Consumption of Plant-Derived Phenolic Acids Modulates Hunger and Satiety Responses Due to Chemical Interactions with Enteroendocrine Mediators
Abstract
1. Introduction
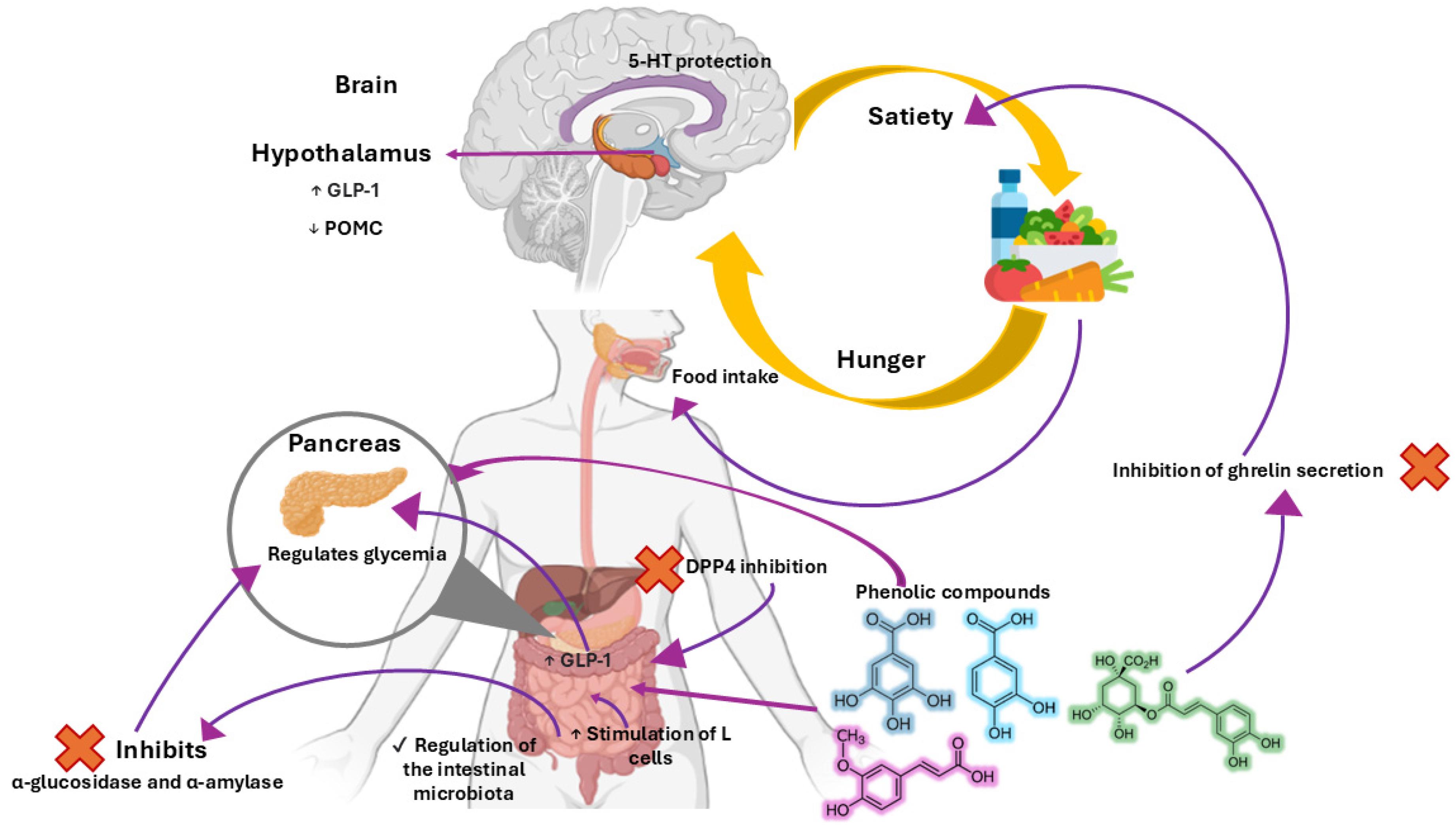
2. Diet-Induced Disturbances on Hunger and Satiety Pathways
3. Impact of Phenolic Compounds on Satiety Regulation
4. Bibliographic Analysis
5. Reported Effects of Four Selected Phenolic Acids on Hunger and Satiety
5.1. Chlorogenic Acid (CGA)
5.2. Gallic Acid (GA)
5.3. Ferulic Acid (FA)
5.4. Protocatechuic Acid (PCA)
6. Chemical Properties of Phenolic Compounds Associated with Their Effects on Satiety
7. Impact of Model and Phenolic Metabolism
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Description |
| 5-HT | 5-hydroxytryptamine, serotonin |
| AgRP | Agouti-related peptide |
| ARC | arcuate nucleus |
| AP | avocado paste |
| CCK1R | CCK type 1 receptor |
| CHO | Chinese hamster ovary |
| CGA | chlorogenic acid |
| CCK | cholecystokinin |
| CART | cocaine and amphetamine regulated transcript |
| DPP4 | dipeptidyl peptidase 4 |
| FFAR1, also known as GPR-40 | fatty acid receptor 1 |
| FA | ferulic acid |
| FFAR2, also known as GPR-43 | free fatty acid receptor 2 |
| FFAR3 | free fatty acid receptor 3 |
| GA | gallic acid |
| GIP | gastric inhibitory polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| GLUT2 | glucose transporter 2 |
| GLUT4 | glucose transporter 4 |
| GPCRs | G-protein-coupled receptors |
| hTAS2R | human bitter taste receptors |
| LEPR | leptin receptor |
| MC3R | melanocortin receptor 3 |
| MAO-A | monoamine oxidase A |
| NPY | neuropeptide Y |
| NF-κB | nuclear factor kappa-light-chain enhancer of activated B cells |
| PYY | peptide tyrosine tyrosine |
| Pck1 | phosphoenolpyruvate carboxykinase 1 |
| POMC | proopiomelanocortin |
| PCA | protocatechuic acid |
| SGLT-1 | sodium-glucose cotransporter-1 |
References
- Hollingworth, S.; Dalton, M.; Blundell, J.E.; Finlayson, G. Evaluation of the influence of raw almonds on appetite control: Satiation, satiety, hedonics and consumer perceptions. Nutrients 2019, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.L.; Baugh, M.E.; Oster, M.E.; DiFeliceantonio, A.G. The impact of caloric availability on eating behavior and ultra-processed food reward. Appetite 2022, 178, 106274. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Wood, W.; Monterosso, J. Healthy eating habits protect against temptations. Appetite 2016, 103, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Ferguson, A.V. Neurophysiology of hunger and satiety. Dev. Disabil. Res. Rev. 2008, 14, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef]
- Jain, S.; Singh, S.N. Regulation of food intake: A complex Process. Def. Life Sci. J. 2018, 3, 182–189. [Google Scholar] [CrossRef]
- Gonzalez-Izundegui, D.; Campos, A.; Calderon, G.; Ricardo-Silgado, M.L.; Cifuentes, L.; Decker, P.A.; Vargas, E.J.; Tran, L.; Burton, D.; Abu Dayyeh, B. Association of gastric emptying with postprandial appetite and satiety sensations in obesity. Obesity 2021, 29, 1497–1507. [Google Scholar] [CrossRef]
