Impact of Cooking Duration on Calcium Oxalate Needle-like Crystals in Plants: A Case Study of Vegetable Taro Flowers in Yunnan
Abstract
1. Introduction
- (1)
- To determine whether taro flowers contain abundant needle-like calcium oxalate crystals similar to other parts of the taro plant, and to examine how these crystals and the total amount of calcium oxalate change with extended cooking time.
- (2)
- To explore the potential for developing and utilizing taro flowers as a vegetable on a global scale, thereby contributing to food security, employment, and development.
2. Materials and Methods
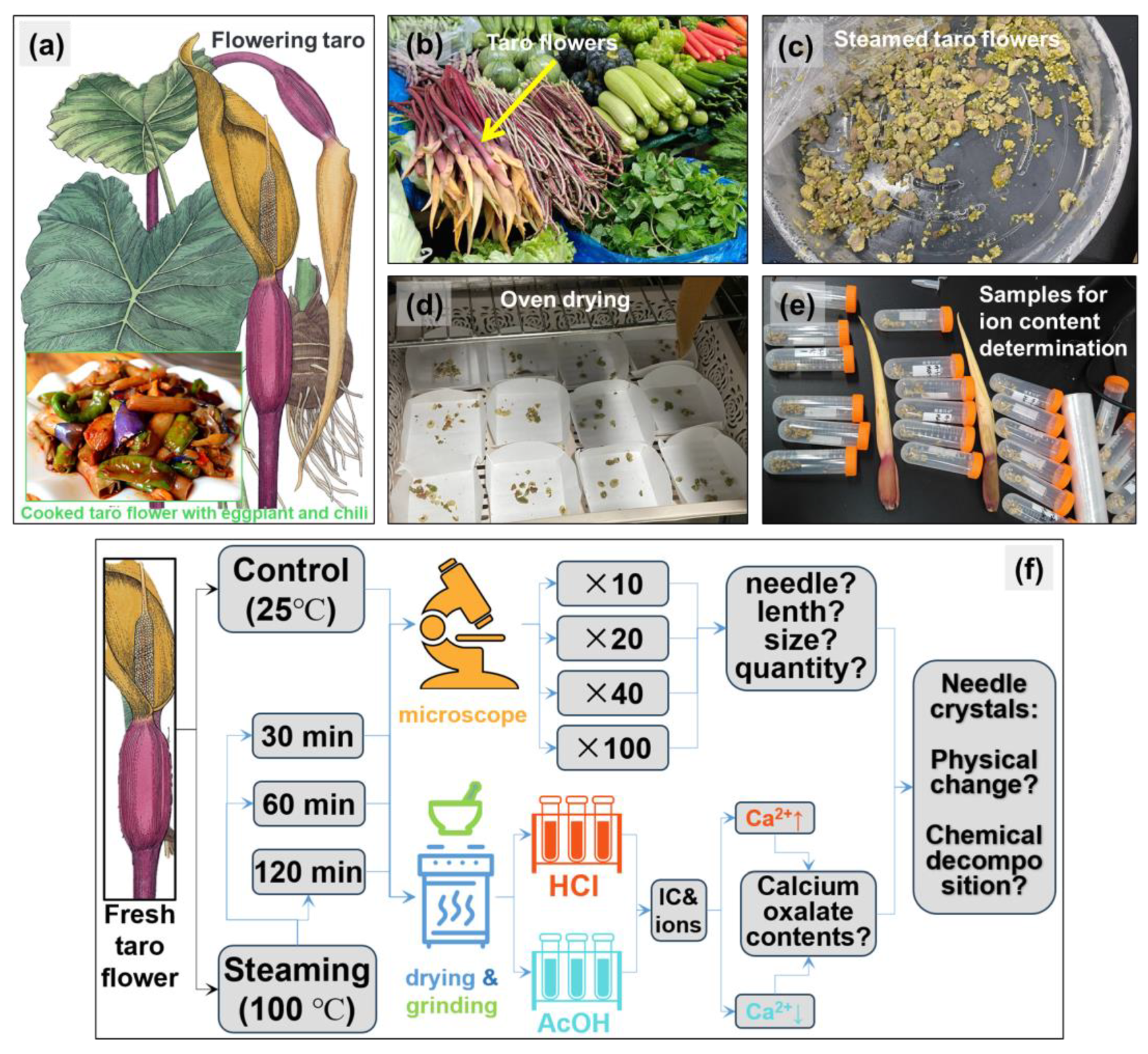
2.1. Taro Flower Samples and Treatments
2.2. Observation of Calcium Oxalate Crystals and Effects of HCl and AcOH Treatments
2.3. Ion Concentration Determination
3. Results
3.1. Variations in Calcium Oxalate Needle-like Crystals Under Different Cooking Durations
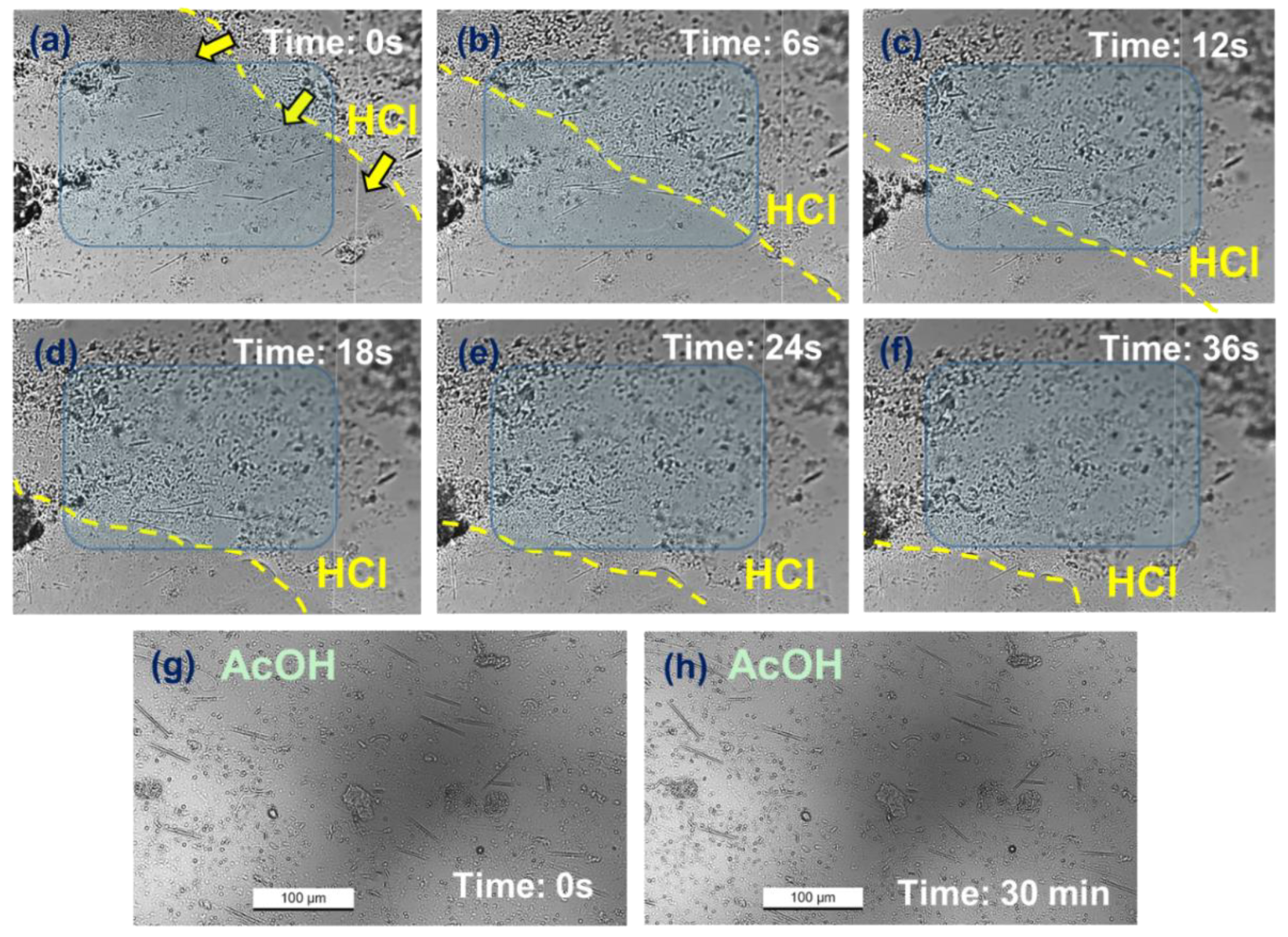
3.2. Changes in Needle-like Crystals Under HCl and AcOH Treatments
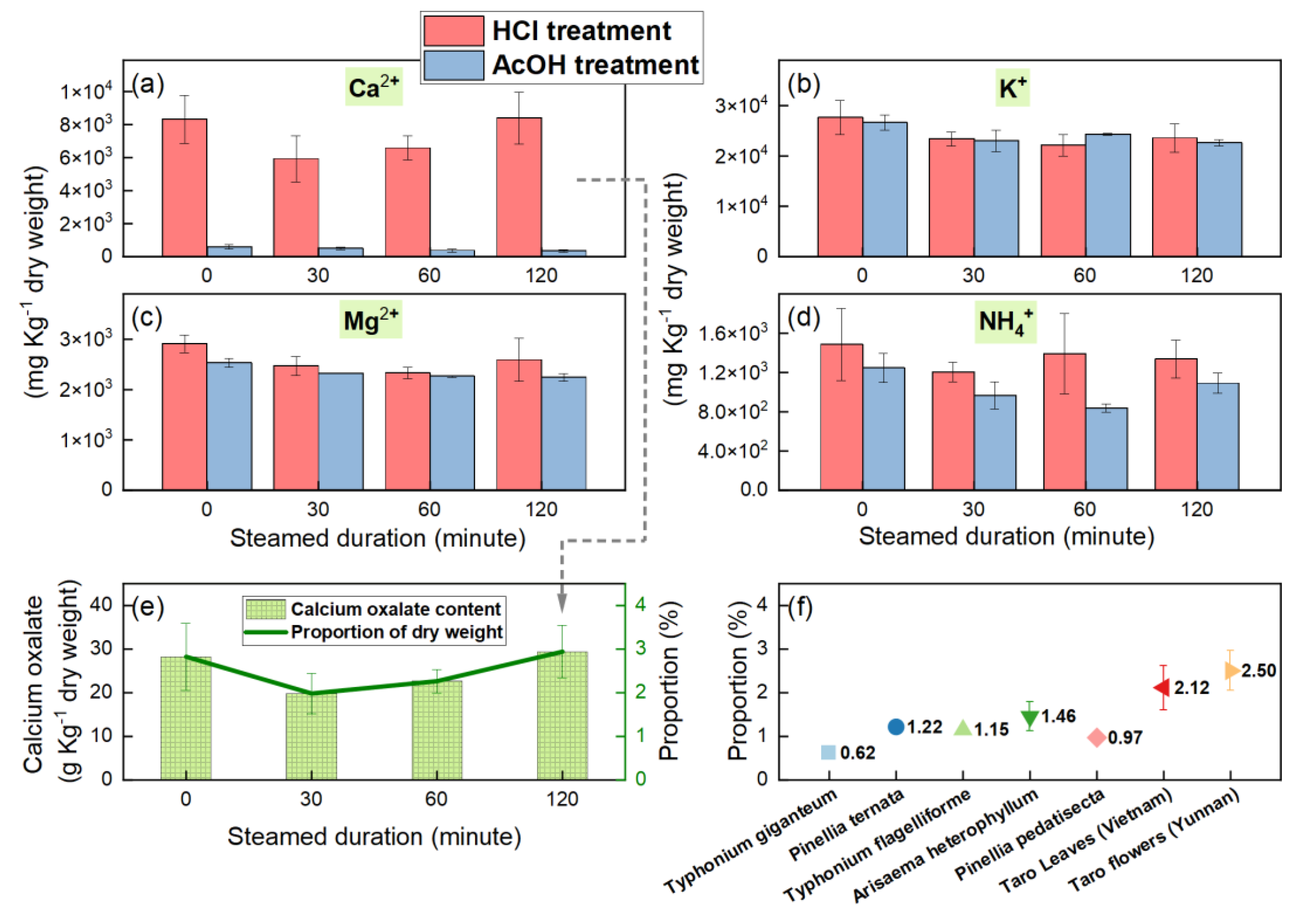
3.3. Ion Concentrations and Calcium Oxalate Contents Under Different Cooking Durations
4. Discussion
4.1. Possible Reasons for the Reduction in Calcium Oxalate Needle Crystals with Cooking
