Enhancement of Nutrient, Trace Element, and Organic Selenium Contents of Ratooning Rice Grains and Straw Through Foliar Application of Selenite
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Soil Characteristics and Experimental Design
2.2.1. Experimental Site and Growth Conditions
2.2.2. Determination of Morphological Indicators and Se Contents in Grains
2.3. Measurement Items and Methods
2.3.1. Sampling and Measurement Times for Physiological Indicators
2.3.2. Measurement of Rice Plant Biomass and Yield
2.3.3. Chlorophyll Content
2.3.4. Determination of Quality Indicators for Forage and Rice
2.3.5. Determination of Se Content and Se Speciation
2.3.6. Digestion of Samples and Determination of Elemental Concentrations
2.3.7. Photosynthetic Rate Measurement
2.3.8. Activity of Enzymes Correlated with Antioxidant Enzyme System
2.3.9. Determination of the Characteristics of the Grain Quality
2.3.10. Determination of Starch, Protein and Amino Acid
2.4. Statistical Analysis
3. Results
3.1. Morphological Attributes
3.2. Se-Content of Different Parts of RR
3.3. Nutritional Quality and Trace Element Content of RR Stems and Leaves
3.3.1. Nutritional Quality of RR Stems and Leaves
3.3.2. Trace Element Content of RR Stems and Leaves
3.4. Photosynthetic Indicators of RR Leaves
3.4.1. Photosynthetic Indicators of RR Leaves in Current Season
3.4.2. Photosynthetic Indicators of RR Leaves in Subsequent Seasons
