HPLC-MS. J. Agric. Food Chem. 2005, 53, 8116–8122. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Li, C.; Wang, B.; Ma, L.; Ren, Y.; Bi, Y.; Li, Y.; Xue, H.; Prusky, D. The effect of benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) treatment on regulation of reactive oxygen species metabolism involved in wound healing of potato tubers during postharvest. Food Chem. 2020, 309, 125608. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.F.; Wu, W.N.; Ba, L.J.; Ma, C.; Ji, N.; Cao, S. Melatonin enhances the postharvest disease resistance of blueberries fruit by modulating the jasmonic acid signaling pathway and phenylpropanoid metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Wu, C.; Fan, G.; Li, T. Methyl jasmonate induces the resistance of postharvest blueberry to gray mold caused by Botrytis cinerea. J. Sci. Food Agric. 2020, 100, 4272–4281. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.T.; Han, Y.; Liang, C.Q.; Jin, M.J.; Zhang, Z.K.; Rao, J.P. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Li, S.E.; Xu, Y.H.; Bi, Y.; Zhang, B.; Shen, S.L.; Jiang, T.J.; Zheng, X.L. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Liu, J.; Qu, L.; Ge, Y. L-glutamate maintains the quality of apple fruit by mediating carotenoid, sorbitol and sucrose metabolisms. J. Sci. Food Agric. 2023, 104, 4944–4955. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zeng, K.; Ming, J. Control of blue and green mold decay of citrus fruit by Pichia membranefaciens and induction of defense responses. Sci. Hortic. 2012, 135, 120–127. [Google Scholar] [CrossRef]
- Yan, S.; Luo, Y.; Zhou, B.; Ingram, D.T. Dual effectiveness of ascorbic acid and ethanol combined treatment to inhibit browning and inactivate pathogens on fresh-cut apples. LWT Food Sci. Technol. 2017, 80, 311–320. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, S.; Ma, Y.; Zhao, W.; Wang, P.; Zhao, S.; Wang, D. Ascorbic acid prevents yellowing of fresh-cut yam by regulating pigment biosynthesis and energy metabolism. Food Res. Int. 2022, 157, 111424. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Machado, A.; Pereira, H.; Teixeira, R.T. Anatomy and development of the endodermis and phellem of Quercus suber L. roots. Microsc. Microanal. 2013, 19, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Han, Y.; Zhang, X.; Wang, Q.; Zheng, X.; Wang, Y.; Li, Y.; Prusky, D.; Bi, Y. Ferulic acid treatment enhances the synthesis, transport and deposition of suberin polyaliphatic monomers on potato tuber wounds. Postharvest Biol. Technol. 2023, 203, 112402. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.; Bi, Y.; He, X.; Wang, Y.; Li, Y.; Zheng, X.; Prusky, D. Preharvest multiple sprays with sodium nitroprusside promote wound healing of harvested muskmelons by activation of phenylpropanoid metabolism. Postharvest Bio. Technol. 2019, 158, 110988. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Cui, P.; Li, Y.X.; Cui, C.K.; Huo, Y.R.; Lu, G.Q.; Yang, H.Q. Proteomic and metabolic profile analysis of low-temperature storage responses in Ipomoea batata Lam. tuberous roots. BMC Plant Biol. 2020, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Bai, S.; Wu, F.; Lu, G.; Yang, H. Effect of nitric oxide on the activity of phenylalanine ammonia-lyase and antioxidative response in sweetpotato root in relation to wound-healing. Postharvest Bio. Technol. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Stark, R.E. Modeling suberization with peroxidase-catalyzed polymerization of hydroxycinnamic acids: Cross-coupling and dimerization reactions. Phytochemistry 2006, 67, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Sutela, S.; Hahl, T.; Tiimonen, H.; Aronen, T.; Ylioja, T.; Laakso, T.; Saranpää, P.; Chiang, V.; Julkunen-Tiitto, R.; Häggman, H. Phenolic compounds and expression of 4CL genes in silver birch clones and Pt4CL1a lines. PLoS ONE 2014, 9, e114434. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.U.; Zhang, X.; Ma, Z.; Huang, M.; Yang, C.; Wang, X.; Peng, J. Contribution of the LAC genes in fruit quality attributes of the fruit-bearing plants: A comprehensive review. Int. J. Mol. Sci. 2023, 24, 15768. [Google Scholar] [CrossRef] [PubMed]
- Mahto, R.; Das, M. Effect of gamma irradiation on the physico-mechanical and chemical properties of potato (Solanum tuberosum L.), cv. ‘Kufri Sindhuri’, in non-refrigerated storage conditions. Postharvest Biol. Technol. 2014, 92, 37–45. [Google Scholar] [CrossRef]
- Korth, K.L.; Blount, J.W.; Chen, F.; Rasmussen, S.; Lamb, C.; Dixon, R.A. Changes in phenylpropanoid metabolites associated with homology-dependent silencing of phenylalanine ammonia-lyase and its somatic reversion in tobacco. Physiol. Plant. 2001, 111, 137–143. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Stout, J.; Weng, J.K.; Humphreys, J.; Ruegger, M.O.; Chapple, C. Mutations in the cinnamate 4-hydroxylase gene impact metabolism, growth and development in Arabidopsis. Plant J. 2009, 60, 771–782. [Google Scholar] [CrossRef]
- Thévenin, J.; Pollet, B.; Letarnec, B.; Saulnier, L.; Gissot, L.; Maia-Grondard, A.; Lapierre, C.; Jouanin, L. The simultaneous repression of CCR and CAD, two enzymes of the lignin biosynthetic pathway, results in sterility and dwarfism in Arabidopsis thaliana. Mol. Plant 2011, 4, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Veronico, P.; Paciolla, C.; Pomar, F.; De Leonardis, S.; García-Ulloa, A.; Melillo, M.T. Changes in lignin biosynthesis and monomer composition in response to benzothiadiazole and root-knot nematode Meloidogyne incognita infection in tomato. J. Plant Physiol. 2018, 230, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Chang, Y.; Li, B.; Shao, S.L.; Zhang, Z.Z. Functional analysis of 4-coumarate: CoA ligase from Dryopteris fragrans in transgenic tobacco enhances lignin and flavonoids. Genet. Mol. Biol. 2020, 43, e20180355. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, Q.E.A.; Rees, D.; Aked, J.; Kihurani, A. Sweetpotato cultivars differ in efficiency of wound healing. Postharvest Biol. Technol. 2006, 42, 65–74. [Google Scholar] [CrossRef]



| Function | Annotation | Gene ID | Gene Expression (FPKM Value) | |
|---|---|---|---|---|
| CK | AA | |||
| Phenylalanine, Tyrosine, Tryptophan biosynthesis | 3-dehydroquinate dehydratase/shikimate dehydrogenase (SKDH) | itf13g19040.t1 | 42.7 ± 5.4 | 75.6 ± 12.3 * |
| phosphoribosylanthranilate isomerase (trpF) | itf13g19040.t2 | 1.2 ± 0.6 | 2.5 ± 0.3 * | |
| itf05g14750.t2 | 1.5 ± 1.7 | 0.0 ± 0.0 | ||
| arogenate/prephenate dehydratase (ADT/PDT) | itf04g31570.t1 | 71.8 ± 19.3 | 126.6 ± 11.5 * | |