4.2. The Potential Recrystallization Process of Dissolved Calcium Oxalate Needle Crystals
4.3. Comparison with Other Araceae Plants That Have High Calcium Oxalate Content
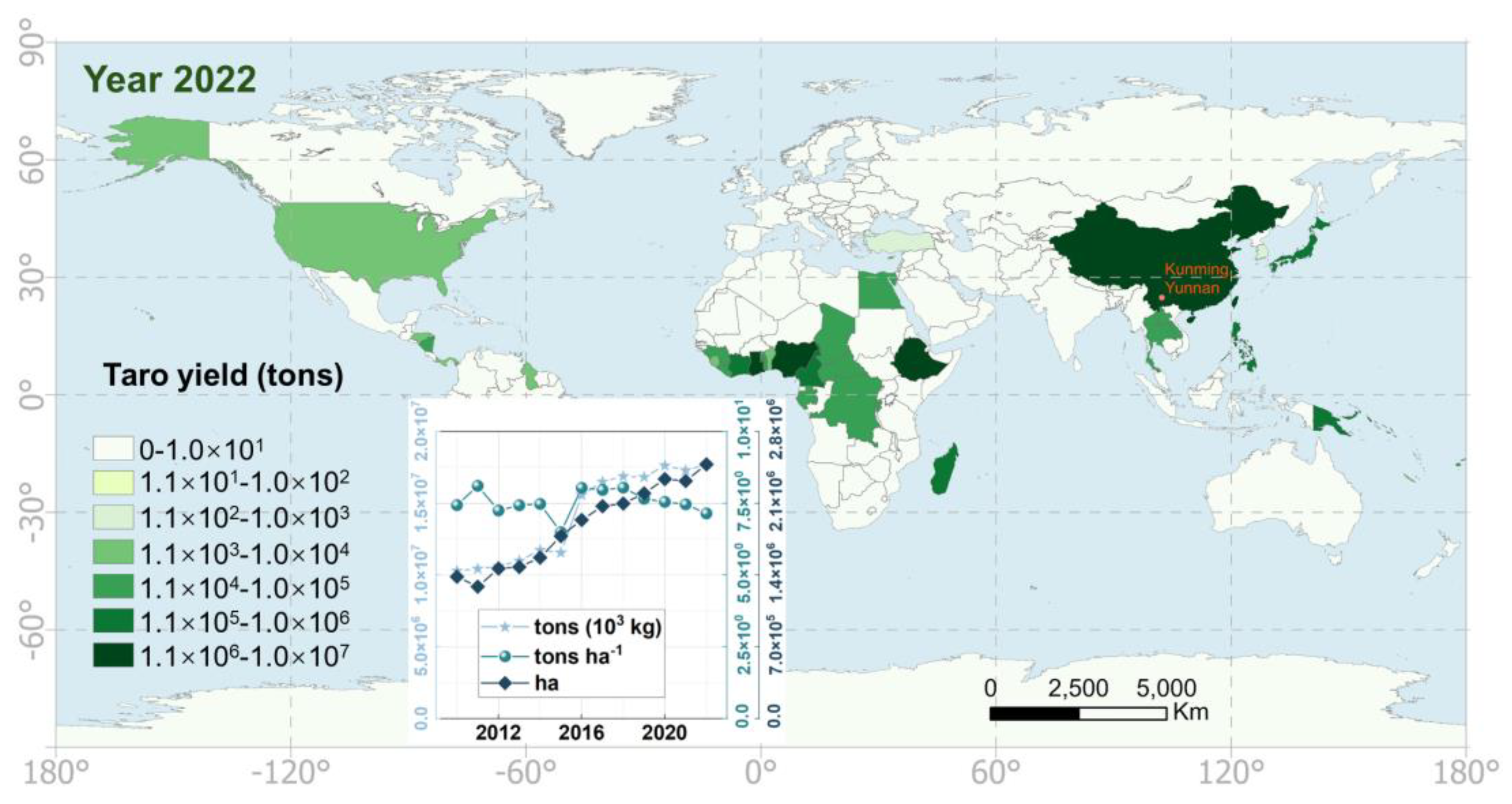
4.4. The Potential Benefits of Taro Flowers as a Global Vegetable and Their Role in Poverty Reduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashmi, D.R.; Raghu, N.; Gopenath, T.S.; Palanisamy, P.; Bakthavatchalam, P.; Karthikeyan, M.; Gnanasekaran, A.; Ranjith, M.S.; Chandrashekrappa, G.K.; Basalingappa, K.M. Taro (Colocasia esculenta): An overview. J. Med. Plants Stud. 2018, 6, 156–161. [Google Scholar]
- Oladimeji, J.J.; Kumar, P.L.; Abe, A.; Vetukuri, R.R.; Bhattacharjee, R. Taro in West Africa: Status, Challenges, and Opportunities. Agronomy 2022, 12, 2094. [Google Scholar] [CrossRef]
- Ferdaus, M.J.; Chukwu-Munsen, E.; Foguel, A.; da Silva, R.C. Taro Roots: An Underexploited Root Crop. Nutrients 2023, 15, 3337. [Google Scholar] [CrossRef]
- Ahmed, I.; Lockhart, P.J.; Agoo, E.M.G.; Naing, K.W.; Nguyen, D.V.; Medhi, D.K.; Matthews, P.J. Evolutionary origins of taro (Colocasia esculenta) in Southeast Asia. Ecol. Evol. 2020, 10, 13530–13543. [Google Scholar] [CrossRef]
- Xu, J.C.; Yang, Y.P.; Pu, Y.D.; Ayad, W.G.; Eyzaguirre, P.B. Genetic diversity in taro (Colocasia esculenta Schott, Araceae) in China: An ethnobotanical and genetic approach. Econ. Bot. 2001, 55, 14–31. [Google Scholar]