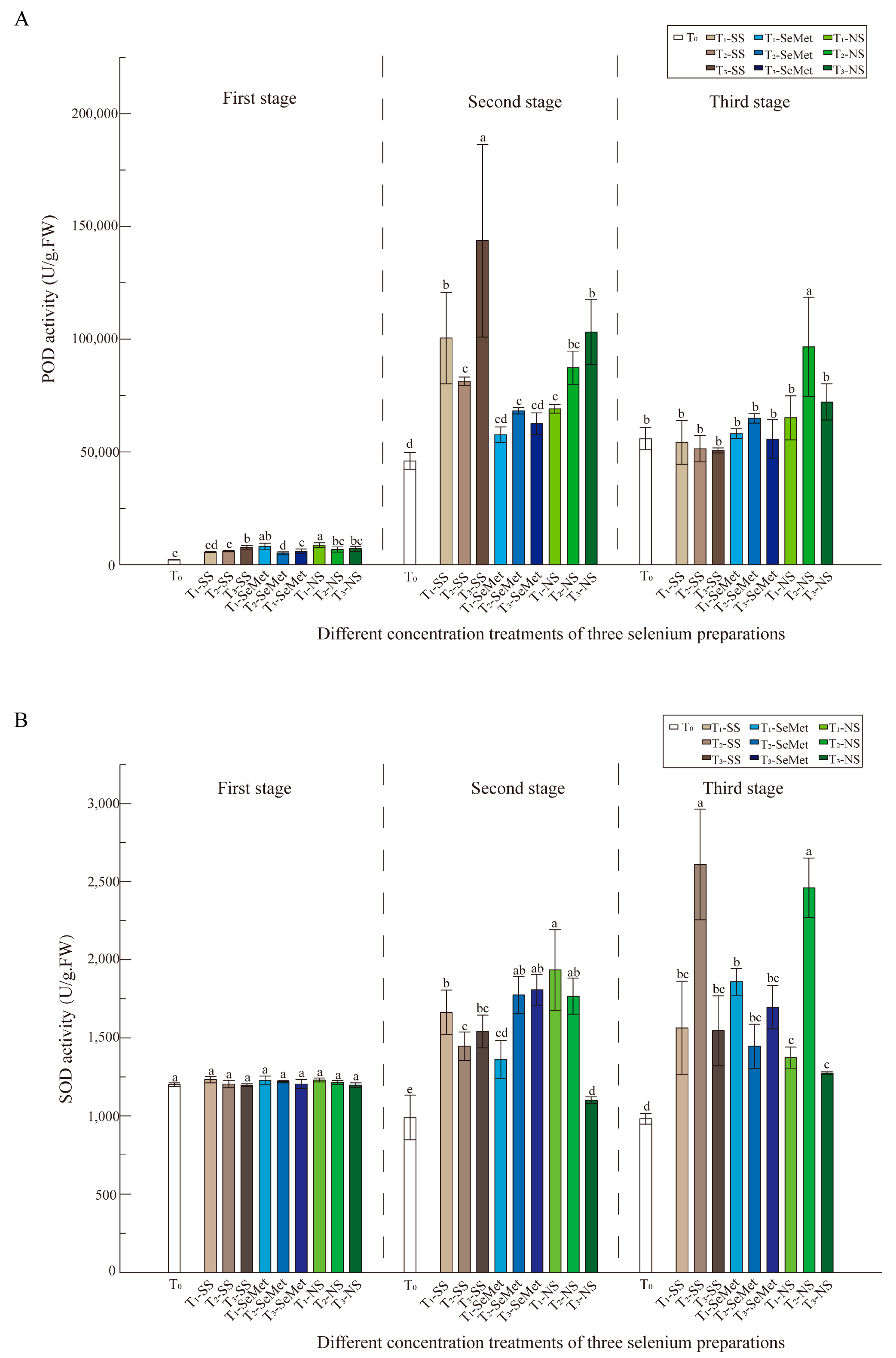
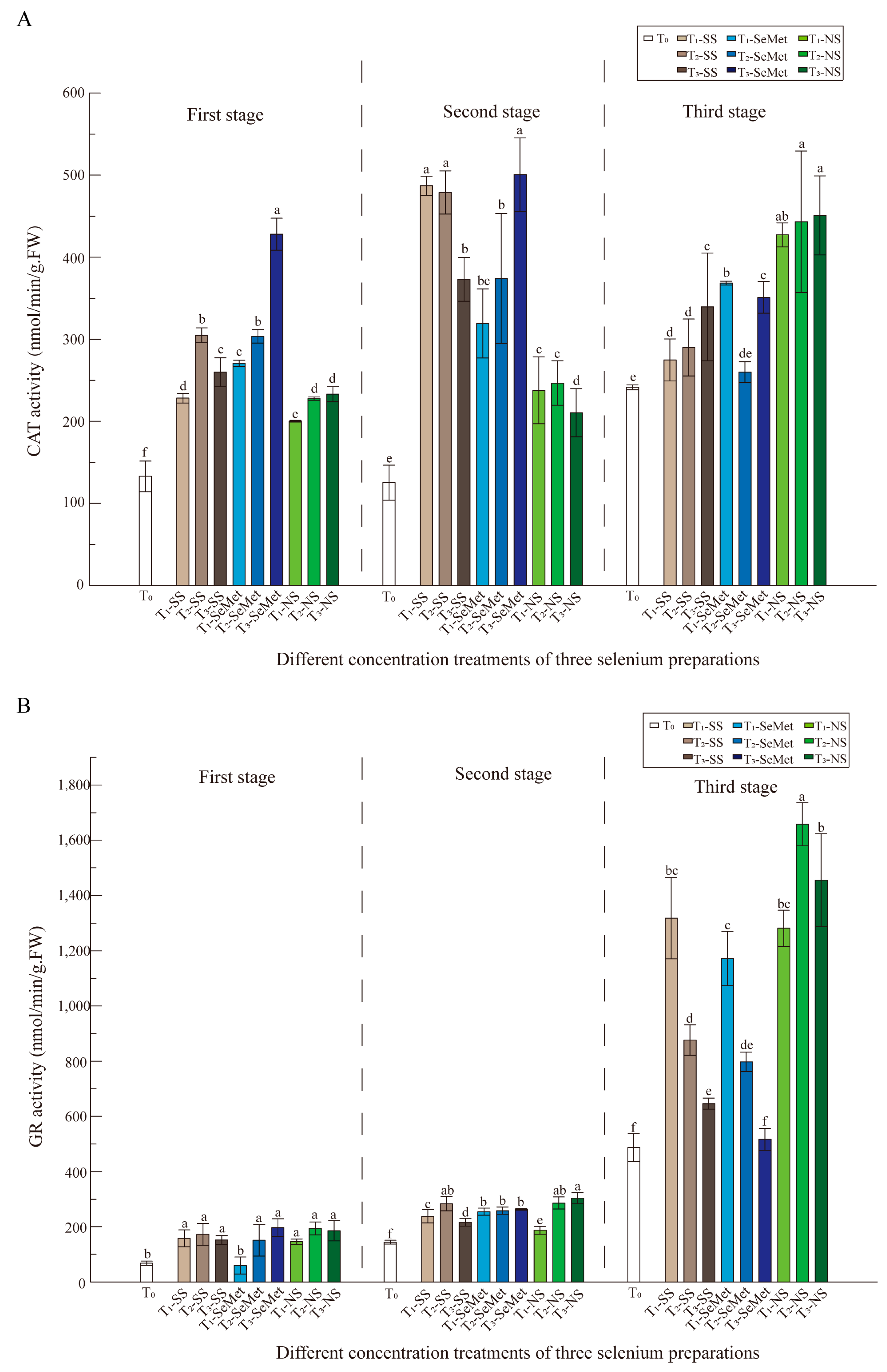
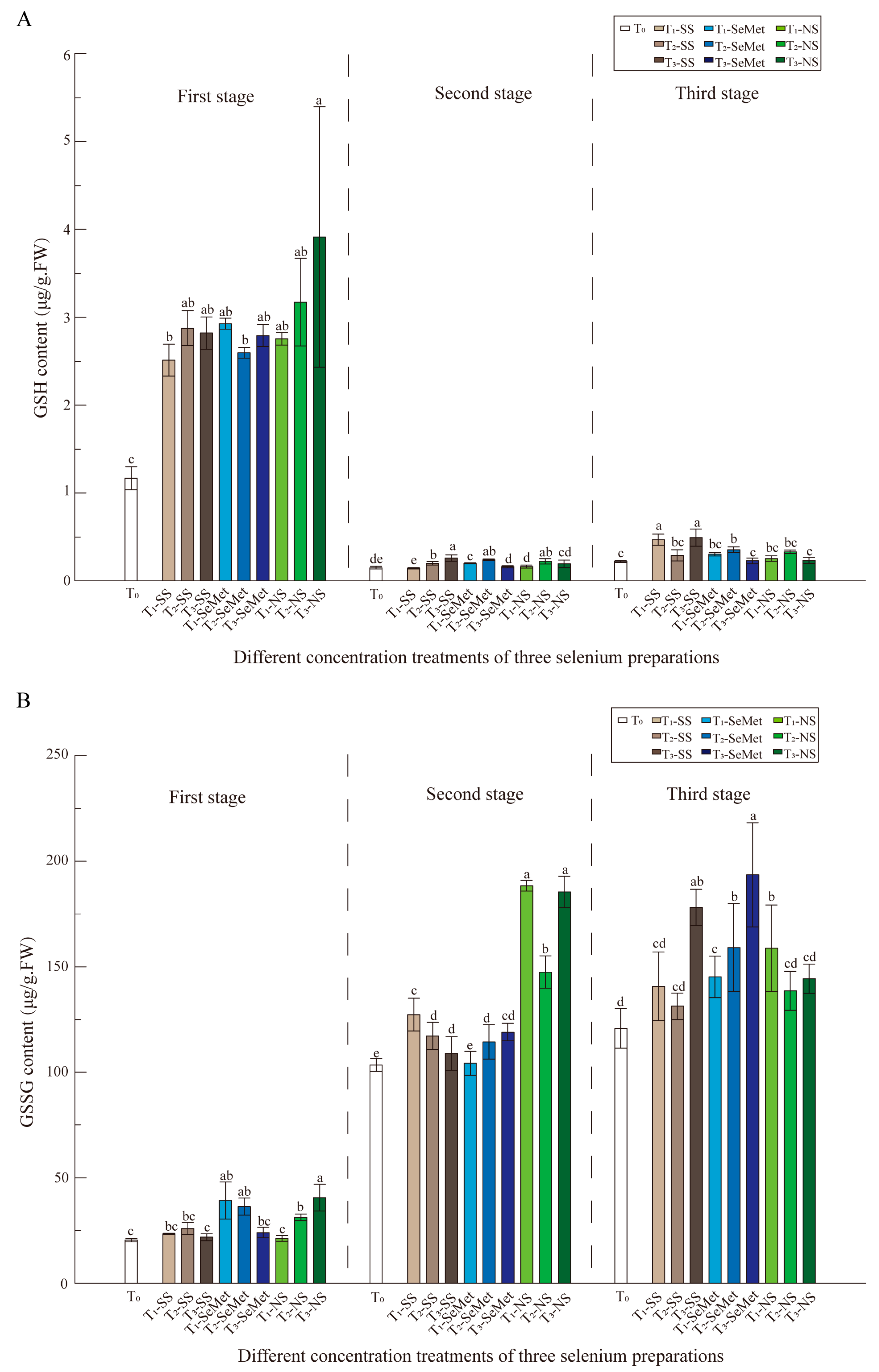
3.5. Antioxidant Enzyme Activities
3.6. Grain Yield and Total Biomass
3.7. Se Concentration and Accumulation Distribution Ratio in RR Grains and Leaves
3.8. Brown Rice Quality of RR
3.8.1. Trace Element Contents
3.8.2. The Processing and Appearance Quality of Brown Rice
3.8.3. The Cooking and Eating Quality
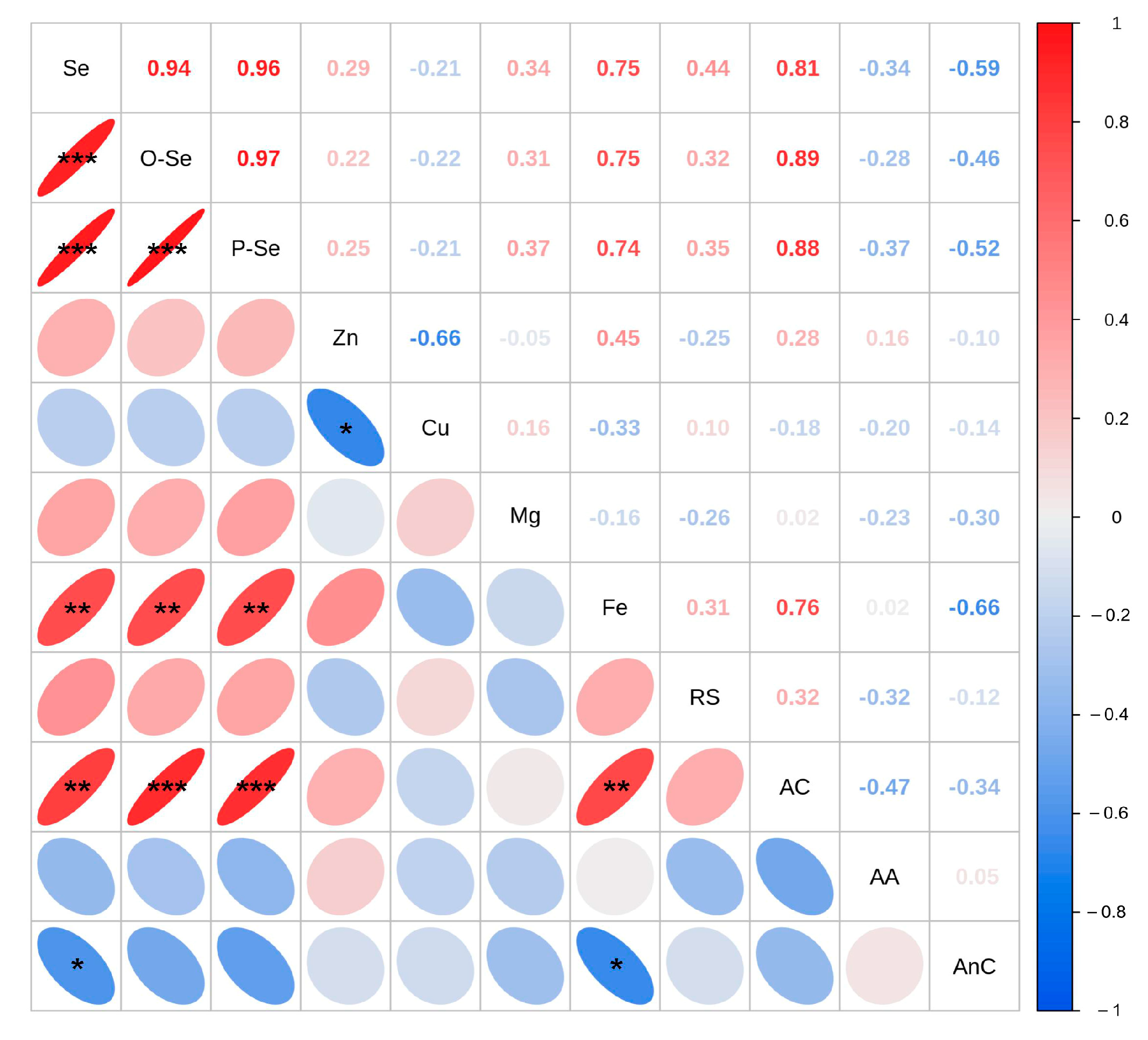
3.9. Correlation Analysis
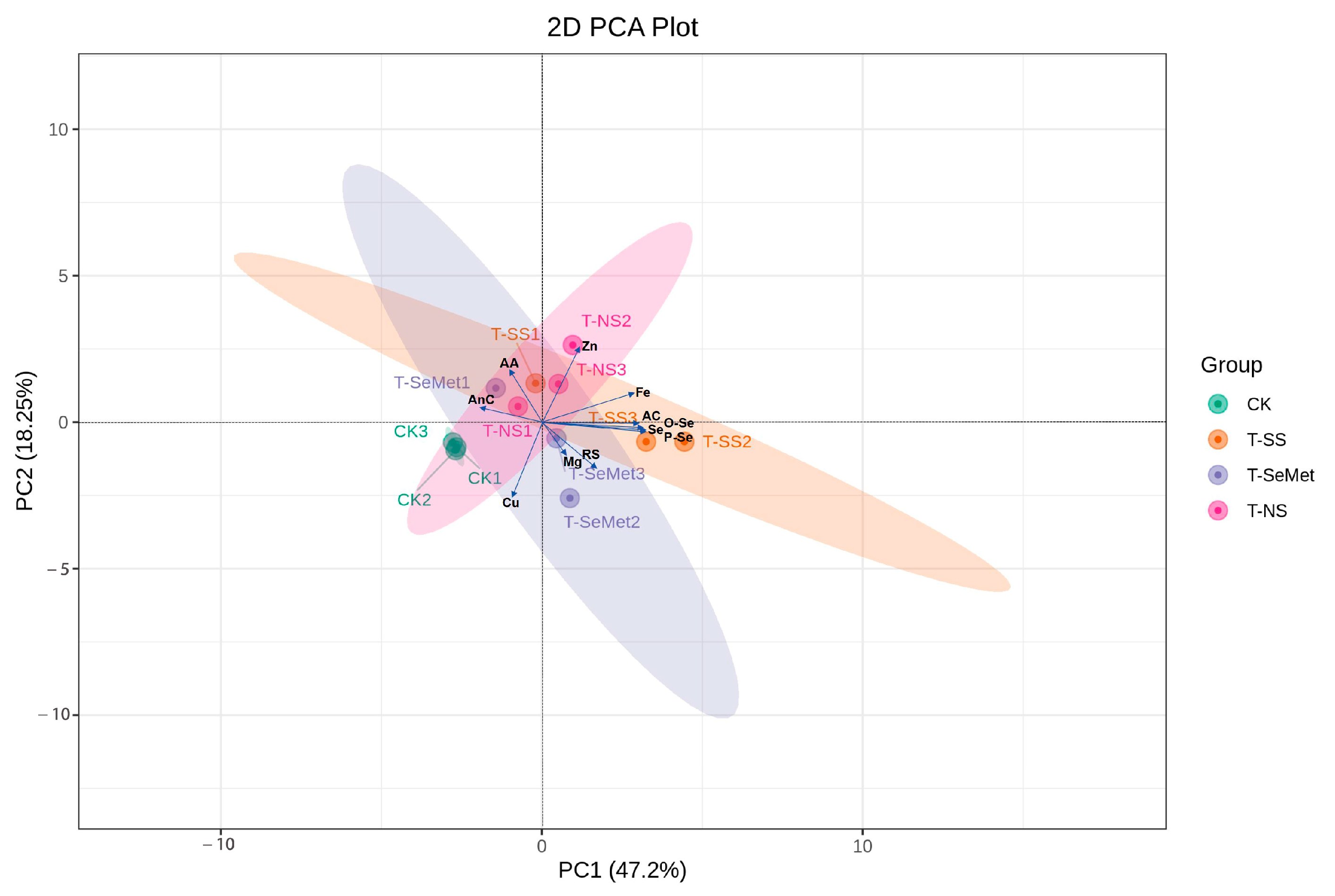
3.10. Principal Component Analysis
4. Discussion
4.1. The Exogenous Application of Se Promoted the Appearance Quality and Photosynthetic Traits of RR
4.2. Exogenous Application of Se Promoted the Antioxidant Properties of RR
4.3. The Potential of Foliar Biofortification to Enhance the Functional Component Contents of RR Grains
4.4. Biostatistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Se | Selenium |
| SS | Sodium selenite |
| SeMet | Selenomethionine |
| NS | Nanoselenium |
| CK | Control |
| T0 | The control treatment (CK, only distilled water) |
| T1-SS | 50 mg L−1 of SS |
| T2-SS | 100 mg L−1 of SS |
| T3-SS | 150 mg L−1 of SS |
| T1-SeMet | 50 mg L−1 of SeMet |
| T2-SeMet | 100 mg L−1 of SeMet |
| T3-SeMet | 150 mg L−1 of SeMet |
| T1-NS | 150 μg L−1 of NS |
| T2-NS | 450 μg L−1 of NS |