- Upadhyay, R.; Rao, L.J.M. An Outlook on Chlorogenic Acids-Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Klepacka, J.; Fornal, L. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Puente, M.; Mateos, R.; Seguido, M.A.; Garcia-Cordero, J.; Gonzalez, S.; Tarradas, R.M.; Bravo-Clemente, L.; Sarria, B. Appetite and Satiety Effects of the Acute and Regular Consumption of Green Coffee Phenols and Green Coffee Phenol/Oat beta-Glucan Nutraceuticals in Subjects with Overweight and Obesity. Foods 2021, 10, 2511. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hakim, B.N.; Yahya, H.M.; Shahar, S.; Abdul Manaf, Z.; Damanhuri, H. Effect of sequence of fruit intake in a meal on satiety. Int. J. Environ. Res. Public Health 2019, 16, 4464. [Google Scholar] [CrossRef]
- Shapira, N. The metabolic concept of meal sequence vs. satiety: Glycemic and oxidative responses with reference to inflammation risk, protective principles and Mediterranean diet. Nutrients 2019, 11, 2373. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Savini, I.; Gasperi, V.; Catani, M.V. Oxidative stress and obesity. In Obesity: A Practical Guide; Springer: Cham, Switzerland, 2016; pp. 65–86. [Google Scholar]
- Gribble, F.M.; Reimann, F.; Roberts, G.P. Gastrointestinal hormones. In Physiology of the Gastrointestinal Tract; Academic Press: Cambridge, MA, USA, 2018; pp. 31–70. [Google Scholar]
- Jais, A.; Brüning, J.C. Arcuate nucleus-dependent regulation of metabolism—Pathways to obesity and diabetes mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.-S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Oteiza, P.I. (–)-Epicatechin and Anthocyanins Modulate GLP-1 Metabolism: Evidence from C57BL/6J Mice and GLUTag Cells. J. Nutr. 2021, 151, 1497–1506. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhalidy, H.; Liu, D. The emerging role of polyphenols in the management of type 2 diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, S.M.; Zhuang, Y.W.; Yun, Y.; Xu, P.Y.; He, X.H.; Guo, J.; Yin, W.C.; Xu, H.E.; Xie, X.; et al. Molecular recognition of an acyl-peptide hormone and activation of ghrelin receptor. Nat. Commun. 2021, 12, 5064. [Google Scholar] [CrossRef] [PubMed]
- Serna, A.; Marhuenda, J.; Arcusa, R.; Pérez-Piñero, S.; Sánchez-Macarro, M.; García-Muñoz, A.M.; Victoria-Montesinos, D.; Cánovas, F.; López-Román, F.J. Effectiveness of a polyphenolic extract (Lippia citriodora and Hibiscus sabdariffa) on appetite regulation in overweight and obese grade I population: An 8-week randomized, double-blind, cross-over, placebo-controlled trial. Eur. J. Nutr. 2021, 61, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Tsai, M.-L.; Badmaev, V.; Jimenez, M.; Ho, C.-T.; Pan, M.-H. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M. Chlorogenic acid protects paraoxonase 1 activity in high density lipoprotein from inactivation caused by physiological concentrations of hypochlorite. Fitoterapia 2009, 80, 138–142. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Celestino, M.M.; Gomes, A.C.; Botelho, P.B.; Gambero, A.; Mesquita, L.M.; Vilegas, W.; Ribeiro, M.L.; Mota, J.F. South American herbal extracts reduce food intake through modulation of gastrointestinal hormones in overweight and obese women. J. Func. Food 2017, 35, 555–563. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022, 59, 6834–6856. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; O’Callaghan, C.; Walpola, I.C.; Wainstein, G.; Taylor, N.; Aru, J.; Huebner, B.; John, Y.J. Understanding the effects of serotonin in the brain through its role in the gastrointestinal tract. Brain 2022, 145, 2967–2981. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.J.; Qi, Z.; Zhong, Y.L.; Xu, S.H.; Zheng, W. Chlorogenic acid maintains glucose homeostasis through modulating the expression of SGLT-1, GLUT-2, and PLG in different intestinal segments of Sprague-Dawley rats fed a high-fat diet. Biomed. Environ. Sci. 2015, 28, 894–903. [Google Scholar]
- Huang, D.-W.; Chang, W.-C.; Wu, J.S.-B.; Shih, R.-W.; Shen, S.-C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef]
- Stamper, C.; Safadi, S.; Gehr, A.; Asuncion, P.; Hong, M.Y. Effects of fresh vs dried mango consumption on satiety and postprandial glucose in healthy adults. Metab. Open 2023, 19, 100253. [Google Scholar] [CrossRef]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; De la Rosa, L.A. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Depoortere, I.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef]
- Grau-Bové, C.; Miguéns-Gómez, A.; González-Quilen, C.; Fernández-López, J.-A.; Remesar, X.; Torres-Fuentes, C.; Ávila-Román, J.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Blay, M.T. Modulation of food intake by differential TAS2R stimulation in rat. Nutrients 2020, 12, 3784. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Moreno, J.J. Bitter taste receptors: Key target to understand the effects of polyphenols on glucose and body weight homeostasis. Pathophysiological and pharmacological implications. Biochem. Pharmacol. 2024, 228, 116192. [Google Scholar] [CrossRef]
- Sousa, J.N.; Paraíso, A.F.; Andrade, J.M.O.; Lelis, D.F.; Santos, E.M.; Lima, J.P.; Monteiro-Junior, R.S.; D’Angelo, M.F.S.V.; de Paula, A.M.B.; Guimarães, A.L.S. Oral gallic acid improve liver steatosis and metabolism modulating hepatic lipogenic markers in obese mice. Exp. Gerontol. 2020, 134, 110881. [Google Scholar] [CrossRef]
- Bashar, S.M.; Elhadidy, M.G.; Mostafa, A.F.; Hamed, B.; Helmy, S.; Abd-Elmoniem, H.A. Hepatoprotective effect of gallic acid against type 2-induced diabetic liver injury in male rats through modulation of fetuin-A and GLP-1 with involvement of ERK1/2/NF-κB and Wnt1/β-catenin signaling pathways. Gen. Physiol. Biophys. 2021, 40, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Cázares-Camacho, R.; Domínguez-Avila, J.A.; Astiazarán-García, H.; Montiel-Herrera, M.; González-Aguilar, G.A. Neuroprotective effects of mango cv. ‘Ataulfo’ peel and pulp against oxidative stress in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2021, 101, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef]
- Martinussen, C.; Veedfald, S.; Dirksen, C.; Bojsen-Møller, K.N.; Svane, M.S.; Wewer Albrechtsen, N.J.; van Hall, G.; Kristiansen, V.B.; Fenger, M.; Holst, J.J. The effect of acute dual SGLT1/SGLT2 inhibition on incretin release and glucose metabolism after gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E956–E964. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Loka, M.; Pandey, A.K.; Bishayee, A. Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv. Protein Chem. Str. Biol. 2021, 125, 215–257. [Google Scholar]