- Ni, L.; Zhao, Q. Studies on Stimulating Components in Taro Petioles. J. Chin. Inst. Food Sci. Technol. 2009, 9, 72–79. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakashima, T.; Mori, K. Formation and distribution of calcium oxalate crystal idioblast in the tissues of taro Colocasia esculenta (L.) Schott. J. Jpn. Soc. Hortic. Sci. 2003, 72, 162–168. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, X. A Case Report of Ventricular Premature Contractions Induced by Taro Flower Poisoning. Acta Acad. Med. Kunming 1988, 9, 74. [Google Scholar]
- Sunell, L.A.; Healey, P.L. Distribution of Calcium Oxalate Crystal Idioblasts in Corms of Taro (Colocasia esculenta). Am. J. Bot. 1979, 66, 1029–1032. [Google Scholar] [CrossRef]
- Liu, M.; Luo, X.; Liu, J. Calcium Oxalate Crystals in the Vegetative Organs of Colocasia esculenta (L.) Schott (Araceae) and Their Possible Physiological Function. J. Trop. Subtrop. Bot. 2012, 20, 126–131. [Google Scholar]
- Ward, D.; Spiegel, M.; Saltz, D. Gazelle Herbivory and Interpopulation Differences in Calcium Oxalate Content of Leaves of a Desert Lily. J. Chem. Ecol. 1997, 23, 333–346. [Google Scholar] [CrossRef]