| T3-NS | 900 μg L−1 of NS |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| GR | Glutathione reductase |
| GSSG | Oxidized glutathione |
| APX | Ascorbate peroxidase |
| GSH | Reduced glutathione |
| Pn | Photosynthetic rate |
| Ci | Intercellular CO2 concentration |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| AC | Amylose content |
| GC | Gelatinization consistency |
| ASV | Alkali spreading value |
| GT | Gelatinization temperature |
| AA | Amino acid |
| RR | Ratooning rice |
| RS | Resistant starch |
| MeSeCys | Methylselenocysteine |
| PCA | Principal component analysis |
| LY | Liangyou 6326 |
References
- Moulick, D.; Mukherjee, A.; Das, A.; Roy, A.; Majumdar, A.; Dhar, A.; Pattanaik, B.K.; Chowardhara, B.; Ghosh, D.; Upadhyay, M.K.; et al. Selenium-An environmentally friendly micronutrient in agroecosystem in the modern era: An overview of 50-year findings. Ecotoxicol. Environ. Saf. 2024, 270, 115832. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.X.; Wu, L.; Hou, M.Y.; Jia, S.F.; Jiang, L.N.; Zhang, D.J. Effects of selenium application on wheat yield and grain selenium content:A global meta-analysis. Field Crops Res. 2024, 307, 109266. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 19, 436–442. [Google Scholar] [CrossRef]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Available online: https://www.docin.com/p-1570008617.html (accessed on 9 November 2024).