- Zhang, Z.Y.; Yang, P.; Zhao, J.B. Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Arnone, A.; Tenore, G.C.; Colao, A.; Savastano, S. Could hop-derived bitter compounds improve glucose homeostasis by stimulating the secretion of GLP-1? Crit. Rev. Food Sci. 2019, 59, 528–535. [Google Scholar] [CrossRef]
- Halter, B.; Ildari, N.; Cline, M.A.; Gilbert, E.R. Ferulic acid, a phytochemical with transient anorexigenic effects in birds. Comp. Biochem. Physiol. A 2021, 259, 111015. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinia, H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.; Reimann, F.; Blottiere, H. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, M.; Sun, J.; Guo, A.; Fernando, R.L.; Chen, Y.; Peng, P.; Zhao, G.; Deng, Y. DPP-4 inhibitor improves learning and memory deficits and AD-like neurodegeneration by modulating the GLP-1 signaling. Neuropharmacol. 2019, 157, 107668. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.A.; Moselhy, S.S. Inhibition of dipeptidyl peptidase-4 (DPP4), antioxidant, antiglycation and anti-inflammatory effect of Ferulic acid against streptozotocin toxicity mediate nephropathy in diabetic rats. Environ. Sci. Pollut. Res. 2022, 30, 33942–33948. [Google Scholar] [CrossRef]
- An, Y.X.; Zhang, L.C.; Fan, X.X.; Cui, K.L.; He, S.L.; Ji, P.Y.; Tian, J.M.; Li, Z.Y. Ferulic acid reduces GLP-1 degradation to ameliorate diet-induced obesity-associated hepatic steatosis. Food Front. 2024, 5, 691–707. [Google Scholar] [CrossRef]
- Corella-Salazar, D.A.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Astiazaran-Garcia, H.; Salazar-López, N.J.; Serafín-García, M.S.; Olivas-Orozco, G.I.; Molina-Corral, F.J.; González-Aguilar, G.A. Sub-chronic consumption of a phenolic-rich avocado paste extract induces GLP-1-, leptin-, and adiponectin-mediated satiety in Wistar rats. J. Food Biochem. 2021, 45, e13957. [Google Scholar] [CrossRef]
- Obaroakpo, J.U.; Liu, L.; Zhang, S.W.; Lu, J.; Liu, L.; Pang, X.Y.; Lv, J.P. modulation of glucagon-like peptide release by DPP-IV inhibitory polyphenol-polysaccharide conjugates of sprouted quinoa yoghurt. Food Chem. 2020, 324, 126857. [Google Scholar] [CrossRef] [PubMed]
- Syama, H.P.; Arun, K.B.; Sinumol, G.; Dhanya, R.; Suseela Anusree, S.; Nisha, P.; Ravi Shankar, L.; Sundaresan, A.; Jayamurthy, P. Syzygium cumini seed exhibits antidiabetic potential via multiple pathways involving inhibition of α-glucosidase, DPP-IV, glycation, and ameliorating glucose uptake in L6 cell lines. J. Food Process. Pres. 2018, 42, e13464. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.O.; Bharuth, V.; Ijomone, O.M.; Islam, M.S. Ferulic acid improves glucose homeostasis by modulation of key diabetogenic activities and restoration of pancreatic architecture in diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 324–339. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Chen, S.Y.; Hu, Y.Y.; Ye, X.Q.; Wang, S.Y.; Tian, J.H. The cooperation of maize starch and ferulic acid under different treatments and its effect on postprandial blood glucose level. Food Hydrocoll. 2024, 157, 110361. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Grau-Bové, C.; González-Quilen, C.; Terra, X.; Blay, M.T.; Beltrán-Debón, R.; Jorba-Martín, R.; Espina, B.; Pinent, M.; Ardévol, A. Effects of Flavanols on Enteroendocrine Secretion. Biomolecules 2020, 10, 844. [Google Scholar] [CrossRef]