- Salinas, M.L.; Ogura, T.; Soffchi, L. Irritant contact dermatitis caused by needle-like calcium oxalate crystals, raphides in Agave tequilana among workers in tequila distilleries and agave plantations. Contact Dermat. 2001, 44, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Molano-Flores, B. Herbivory and Calcium Concentrations Affect Calcium Oxalate Crystal Formation in Leaves of Sida (Malvaceae). Ann. Bot. 2001, 88, 387–391. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Prychid, C.J.; Rudall, P.J. Calcium Oxalate Crystals in Monocotyledons: A Review of their Structure and Systematics. Ann. Bot. 1999, 84, 725–739. [Google Scholar] [CrossRef]
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Chang, H.; Huang, P.J. Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA. Anal. Chem. 1997, 69, 1485–1491. [Google Scholar] [CrossRef]
- Zhang, W.J.; Fan, Y.K.; Chi, J.L. The synergistic effect of multiple organic macromolecules on the formation of calcium oxalate raphides of Musa spp. J. Exp. Bot. 2024, 75, 2470–2480. [Google Scholar] [CrossRef]
- Ishii, Y. Needle Crystal of Calcium Oxalate Monohydrate Found in Plant. J. Electron Microsc. 1992, 41, 53–56. [Google Scholar]
- He, H.; Li, D.H.; Li, X.X.; Fu, L. Research progress on the formation, function, and impact of calcium oxalate crystals in plants. Crystallogr. Rev. 2024, 30, 31–60. [Google Scholar] [CrossRef]
- Khan, M.I.; Pandith, S.A.; Shah, M.A.; Reshi, Z.A. Calcium Oxalate Crystals, the Plant ‘Gemstones’: Insights into Their Synthesis and Physiological Implications in Plants. Plant Cell Physiol. 2023, 64, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Keating, R.C. Systematic Occurrence of Raphide Crystals in Araceae. Ann. Mo. Bot. Gard. 2004, 91, 495–504. [Google Scholar]
- Thanh, H.D.; Vu, H.P.; Van, H.V.; Duc, N.L.; Minh, T.L.; Savage, G. Oxalate Content of Taro Leaves Grown in Central Vietnam. Foods 2017, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Pueschel, C.M.; West, J.A. Cytoplasmic streaming of calcium oxalate crystals in Callipsygma wilsonis (Bryopsidales, Chlorophyta). Phycol. Res. 2007, 55, 278–285. [Google Scholar] [CrossRef]
- Nair, C.G.R.; Ninan, K. Thermal decomposition studies: Part X. Thermal decomposition kinetics of calcium oxalate monohydrate—Correlations with heating rate and samples mass. Thermochim. Acta 1978, 23, 161–169. [Google Scholar] [CrossRef]
- Ibis, F.; Dhand, P.; Suleymanli, S.; van der Heijden, A.E.D.M.; Kramer, H.J.M.; Eral, H.B. A Combined Experimental and Modelling Study on Solubility of Calcium Oxalate Monohydrate at Physiologically Relevant pH and Temperatures. Crystals 2020, 10, 924. [Google Scholar] [CrossRef]