- Wu, Z.L.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.X.; Yin, X.B.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Umont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Williams, P.N.; Lombi, E.; Sun, G.X.; Scheckel, K.; Zhu, Y.G.; Feng, X.B.; Zhu, J.M.; Carey, A.M.; Adomako, E.; Lawgali, Y.; et al. Selenium characterization in the global rice supply chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- Hu, Y.; Norton, G.J.; Duan, G.L.; Huang, Y.C.; Liu, Y.X. Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 2014, 384, 131–140. [Google Scholar] [CrossRef]
- Farooq, M.U.; Tang, Z.; Zeng, R.; Liang, Y.K.; Zhang, Y.J.; Zheng, T.D.; Ei, H.H.; Ye, X.Y.; Jia, X.M.; Zhu, J.Q. Accumulation, mobilization, and transformation of selenium in rice grain provided with foliar sodium selenite. J. Sci. Food Agric. 2019, 99, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.K.; Farooq, M.U.; Zeng, R.; Tang, Z.C.; Zhang, Y.J.; Zheng, T.D.; Ei, H.H.; Ye, X.Y.; Jia, X.M.; Zhu, J.Q. Breeding of selenium rich red glutinous rice, protein extraction and analysis of the distribution of selenium in grain. Int. J. Agric. Biol. 2018, 20, 1005–1011. [Google Scholar]
- Su, Y.; Huang, X.; Li, L.; Muhammad, Z.A.; Li, M.L.; Zheng, T.D.; Luo, D.; Ye, X.Y.; Jia, X.M.; Panhwar, F.H.; et al. Comparative responses of silicon to reduce cadmium and enrich selenium in rice varieties. Foods 2023, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Long, W.X.; Liang, T.; Zhu, M.H.; Zhu, A.Y.; Luo, X.Y.; Wu, X.T. Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 2023, 486, 87–101. [Google Scholar] [CrossRef]
- Ros, G.; Van Rotterdam, A.; Bussink, D.; Bindraban, P. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Yin, H.Q.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Chu, X.Y.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environm Ental Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Dong, H.L.; Chen, Q.; Wang, W.Q.; Peng, S.B.; Huang, J.L.; Cui, K.H.; Nie, L.X. The growth and yield of a wet-seeded rice-ratoon rice system in central China. Field Crops Res. 2017, 208, 55–59. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Zhang, J.B. Ratoon rice production in central China: Environmental sustainability and food production. Sci. Total Environ. 2021, 764, 142850. [Google Scholar] [CrossRef]
- Lin, W.X. Developmental status and problems of rice ratooning. J. Integr. Agric. 2019, 18, 246–247. [Google Scholar] [CrossRef]
- Golam, F.; Rosna, T.; Zakaria, P. Rice ratoon crop: A sustainable rice production system for tropical hill agriculture. Sustainability 2014, 6, 5785–5800. [Google Scholar] [CrossRef]
- Peng, S.B.; Zheng, C.; Yu, X. Progress and challenges of rice ratooning technology in China. Crop Environ. 2023, 2, 5–11. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, C.; Yu, X.; Zheng, C.; Xiao, S.; Xu, L.; Cui, K.H.; Huang, J.L.; Peng, S.B. On-farm comparison in grain quality between main and ratoon crops of ratoon rice in Hubei province, central China. J. Sci. Food Agri. 2022, 102, 7259–7267. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Yang, F.; Li, Q.P.; Zeng, Y.L.; Li, B.; Zhong, X.Y.; Lu, H.; Wang, L.; Chen, H.; Chen, Y.; et al. Differences in starch structural and physicochemical properties and texture characteristics of cooked rice between the main crop and ratoon rice. Food Hydrocoll. 2021, 116, 106643. [Google Scholar] [CrossRef]
- Zhang, W.; Zhan, Z.; Wang, H.X.; Shu, Z.X.; Wang, P.P.; Zeng, X.F. Structural, pasting and sensory properties of rice from main and ratoon crops. Int. J. Food Prop. 2021, 24, 965–975. [Google Scholar] [CrossRef]
- Lin, C.J.; Li, C.Y.; Lin, S.K.; Yang, F.H.; Huang, J.J.; Liu, Y.H.; Lur, H.S. Influence of high temperature during grain filling on the accumulation of storage proteins and grain quality in rice (Oryza sativa L.). J. Agric. Food Chem. 2010, 58, 10545–10552. [Google Scholar] [CrossRef]
- Lin, F.F.; Huang, J.W.; Lin, S.; Letuma, P.; Xie, D.X.; Rensing, C.; Lin, W.X. Physiological and transcriptomic analysis reveal the regulatory mechanism under lying grain quality improvement induced by rice ratooning. J. Sci. Food Agric. 2023, 103, 3569–3578. [Google Scholar] [CrossRef]
- Lin, F.F.; Rensing, C.; Pang, Z.Q.; Zou, J.N.; Lin, S.; Lin, W.X. Metabolomic analysis reveals differential metabolites and pathways involved in grain chalkiness improvement under rice ratooning. Field Crops Res. 2022, 283, 108521. [Google Scholar] [CrossRef]
- Peng, T.; Wang, T.T.; Li, L.Z.; Zhao, Q.Z. Effect of clipping stage and stubble height on quality and yield of herbage and grain yield of ratoon rice. J. Henan Agric. Sci. 2020, 49, 27–33, (In Chinese with English abstract). [Google Scholar]
- Royo, C.; Lopez, A.; Serra, J.; Tribo, F. Effect of sowing date and cutting stage on yield and quality of irrigated barley and triticale used for forage and grain. J. Agron. Crop Sci. 1997, 179, 227–234. [Google Scholar] [CrossRef]
- McMullen, K.G.; Virgona, J.M. Dry matter production and grain yield from grazed wheat in southern New South Wales. Anim. Prod. Sci. 2009, 49, 769–776. [Google Scholar] [CrossRef]
- Sakai, M.; Iida, S.; Maeda, H.; Sunohara, Y.; Nemoto, H.; Imbe, T. New rice varieties for whole crop silage use in Japan. Breed. Sci. 2003, 53, 271–275. [Google Scholar] [CrossRef]
- Nakano, H.; Hattori, I.; Morita, S. Dry matter yield response to seeding rate and row spacing in direct-seeded and double-harvested forage rice. Jpn. Agric. Res. Q. 2019, 53, 255–264. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of different forms of selenium fertilizers on Se accumulation, distribution, and residual effect in winter wheat–summer maize rotation system. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef]