- Grzelak-Blaszczyk, K.; Milala, J.; Kolodziejczyk, K.; Sójka, M.; Czarnecki, A.; Kosmala, M.; Klewicki, R.; Fotschki, B.; Jurgonski, A.; Juskiewicz, J. Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. 2020, 11, 3585–3597. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, R.; Wang, Y.; Han, S.; Qin, X.; Li, Z.; Wang, X.; Han, Y.; Wang, T.; Xia, B. Protocatechuic Acid Ameliorates High Fat Diet-Induced Obesity and Insulin Resistance in Mice. Mol. Nutr. Food Res. 2022, 67, 2200244. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Teralı, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 2019, 43, e12927. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Subhedar, N.K.; Kokare, D.M. Involvement of neuropeptide CART in the central effects of insulin on feeding and body weight. Pharmacol. Biochem. Behav. 2019, 181, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Al Shukor, N.; Raes, K.; Van Camp, J.; Smagghe, G. Analysis of interaction of phenolic compounds with the cholecystokinin signaling pathway to explain effects on reducing food intake. Peptides 2014, 53, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Araújo, R.L.; Eidenberger, T.; Brandão, M.G. Chemical composition and inhibitory activities on dipeptidyl peptidase IV and pancreatic lipase of two underutilized species from the Brazilian Savannah: Oxalis cordata A. St.-Hil. and Xylopia aromatica (Lam.) Mart. Food Res. Int. 2018, 105, 989–995. [Google Scholar] [CrossRef]
- Bourdillon, N.; Eugster, P.J.; Vocat, C.; Nguyen, T.; Wuerzner, G.; Grouzmann, E.; Millet, G.P. Saxagliptin: A potential doping agent? A randomized, double-blinded, placebo-controlled, and crossover pilot study in young active men. Physiol. Rep. 2022, 10, e15515. [Google Scholar] [CrossRef]
- Tuersuntuoheti, T.; Pan, F.; Zhang, M.; Wang, Z.H.; Han, J.X.; Sun, Z.Z.; Song, W. Prediction of DPP-IV inhibitory potentials of polyphenols existed in Qingke barley fresh noodles: In vitro and in silico analyses. J. Food Process. Pres. 2022, 46, e16808. [Google Scholar] [CrossRef]
- Gao, F.; Fu, Y.; Yi, J.; Gao, A.; Jia, Y.; Cai, S. Effects of different dietary flavonoids on dipeptidyl peptidase-IV activity and expression: Insights into structure–activity relationship. J. Agric. Food Chem. 2020, 68, 12141–12151. [Google Scholar] [CrossRef]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef]
- Goudar, G.; Manne, M.; Sathisha, G.; Sharma, P.; Mokalla, T.R.; Kumar, S.B.; Ziouzenkova, O. Phenolic, nutritional and molecular interaction study among different millet varieties. Food Chem. Adv. 2023, 2, 100150. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Ejakpovi, I.I.; Oyeleye, S.I. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure–function perspective. Comp. Clin. Pathol. 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- Pollini, L.; Riccio, A.; Juan, C.; Tringaniello, C.; Ianni, F.; Blasi, F.; Mañes, J.; Macchiarulo, A.; Cossignani, L. Phenolic acids from Lycium barbarum leaves: In vitro and in silico studies of the inhibitory activity against porcine pancreatic α-amylase. Processes 2020, 8, 1388. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Wu, B.J.; Kulkarni, K.; Basu, S.; Zhang, S.X.; Hu, M. First-Pass Metabolism via UDP-Glucuronosyltransferase: A Barrier to Oral Bioavailability of Phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013, 4, 52–62. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Identification of (Poly)phenolic Compounds in Concord Grape Juice and Their Metabolites in Human Plasma and Urine after Juice Consumption. J. Agric. Food Chem. 2011, 59, 9512–9522. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite Profiling of Hydroxycinnamate Derivatives in Plasma and Urine after the Ingestion of Coffee by Humans: Identification of Biomarkers of Coffee Consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
| Phenolic Acid | Chemical Structure | Log P | Hydrogen Bond Donor Count | Hydrogen Bond Acceptor Count | Reported Effects | References |
|---|---|---|---|---|---|---|
| Chlorogenic acid (CGA) |  | −0.4 | 6 | 9 | ↑ Secretion of GLP-1 ↑ Amylin ↓ MAO-A 5-HT protection ↑ SGLT-1 ↑ Stimulation of L cells | [29,31,32,33,36] |
| Gallic acid (GA) |  | 0.7 | 4 | 5 | ↓ Food intake ↓ Octanoyl ghrelin Potential interaction with bitter taste receptors ↓ Body weight ↑ Insulin sensitivity ↓ Diabetic hyperphagia | [40,41,43,44,45] |
| Ferulic acid (FA) | 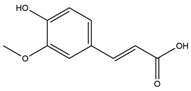 | 1.5 | 2 | 4 | May secrete GLP-1 ↓ POMC mRNA ↓ Body weight Modulates intestinal microbiota Inhibition of DPP4 Interactions with GLP-1 ↑ Satiety Modulates GLP-1, leptin, adiponectin mRNA and/or protein Inhibits α-amylase and α -glucosidase | [52,53,55,60,61,62,65] |
| Protocatechuic acid (PCA) | 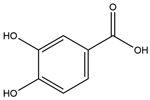 | 1.1 | 3 | 4 | ↑ PYY, CCK, or GLP1 Modulates intestinal microbiota ↓ Body weight Protects pancreas | [69,70,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Villegas-Ochoa, M.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Viuda-Martos, M.; González-Aguilar, G.A. Consumption of Plant-Derived Phenolic Acids Modulates Hunger and Satiety Responses Due to Chemical Interactions with Enteroendocrine Mediators. Foods 2024, 13, 3640. https://doi.org/10.3390/foods13223640
Zuñiga-Martínez BS, Domínguez-Avila JA, Montiel-Herrera M, Villegas-Ochoa MA, Robles-Sánchez RM, Ayala-Zavala JF, Viuda-Martos M, González-Aguilar GA. Consumption of Plant-Derived Phenolic Acids Modulates Hunger and Satiety Responses Due to Chemical Interactions with Enteroendocrine Mediators. Foods. 2024; 13(22):3640. https://doi.org/10.3390/foods13223640
Chicago/Turabian StyleZuñiga-Martínez, B. Shain, J. Abraham Domínguez-Avila, Marcelino Montiel-Herrera, Mónica A. Villegas-Ochoa, Rosario Maribel Robles-Sánchez, J. Fernando Ayala-Zavala, Manuel Viuda-Martos, and Gustavo A. González-Aguilar. 2024. "Consumption of Plant-Derived Phenolic Acids Modulates Hunger and Satiety Responses Due to Chemical Interactions with Enteroendocrine Mediators" Foods 13, no. 22: 3640. https://doi.org/10.3390/foods13223640
APA StyleZuñiga-Martínez, B. S., Domínguez-Avila, J. A., Montiel-Herrera, M., Villegas-Ochoa, M. A., Robles-Sánchez, R. M., Ayala-Zavala, J. F., Viuda-Martos, M., & González-Aguilar, G. A. (2024). Consumption of Plant-Derived Phenolic Acids Modulates Hunger and Satiety Responses Due to Chemical Interactions with Enteroendocrine Mediators. Foods, 13(22), 3640. https://doi.org/10.3390/foods13223640