- Løge, I.A.; Anabaraonye, B.U.; Bovet, N.; Fosbøl, P.L. Crystal Nucleation and Growth: Supersaturation and Crystal Resilience Determine Stickability. Cryst. Growth Des. 2023, 23, 2619–2627. [Google Scholar] [CrossRef]
- Garside, J. The concept of effectiveness factors in crystal growth. Chem. Eng. Sci. 1971, 26, 1425–1431. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Wu, H.; Xie, Y.; Tao, X.; Zeng, P.; Cheng, Q.; Wang, C.; Liu, B.; Zhang, P. Effect of Arisaematis Rhizoma Processed on the Contents of Lectin Protein and Calcium Oxalate Crystal as Toxic Components. J. Nanjing Univ. Tradit. Chin. Med. 2022, 38, 375–381. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, H. RP-HPLC Determination of Calcium Oxalate Needle Crystal Content in Medicinal Plants of the Araceae Family. Chin. Tradit. Pat. Med. 2008, 30, 260–262. [Google Scholar] [CrossRef]
- Zhong, L. Studies on Irritant Component of Pinelliae Tuber and the Attenuation Mechanism and Technology of Processing. Ph.D. Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2007. Available online: https://cdmd.cnki.com.cn/Article/CDMD-10315-2007223579.htm (accessed on 19 November 2024).