- Lyu, L.H.; Wang, H.Q.; Liu, R.F.; Xing, W.J.; Li, J.; Man, Y.B.; Wu, F.Y. Size-dependent transformation, uptake, and transportation of SeNPs in a wheat–soil system. J. Hazard. Mater. 2022, 424, 127323. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.R.; Zou, N.; Wu, Y.L.; Li, J.Q.; Pan, C.P. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Pan, C.P. Chitosan Functionalized Nano Selenium Compatible Bacterial Enzyme System and Its Preparation Method and Application. China Patent ZL 2015 1 0698215.X, 11 January 2019. [Google Scholar]
- Miao, S.Y.; Liang, D.L.; Zhao, W.L.; Hu, B. Phytotoxicity differences among 7 winter wheat genotypes in China when amended selenate and selenite. J. Agro-Environ. Sci. 2013, 32, 1934–1940, (In Chinese with English abstract). [Google Scholar]
- Standardization Administration of the People’s Republic of China. GB/T 23387-2009; Evaluation of forage nutritional quality—grading index (G I) method. Standard Press: Beijing, China, 2017. (In Chinese)
- Zhang, Y.; Frankenberger, W.T. Speciation of selenium in plant water extracts by ion exchange chromatography-hydride generation atomic absorption spectrometry. Sci. Total Environ. 2001, 269, 39–47. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Wu, Q.H.; Lv, H.Q.; Chen, W.X.; Wang, L.Z.; Shi, S.J.; Yang, J.G.; Zhao, P.P.; Li, Y.P.; Christopher, R.; et al. Toxicity of different forms of antimony to rice plants: Effects on reactive oxidative species production, antioxidative systems, and uptake of essential elements. Environ. Pollut. 2020, 263, 114544. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 Silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II Activity, and electron transport efficiency in tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.K.; Li, X.B.; He, H.Z.; Li, H.S.; Zhang, Z.M. Varietal differences in the growth of rice seedlings exposed to perchlorate and their antioxidative defense mechanisms. Environ. Toxicol. Chem. 2015, 34, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Vasanthem, T.; Bhatty, R.S. Physicochemical properties of small- and large- granule starches of waxy, regular, and high-amylose barleys. Cereal Chem. 1996, 7, 199–207. [Google Scholar]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Chengdu Institute of Grain Storage Science. National Grain Reserve Administration Grain and Oil Inspection—Determination of Rice Gum Consistency: GB/T 22294-2008; China Standard Press: Beijing, China, 2008. [Google Scholar]
- Lu, B.Y.; An, H.Y.; Song, X.L.; Yang, B.S.; Jian, Z.Q.; Cui, F.Z.; Xue, J.F.; Gao, Z.Q.; Du, T.Q. Enhancement of nutritional substance, trace elements, and pigments in waxy maize grains through foliar application of selenite. Foods 2024, 13, 1337. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; Garcia-Morales, S. The role of selenium nano particles in agriculture and food technology. Biol. Trace Elem. Res. 2022, 200, 2528–2548. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Huang, Y.; Lin, Z.; Bañuelos, G.S.; Lam, M. H-W.; Yin, X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011, 126, 1088–1093. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Influence of selenite and selenate on growth, leaf physiology and antioxidant defense system in wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 5700–5710. [Google Scholar] [CrossRef]
- Li, J.H.; Yang, W.P.; Guo, A.N.; Qi, Z.W.; Chen, J.; Huang, T.M.; Yang, Z.P.; Gao, Z.Q.; Sun, M.; Wang, J.W. Combined foliar and soil selenium fertilizer increased the grain yield, quality, total Se, and organic Se content in naked oats. J. Cereal Sci. 2021, 100, 103265. [Google Scholar] [CrossRef]
- Luo, H.W.; He, L.X.; Du, B.; Pan, S.G.; Mo, Z.W.; Duan, M.Y.; Tian, H.; Tang, X.R. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crops Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.B.; Liu, J.; Chen, Y.L.; Zhang, X.J. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lara, T.S.; Lessa, J.H.D.L.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Liang, K.; Liang, S.; Zhu, H. Comparative proteomics analysis of the effect of selenium treatment on the quality of foxtail millet. LWT 2020, 131, 109691. [Google Scholar] [CrossRef]
- Shi, L.J.; Bai, W.B.; Cao, C.L.; Gao, P. Effects of exogenous selenium application on yield, grain selenium content and quality in sorghum. Crops 2020, 3, 191–196. [Google Scholar]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic biofortification of significant cereal crops with selenium—A review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Barman, F.; Kundu, R. Foliar application of selenium affecting pollen viability, grain chalkiness, and transporter genes in cadmium accumulating rice cultivar: A pot study. Chemosphere 2023, 313, 137538.54. [Google Scholar] [CrossRef]
- Auobi Amirabad, S.; Behtash, F.; Vafaee, Y. Selenium mitigates cadmium toxicity by preventing oxidative stress and enhancing photosynthesis and micronutrient availability on radish (Raphanus sativus L.) cv. Cherry Belle. Environ. Sci. Pollut. Res. 2020, 27, 12476–12490. [Google Scholar] [CrossRef]
- Bao, K.M.; Wang, Y.R.; Du, X.P.; Wuriyanghan, H.; Wang, X.; Xie, J.T.; Zhao, X.H.; Jia, W. Comparison of selenium accumulation in edible parts of wheat and broad bean. Agronomy 2023, 13, 1939. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Liu, C.; Han, Y. Effects of selenate and selenite on selenium accumulation and speciation in lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- Hu, C.X.; Nie, Z.J.; Shi, H.Z.; Peng, H.Y.; Li, G.X.; Liu, H.Y.; Li, C.; Liu, H.E. Selenium uptake, translocation, subcellular distribution and speciation in winter wheat in response to phosphorus application combined with three types of selenium fertilizer. BMC Plant Biol. 2023, 23, 224. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wei, S.; Skuza, L.; Jia, G. Selenium spiked in soil promoted zinc accumulation of Chinese cabbage and improved antioxidant system and lipid peroxidation. Ecotoxicol. Environ. Saf. 2019, 180, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Prom-U-Thai, C.; Rashid, A.; Ram, H.; Zou, C.Q.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.W.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Silva, V.M.; Montanha, G.S.; Reis, A.R.D. Selenium increases photosynthetic capacity, daidzein biosynthesis, nodulation and yield of peanuts plants (Arachis hypogaea L.). Plant Physiol. Biochem. 2022, 190, 231–239. [Google Scholar] [CrossRef]