| tyrosine aminotransferase (TAT) | itf07g12100.t1 | 15.8 ± 2.8 | 25.9 ± 2.4 ** | |
| itf02g12580.t1 | 0.0 ± 0.0 | 8.7 ± 5.1 * | ||
| Phenylpropanoid Biosynthesis | phenylalanine ammonia-lyase (PAL) | itf06g07070.t1 | 110.8 ± 30.7 | 217.9 ± 58.4 * |
| feruloyl-CoA 6-hydroxylase (F6H) | itf07g02770.t1 | 14.0 ± 4.6 | 39.8 ± 35.2 | |
| itf14g07470.t1 | 1.3 ± 0.8 | 4.8 ± 3.0 | ||
| itf14g07440.t1 | 1.2 ± 1.0 | 4.6 ± 2.2 | ||
| itf14g07430.t1 | 1.7 ± 2.0 | 7.0 ± 1.5 * | ||
| 4-coumarate-CoA ligase (4CL) | itf03g10110.t1 | 15.6 ± 1.9 | 25.4 ± 3.6 * | |
| peroxidase (POD) | itf07g05260.t1 | 0.0 ± 0.0 | 0.4 ± 0.2 * | |
| itf14g19200.t1 | 2.9 ± 0.8 | 5.7 ± 1.6 | ||
| itf09g05830.t1 | 0.1 ± 0.0 | 1.1 ± 0.4 ** | ||
| MAPK signaling pathway—plant | LRR receptor-like serine/threonine-protein kinase FLS2 (FLS2) | itf10g03920.t1 | 0.6 ± 0.1 | 2.1 ± 0.9 * |
| mitogen-activated protein kinase kinase 3 (MKK3) | itf02g25910.t7 | 1.1 ± 1.0 | 0.0 ± 0.0 | |
| transmembrane protein 222 (TMEM222) | itf06g10170.t3 | 0.0 ± 0.0 | 0.5 ± 0.4 | |
| ethylene receptor (ETR, ERS) | itf13g20890.t4 | 16.0 ± 5.2 | 35.4 ± 7.9 * | |
| itf04g07670.t1 | 5.6 ± 2.5 | 12.4 ± 2.2 * | ||
| itf03g16670.t1 | 12.3 ± 0.7 | 19.3 ± 5.0 | ||
| itf04g24110.t1 | 30.9 ± 8.2 | 59.8 ± 15.9 * | ||
| mitogen-activated protein kinase kinase 9 (MKK9) | itf04g16830.t1 | 85.6 ± 21.7 | 139.5 ± 19.3 * | |
| EIN3-binding F-box protein (EBF1_2) | itf03g20590.t1 | 26.8 ± 6.7 | 59.2 ± 6.4 ** | |
| itf03g20570.t1 | 110.3 ± 21.8 | 225.3 ± 11.3 ** | ||
| itf15g19520.t1 | 77.9 ± 15.0 | 132.9 ± 22.7 * | ||
| itf13g01870.t1 | 33.5 ± 14.5 | 90.7 ± 24.3 * | ||
| ethylene-responsive transcription factor 1 (ERF1) | itf13g14940.t1 | 91.1 ± 49.6 | 184.8 ± 36.1 | |
| itf13g21010.t1 | 109.6 ± 60.7 | 249.6 ± 81.4 | ||
| itf14g18080.t1 | 1.3 ± 0.8 | 4.3 ± 2.4 | ||
| itf04g07800.t1 | 5.5 ± 2.6 | 13.1 ± 5.2 | ||
| 5′-3′ exoribonuclease 2 (XRN2, RAT1) | itf02g03750.t3 | 0.0 ± 0.0 | 0.4 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, H.; Cheng, J.; Pang, L.; Yin, L.; Guan, Y.; Cheng, J.; Lu, X.; Lu, G. Physiological–Biochemical Characteristics and a Transcriptomic Profiling Analysis Reveal the Postharvest Wound Healing Mechanisms of Sweet Potatoes under Ascorbic Acid Treatment. Foods 2024, 13, 2569. https://doi.org/10.3390/foods13162569
Xuan H, Cheng J, Pang L, Yin L, Guan Y, Cheng J, Lu X, Lu G. Physiological–Biochemical Characteristics and a Transcriptomic Profiling Analysis Reveal the Postharvest Wound Healing Mechanisms of Sweet Potatoes under Ascorbic Acid Treatment. Foods. 2024; 13(16):2569. https://doi.org/10.3390/foods13162569
Chicago/Turabian StyleXuan, Hongxia, Jiyu Cheng, Linjiang Pang, Liqing Yin, Yuge Guan, Junfeng Cheng, Xinghua Lu, and Guoquan Lu. 2024. "Physiological–Biochemical Characteristics and a Transcriptomic Profiling Analysis Reveal the Postharvest Wound Healing Mechanisms of Sweet Potatoes under Ascorbic Acid Treatment" Foods 13, no. 16: 2569. https://doi.org/10.3390/foods13162569
APA StyleXuan, H., Cheng, J., Pang, L., Yin, L., Guan, Y., Cheng, J., Lu, X., & Lu, G. (2024). Physiological–Biochemical Characteristics and a Transcriptomic Profiling Analysis Reveal the Postharvest Wound Healing Mechanisms of Sweet Potatoes under Ascorbic Acid Treatment. Foods, 13(16), 2569. https://doi.org/10.3390/foods13162569