| Case | Fresh Weight (g) | Dried Weight (g) | Acid and Amount (1 mol L−1) |
|---|---|---|---|
| F-1-HCl | 1.003 | 0.163 | HCl 2 mL |
| F-2-HCl | 1.001 | 0.176 | HCl 2 mL |
| F-3-HCl | 0.996 | 0.181 | HCl 2 mL |
| F-1-AcOH | 0.999 | 0.146 | AcOH 2 mL |
| F-2-AcOH | 1.000 | 0.178 | AcOH 2 mL |
| F-3-AcOH | 1.011 | 0.156 | AcOH 2 mL |
| S-30-1-HCl | 1.010 | 0.200 | HCl 2 mL |
| S-30-2-HCl | 1.011 | 0.180 | HCl 2 mL |
| S-30-3-HCl | 0.992 | 0.186 | HCl 2 mL |
| S-30-1-AcOH | 0.992 | 0.177 | AcOH 2 mL |
| S-30-2-AcOH | 0.998 | 0.196 | AcOH 2 mL |
| S-30-3-AcOH | 1.010 | 0.188 | AcOH 2 mL |
| S-60-1-HCl | 1.008 | 0.197 | HCl 2 mL |
| S-60-2-HCl | 1.013 | 0.191 | HCl 2 mL |
| S-60-3-HCl | 0.996 | 0.218 | HCl 2 mL |
| S-60-1-AcOH | 0.999 | 0.181 | AcOH 2 mL |
| S-60-2-AcOH | 1.008 | 0.180 | AcOH 2 mL |
| S-60-3-AcOH | 0.992 | 0.171 | AcOH 2 mL |
| S-120-1-HCl | 1.009 | 0.225 | HCl 2 mL |
| S-120-2-HCl | 1.009 | 0.241 | HCl 2 mL |
| S-120-3-HCl | 0.999 | 0.235 | HCl 2 mL |
| S-120-1-AcOH | 1.007 | 0.257 | AcOH 2 mL |
| S-120-2-AcOH | 1.007 | 0.242 | AcOH 2 mL |
| S-120-3-AcOH | 0.999 | 0.212 | AcOH 2 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zi, H.; Chen, R.; Jia, N.; Ma, Y.; Zhao, C.; Chen, Z.; Zhang, J. Impact of Cooking Duration on Calcium Oxalate Needle-like Crystals in Plants: A Case Study of Vegetable Taro Flowers in Yunnan. Foods 2024, 13, 3730. https://doi.org/10.3390/foods13233730
Zi H, Chen R, Jia N, Ma Y, Zhao C, Chen Z, Zhang J. Impact of Cooking Duration on Calcium Oxalate Needle-like Crystals in Plants: A Case Study of Vegetable Taro Flowers in Yunnan. Foods. 2024; 13(23):3730. https://doi.org/10.3390/foods13233730
Chicago/Turabian StyleZi, Haoyu, Rui Chen, Nan Jia, Yuxuan Ma, Chunchang Zhao, Zhe Chen, and Jingwei Zhang. 2024. "Impact of Cooking Duration on Calcium Oxalate Needle-like Crystals in Plants: A Case Study of Vegetable Taro Flowers in Yunnan" Foods 13, no. 23: 3730. https://doi.org/10.3390/foods13233730
APA StyleZi, H., Chen, R., Jia, N., Ma, Y., Zhao, C., Chen, Z., & Zhang, J. (2024). Impact of Cooking Duration on Calcium Oxalate Needle-like Crystals in Plants: A Case Study of Vegetable Taro Flowers in Yunnan. Foods, 13(23), 3730. https://doi.org/10.3390/foods13233730