- Hunan Selenium Enriched Biological Industry Association in China. T/HNFX 001-2017 (v01) Selenium Content Requirements for Selenium Rich Agricultural Products. 2017. Available online: http://down.foodmate.net/standard/sort/12/52694.html (accessed on 9 November 2024).
- Zhang, H.Q.; Zhao, Z.Q.; Zhang, X.; Zhang, W.; Huang, L.Q.; Zhang, Z.Z.; Yuan, L.X.; Liu, X.W. Effects of foliar application of selenate and selenite at different growth stages on selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Mohtashami, R.; Dehnavi, M.M.; Balouchi, H.; Faraji, H. Improving yield, oil content and water productivity of dryland canola by supplementary irrigation and selenium spraying. Agric. Water Manag. 2020, 232, 106046. [Google Scholar] [CrossRef]
- Silva, V.M.; Tavanti, R.F.R.; Gratão, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- de Brito Mateus, M.P.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; dos Reis, A.R. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef]
- Yu, H.; Miao, P.J.; Li, D.; Wu, Y.L.; Zhou, C.R.; Pan, C.P. Improving red pitaya fruit quality by nano-selenium biofortification to enhance phenylpropanoid and betalain biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef]
- Gu, Q.C.; Luo, H.W.; Lin, L.; Zhang, Q.Q.; Yi, W.T.; Liu, Z.F.; Yu, X.H.; Zuo, C.J.; Qi, J.Y.; Tang, X.R. Effects of biological nano-selenium on yield, grain quality, aroma, and selenium content of aromatic rice. Agronomy 2024, 14, 1778. [Google Scholar] [CrossRef]
- Huang, S.; Qin, H.; Jiang, D.; Lu, J.; Zhu, Z.; Huang, X. Bio-nano selenium fertilizer improves the yield, quality, and organic selenium content in rice. J. Food Compos. Anal. 2024, 132, 106348. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Cassman, K.G.; Huang, J.; Peng, S.; Grassini, P. Can ratoon cropping improve resource use efficiencies and profitability of rice in central China? Field Crops Res. 2019, 234, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Huang, S.H.; Jiang, Z.H.; Wang, Y.Z.; Zhang, Z.M. Selenium biofortification modulates plant growth, microelement and heavy metal concentrations, selenium uptake, and accumulation in black-grained wheat. Front. Plant Sci. 2021, 12, 748523. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; de Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H.; Guilherme, L.R.G. Selenium speciation in Se-enriched soybean grains from biofortified plants grown under different methods of selenium application. Foods 2023, 12, 1214. [Google Scholar] [CrossRef]
- Ragályi, P.; Takács, T.; Soós, Á.; Kovács, B.; Dernovics, M.; Lončarić, Z.; Dobosy, P.; Záray, G.; Rékási, M. Quantitative analysis of selenium species in the edible parts of cabbage, carrot, tomato and green pea treated with selenate-enriched irrigation water. Plant Soil 2024, 496, 341–360. [Google Scholar] [CrossRef]
- Kowalska, I.; Smoleń, S.; Czernicka, M.; Halka, M.; Kęska, K.; Pitala, J. Effect of selenium form and salicylic acid on the accumulation of selenium speciation forms in hydroponically grown lettuce. Agriculture 2020, 10, 584. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Yang, P.; Gui, R.Y. Research progress on speciation of selenium compounds in plants. J. Zhejiang A&F Univ. 2012, 29, 288–295, (In Chinese with English abstract). [Google Scholar]
- Wang, W.Q.; He, A.B.; Jiang, G.L.; Sun, H.J.; Jiang, M.; Man, J.G.; Ling, X.X.; Cui, K.H.; Huang, J.L.; Peng, S.B.; et al. Ratoon rice technology: A green and resource-efficient way for rice production. Adv. Agron. 2020, 159, 135–167. [Google Scholar]
- Bao, J.S.; Ying, Y.N.; Zhou, X.; Xu, Y.J.; Wu, P.; Xu, F.F.; Pang, Y.H. Relationships among starch biosynthesizing protein content, fine structure and functionality in rice. Carbohydr. Polym. 2020, 237, 116118. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1980, 107, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Pautong, P.A.; Liu, Q.Q.; Sreenivasulu, N. Low glycemic index rice—A desired trait in starchy staples. Trends Food Sci. Technol. 2020, 106, 132–149. [Google Scholar] [CrossRef]
- Noda, T.; Takahata, Y.; Sato, T.; Suda, I.; Morishita, T.; Ishiguro, K.; Yamakawa, O. Relationships between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweet potato and buckwheat. Carbohydr. Polym. 1998, 37, 153–158. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Yadav, B.S.; Sharma, A.; Yadav, R.B. Resistant starch content of conventionally boiled and pressure-cooked cereals, legumes and tubers. J. Food Sci. Technol. 2010, 47, 84–88. [Google Scholar] [CrossRef]
- Huang, L.C.; Xiao, Y.; Zhao, W.; Rao, Y.A.; Shen, H.M.; Gu, Z.W.; Fan, X.L.; Li, Q.F.; Zhang, C.Q.; Liu, Q.Q. Creating high-resistant starch rice by simultaneous editing of SS3a and SS3b. Plant Biotechnol. J. 2024, 22, 787–789. [Google Scholar] [CrossRef]
- Ding, C.; Xu, C.S.; Lu, B.; Zhu, X.H.; Luo, X.K.; He, B.; Elidio, C.; Liu, Z.H.; Ding, Y.F.; Yang, J.; et al. Comprehensive evaluation of rice qualities under different nitrogen levels in South China. Foods 2023, 12, 697. [Google Scholar] [CrossRef]

| Treatment | Seedling Height (cm) | Chlorophyll Value | Adult Plant Height (cm) | Spike Length (cm) | Number of Grains per Spike | 1000-Grain Weight (g) |
|---|---|---|---|---|---|---|
| CK | 80.07 ± 1.25 g | 38.17 ± 2.16 e | 114.82 ± 9.2 e | 23.75 ± 6.11 c | 20.58 ± 3.54 d | 17.40 ± 3.01 c |
| T1-SS | 83.97 ± 2.72 e | 44.58 ± 2.6 b | 120.37 ± 7.3 c | 25.24 ± 5.61 b | 25.58 ± 3.5 ab | 16.60 ± 1.17 db |
| T2-SS | 93.40 ± 3.62 b | 45.27 ± 4.11 a | 126.62 ± 7.23 a | 24.92 ± 3.68 b | 26.05 ± 4.33 a | 20.11 ± 3.17 b |
| T3-SS | 83.95 ± 3.21 e | 44.37 ± 3.25 c | 116.73 ± 6.25 d | 23.51 ± 6.1 c | 26.28 ± 4.36 a | 17.41 ± 1.56 c |
| T1-SeMet | 90.40 ± 2.18 c | 44.15 ± 4.2 c | 110.05 ± 8.8 f | 24.55 ± 7.62 b | 24.60 ± 5.12 b | 18.43 ± 2.2 c |
| T2-SeMet | 84.15 ± 3.11 e | 47.37 ± 3.52 a | 126.12 ± 7.23 a | 22.06 ± 5.02 d | 26.64 ± 6.13 a | 17.78 ± 3.27 c |
| T3-SeMet | 86.70 ± 3.32 d | 45.15 ± 5.3 ab | 122.71 ± 4.34 b | 27.46 ± 8.21 a | 24.12 ± 3.43 c | 16.23 ± 2.19 d |
| T1-NS | 94.75 ± 5.27 a | 42.76 ± 3.41 d | 122.80 ± 6.27 b | 27.37 ± 6.2 a | 23.29 ± 7.2 c | 22.31 ± 3.26 a |
| T2-NS | 82.21 ± 2.34 f | 45.83 ± 7.03 a | 116.90 ± 3.5 d | 24.26 ± 4.4 c | 23.48 ± 4.33 c | 20.00 ± 2.15 b |
| T3-NS | 95.60 ± 5.17 a | 44.39 ± 6.41 bc | 116.55 ± 11.2 d | 24.38 ± 6.33 b | 24.51 ± 7.14 b | 15.81 ± 1.97 de |
| Treatments | Roots (mg·kg−1) | Straw (mg·kg−1) | Spike Axis (mg·kg−1) | Grains (mg·kg−1) |
|---|---|---|---|---|
| CK | 0.276 ± 0.02 f | 0.078 ± 0.01 i | 0.068 ± 0.01 f | 0.063 ± 0.02 f |
| T1-SS | 0.431 ± 0.23 c | 0.356 ± 0.12 g | 0.236 ± 0.05 d | 0.295 ± 0.1 d |
| T2-SS | 0.657 ± 0.52 b | 0.929 ± 0.33 a | 1.048 ± 0.42 a | 0.757 ± 0.2 a |
| T3-SS | 0.368 ± 0.33 d | 0.516 ± 0.21 e | 0.356 ± 0.13 c | 0.542 ± 0.12 b |
| T1-SeMet | 0.405 ± 0.42 cd | 0.247 ± 0.08 h | 0.117 ± 0.03 e | 0.137 ± 0.03 e |
| T2-SeMet | 0.468 ± 0.34 c | 0.461 ± 0.22 f | 0.179 ± 0.03 de | 0.402 ± 0.11 c |
| T3-SeMet | 0.332 ± 0.12 d | 0.594 ± 0.14 d | 0.403 ± 0.13 c | 0.263 ± 0.07 d |
| T1-NS | 0.351 ± 0.09 d | 0.288 ± 0.05 h | 0.126 ± 0.03 e | 0.112 ± 0.02 e |
| T2-NS | 0.708 ± 0.22 a | 0.684 ± 0.17 c | 0.368 ± 0.06 c | 0.382 ± 0.06 c |
| T3-NS | 0.327 ± 0.13 e | 0.887 ± 0.24 b | 0.770 ± 0.15 b | 0.256 ± 0.07 d |
| Treatments | Dry Matter Content (%) | Crude Protein Content (%) | Crude Ash Content (%) | Crude Fiber Content (%) | Starch Content (%) |
|---|---|---|---|---|---|
| CK | 70.32 ± 6.8 h | 8.11 ± 0.21 b | 4.23 ± 0.33 c | 8.40 ± 0.85 c | 65.84 ± 3.21 c |
| T1-SS | 69.39 ± 5.42 g | 7.86 ± 0.7 b | 3.66 ± 0.74 d | 8.86 ± 0.77 b c | 62.31 ± 4.82 d |
| T2-SS | 89.22 ± 5.43 a | 7.96 ± 0.41 b | 5.01 ± 0.28 b | 9.62 ± 0.8 a | 68.27 ± 6.11 a |
| T3-SS | 88.15 ± 3.41 b | 8.60 ± 0.21 a | 4.12 ± 0.34 c | 9.06 ± 1.32 b | 67.60 ± 5.31 a |
| T1-SeMet | 71.38 ± 6.42 g | 7.90 ± 0.42 b | 5.20 ± 0.43 b | 6.34 ± 0.65 e | 66.86 ± 4.32 b |
| T2-SeMet | 77.67 ± 5.76 e | 7.93 ± 0.23 b | 3.91 ± 1.1 c d | 5.71 ± 0.63 f | 65.24 ± 3.51 c |
| T3-SeMet | 73.84 ± 7.32 f | 5.97 ± 0.2 d | 5.02 ± 0.2 b | 8.44 ± 0.75 c | 63.21 ± 5.06 d |
| T1-NS | 79.80 ± 6.05 d | 6.75 ± 1.1 c | 4.31 ± 0.73 c | 7.97 ± 1.01 d | 61.78 ± 6.7 d |
| T2-NS | 81.48 ± 7.52 c | 5.33 ± 0.57 e | 4.61 ± 0.27 c | 8.02 ± 0.21 d | 61.79 ± 6.44 d |
| T3-NS | 77.51 ± 8.45 e | 5.87 ± 0.66 d | 5.60 ± 0.7 a | 8.30 ± 2.3 d | 66.03 ± 3.33 b |
| Treatments | Iron (mg·kg−1) | Copper (mg·kg−1) | Zinc (mg·kg−1) | Manganese(mg·kg−1) |
|---|---|---|---|---|
| CK | 29.35 ± 3.25 h | 38.06 ± 4.31 c | 6.84 ± 0.7 e | 22.2 ± 1.21 d |
| T1-SS | 46.38 ± 2.17 b | 41.07 ± 1.74 b | 8.53 ± 2.27 c | 31.53 ± 2.01 a |
| T2-SS | 49.16 ± 3.26 a | 39.53 ± 2.35 b | 10.33 ± 1.21 b | 18.6 ± 3.01 e |
| T3-SS | 37.32 ± 6.13 e | 38.61 ± 7.21 c | 11.3 ± 2.07 a | 29.33 ± 3.61 b |
| T1-SeMet | 41.66 ± 4.23 c | 38.63 ± 3.65 c | 7.36 ± 1.02 d | 26.8 ± 4.2 c |
| T2-SeMet | 37.86 ± 8.01 e | 35.43 ± 5.24 e | 8.12 ± 0.67 c d | 23 ± 3.4 d |
| T3-SeMet | 33.85 ± 3.56 f | 35.59 ± 3.83 e | 7.86 ± 1.2 d | 21.55 ± 3.3 d |
| T1-NS | 30.82 ± 6.13 g | 42.4 ± 6.1 a | 11.06 ± 3.02 a | 18.32 ± 2.41 e |
| T2-NS | 41.48 ± 4.02 c | 31.55 ± 4.26 f | 8.33 ± 2.05 c | 18.9 ± 2.3 e |
| T3-NS | 39.85 ± 2.42 d | 37.33 ± 33.6 d | 7.98 ± 1.15 d | 26.1 ± 4.38 c |
| Treatments | Pn (μmol·CO2·m−2·s−1) | Ci (μmol·mol −1) | Tr (mmol H2O·m−2·s−1) | Gs (μmol·m−2·s−1) |
|---|---|---|---|---|
| CK | 19.31 ± 0.05 c | 110.21 ± 0.06 d | 3.47 ± 0.20 c | 0.35 ± 0.02 b |
| T1-SS | 20.93 ± 0.05 c | 52.46 ± 0.72 f | 5.08 ± 0.1 a | 0.36 ± 0.01 b |
| T2-SS | 23.25 ± 0.04 a | 25.79 ± 1.2 h | 2.33 ± 0.01 d | 0.48 ± 0.01 a |
| T3-SS | 21.84 ± 0.03 b | 52.78 ± 0.46 f | 2.48 ± 0.01 d | 0.29 ± 0.00 c |
| T1-SeMet | 21.83 ± 0.03 b | 121.75 ± 0.13 b | 4.63 ± 0.02 ab | 0.36 ± 0.00 b |
| T2-SeMet | 17.89 ± 0.06 d | 107.39 ± 1.21 e | 4.84 ± 0.7 a | 0.25 ± 0.00 d |
| T3-SeMet | 10.22 ± 0.04 f | 106.57 ± 0.16 e | 1.84 ± 0.02 e | 0.21 ± 0.00 e |
| T1-NS | 19.36 ± 0.05 c | 42.68 ± 0.17 g | 1.79 ± 0.02 e | 0.18 ± 0.01 f |
| T2-NS | 17.09 ± 0.02 e | 125.66 ± 0.21 a | 1.13 ± 0.03 f | 0.18 ± 0.02 f |
| T3-NS | 19.32 ± 0.14 c | 117.66 ± 0.14 c | 3.96 ± 0.02 b | 0.12 ± 0.01 g |
| Treatments | Pn (μmol·CO2·m−2·s−1) | Ci (μmol·mol−1) | Tr (mmol·H2O·m−2·s−1) | Gs (μmol·m−2·s−1) |
|---|---|---|---|---|
| CK | 17.02 ± 0.04 d | 113.41 ± 0.08 c | 4.54 ± 0.06 a | 0.34 ± 0.02 b |
| T1-SS | 21.52 ± 0.03 a | 51.88 ± 0.14 i | 4.38 ± 0.02 ab | 0.34 ± 0.01 b |
| T2-SS | 20.23 ± 0.12 b | 81.77 ± 0.64 f | 2.13 ± 0.02 e | 0.11 ± 0.01 e |
| T3-SS | 17.12 ± 0.03d | 104.55 ± 0.17 e | 1.80 ± 0.02 f | 0.31 ± 0.00 c |
| T1-SeMet | 18.73 ± 0.03 c | 63.38 ± 0.71 g | 4.36 ± 0.01 b | 0.38 ± 0.00 b |
| T2-SeMet | 16.28 ± 0.08 e | 105.64 ± 1.15 e | 3.44 ± 0.21 c | 0.37 ± 0.00 b |
| T3-SeMet | 21.44 ± 0.05 a | 55.97 ± 1.14 h | 1.95 ± 0.02 e | 0.21 ± 0.00 d |
| T1-NS | 18.66 ± 0.03c | 111.54 ± 0.12 d | 2.49 ± 0.01 d | 0.19 ± 0.01 d |
| T2-NS | 19.86 ± 0.05 b | 117.35 ± 0.14 b | 2.21 ± 0.01 e | 0.18 ± 0.01 d |
| T3-NS | 19.12 ± 0.02 c | 127.83 ± 0.27 a | 4.74 ± 0.0 a | 0.44 ± 0.01 a |
| Treatments | Total Biomass (t·ha−1) | Grain Yield (t·ha−1) |
|---|---|---|
| Se source (S) | ||
| CK | 17.21 | 6.94 |
| T1-SS | 17.13 | 7.69 |
| T2-SS | 18.42 | 8.53 |
| T3-SS | 17.16 | 7.63 |
| T1-SeMet | 17.76 | 6.68 |
| T2-SeMet | 17.95 | 5.83 |
| T3-SeMet | 15.43 | 7.21 |
| T1-NS | 16.56 | 7.08 |
| T2-NS | 16.62 | 6.98 |
| T3-NS | 16.97 | 7.11 |
| LSD (p = 0.05) | 1.36 | NS |
| Concentration (C) | ||
| T1-SS | 17.13 | 7.69 |
| T1-SeMet | 17.76 | 6.68 |
| T1-NS | 16.56 | 7.08 |
| T2-SS | 18.42 | 8.53 |
| T2-SeMet | 17.95 | 5.83 |
| T2-NS | 16.62 | 7.11 |
| T3-SS | 17.16 | 7.63 |
| T3-SeMet | 15.43 | 7.21 |
| T3-NS | 16.97 | 7.11 |
| LSD (p = 0.05) | NS | NS |
| S × C | * | NS |
| Treatments | Organic Se (mg·kg−1) | Proportion(%) Organic Se/Total Se | Protein Se (mg·kg−1) | Proportion(%) Protein Se/Total Se |
|---|---|---|---|---|
| Se Source(S)–Concentration(C) | ||||
| CK | 0.055 | 79.84 | 0.0264 | 44.18 |
| T1-SS | 0.281 | 90.00 | 0.1328 | 42.54 |
| T2-SS | 0.817 | 90.62 | 0.328 | 36.40 |
| T3-SS | 0.575 | 88.75 | 0.3064 | 40.68 |
| T1-SeMet | 0.152 | 89.25 | 0.061 | 43.24 |
| T2-SeMet | 0.256 | 85.22 | 0.1425 | 46.32 |
| T3-SeMet | 0.363 | 90.52 | 0.1383 | 38.61 |
| T1-NS | 0.144 | 87.23 | 0.0684 | 44.22 |
| T2-NS | 0.239 | 85.36 | 0.1232 | 41.14 |
| T3-NS | 0.367 | 90.28 | 0.1466 | 33.86 |
| LSD (p = 0.05) | 0.168 | 5.14 | 0.0795 | 5.89 |
| S × C | NS | NS | NS | NS |
| Treatments | Zinc (mg·kg−1) | Iron (mg·kg−1) | Copper (mg·kg−1) | Manganese (mg·kg−1) |
|---|---|---|---|---|
| CK | 14.22 ± 0.31 e | 16.41 ± 0.52 e | 9.36 ± 1.85 a | 16.97 ± 3.42 b |
| T1-SS | 17.18 ± 0.6 b | 17.44 ± 1.06 d | 6.48 ± 0.57 d | 16.43 ± 1.22 b |
| T2-SS | 15.62 ± 0.36 d | 21.77 ± 3.22 a | 8.33 ± 0.1 b | 18.87 ± 0.52 a |
| T3-SS | 15.91 ± 0.12 c | 21.05 ± 1.6 a | 8.33 ± 0.01 b | 18.56 ± 0.16 a |
| T1-SeMet | 15.13 ± 0.53 d | 19.30 ± 0.84 c | 7.66 ± 0.0.12 c | 10.45 ± 0.81 e |
| T2-SeMet | 14.11 ± 0.23 e | 18.93 ± 3.43 c | 9.68 ± 0.52 a | 13.93 ± 1.64 cd |
| T3-SeMet | 13.52 ± 0.12 f | 20.55 ± 2.03 b | 8.89 ± 0.33 b | 12.75 ± 1.67 d |
| T1-NS | 16.18 ± 1.54 c | 19.27 ± 1.61 c | 8.12 ± 0.64 bc | 14.62 ± 0.47 c |
| T2-NS | 18.65 ± 0.94 a | 21.88 ± 0.41 a | 7.39 ± 0.36 c | 14.36 ± 0.71 c |
| T3-NS | 17.38 ± 0.67 b | 20.02 ± 3.21 b | 8.35 ± 0.3 b | 12.56 ± 1.24 d |
| Treatments | Processing Quality | Appearance Quality | ||||
|---|---|---|---|---|---|---|
| Brown Rice Rate/% | Milled Rice Rate/% | Head Milled Rice Rate/% | Chalkiness Rate/% | Chalkiness Size/% | Chalkiness Degree/% | |
| CK | 83.21 a | 76.20 c | 65.91 c | 27.02 a | 19.61 a | 3.22 c |
| T1-SS | 82.48 b | 75.01 c | 46.90 g | 18.20 d | 10.33 h | 3.06 c |
| T2-SS | 81.82 c | 79.37 a | 68.41 a | 16.54 f | 12.05 g | 2.04 d |
| T3-SS | 82.34 b | 74.91 c | 54.13 e | 17.06 e | 13.03 f | 1.63 f |
| T1-SeMet | 83.02 a | 75.53 c | 52.05 e | 25.47 b | 15.50 d | 3.91 a |
| T2-SeMet | 82.83 a | 75.12 c | 51.14 f | 19.05 c | 13.32 f | 2.02 d |
| T3-SeMet | 81.64 c | 73.93 d | 52.81 f | 17.51 e | 16.73 c | 1.87 e |
| T1-NS | 82.16 b | 74.51 d | 55.30 e | 20.04 b | 17.51 b | 3.54 b |
| T2-NS | 83.22 a | 77.03 b | 66.80 b | 12.06 h | 16.75 c | 2.03 d |
| T3-NS | 82.14 b | 75.92 c | 59.41 d | 17.62 e | 14.54 e | 2.14 d |
| Treatments | RS (%) | AC (%) | GC (mm) | GT (ASV) | AA (µg·g−1) | Antioxidant Activity (% Inhibition) |
|---|---|---|---|---|---|---|
| CK | 0.78 ± 0.12 h | 14.8 ± 1.84 g | 50.00 ± 3.86 g | 1.93 ± 0.33 bc | 8.95 ± 1.02 b | 38.66 ± 1.73 d |
| T1-SS | 0.86 ± 0.31 g | 19.18 ± 3.6 d | 66.45 ± 3.62 f | 1.78 ± 1.46 d | 7.84 ± 2.33 e | 46.58 ± 3.47 a |
| T2-SS | 2.76 ± 0.32 c | 24.12 ± 5.23 a | 75.55 ± 4.5 d | 2.71 ± 2.31 a | 8.55 ± 0.64 c | 28.76 ± 5.13 g |
| T3-SS | 1.86 ± 0.12 d | 22.91 ± 3.21 b | 77.43 ± 6.14 c | 2.15 ± 1.6 b | 7.83 ± 2.02 e | 28.46 ± 3.43 g |
| T1-SeMet | 3.58 ± 0.62 b | 16.83 ± 2.76 f | 79.24 ± 6.41 b | 1.39 ± 0.14 e | 9.74 ± 0.68 a | 44.54 ± 2.77 b |
| T2-SeMet | 4.18 ± 0.73 a | 20.60 ± 4.63 d | 67.36 ± 6.3 f | 1.83 ± 0.75 c | 6.78 ± 1.32 f | 33.35 ± 4.34 f |
| T3-SeMet | 1.27 ± 0.33 e | 21.44 ± 5.2 c | 78.24 ± 6.14 b | 2.06 ± 0.42 b | 8.55 ± 2.07 c | 32.57 ± 4.7 f |
| T1-NS | 0.98 ± 0.32 f | 18.71 ± 5.71 e | 80.37 ± 1.53 a | 1.29 ± 0.17 e | 8.22 ± 1.46 d | 34.66 ± 5.74 e |
| T2-NS | 0.85 ± 0.11 g | 17.88 ± 4.2 e | 71.76 ± 6.16 e | 2.81 ± 0.57 a | 9.73 ± 1.72 a | 24.57 ± 1.22 h |
| T3-NS | 0.71 ± 0.09 g | 23.13 ± 4.35 c | 83.24 ± 5.25 f | 2.06 ± 0.23 b | 8.58 ± 0.41 c | 42.64 ± 3.76 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Qi, D.; Chen, Y.; Wang, J.; Wang, Q.; Yang, Y.; Niu, H.; Zhao, Q.; Peng, T. Enhancement of Nutrient, Trace Element, and Organic Selenium Contents of Ratooning Rice Grains and Straw Through Foliar Application of Selenite. Foods 2024, 13, 3637. https://doi.org/10.3390/foods13223637
Wu W, Qi D, Chen Y, Wang J, Wang Q, Yang Y, Niu H, Zhao Q, Peng T. Enhancement of Nutrient, Trace Element, and Organic Selenium Contents of Ratooning Rice Grains and Straw Through Foliar Application of Selenite. Foods. 2024; 13(22):3637. https://doi.org/10.3390/foods13223637
Chicago/Turabian StyleWu, Wenjiang, Deqiang Qi, Yalong Chen, Jiaqi Wang, Qinghua Wang, Yanjun Yang, Hongbin Niu, Quanzhi Zhao, and Ting Peng. 2024. "Enhancement of Nutrient, Trace Element, and Organic Selenium Contents of Ratooning Rice Grains and Straw Through Foliar Application of Selenite" Foods 13, no. 22: 3637. https://doi.org/10.3390/foods13223637
APA StyleWu, W., Qi, D., Chen, Y., Wang, J., Wang, Q., Yang, Y., Niu, H., Zhao, Q., & Peng, T. (2024). Enhancement of Nutrient, Trace Element, and Organic Selenium Contents of Ratooning Rice Grains and Straw Through Foliar Application of Selenite. Foods, 13(22), 3637. https://doi.org/10.3390/foods13223637




