Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of pH Indicator Films
2.3. Color Stability of pH Indicator Films Against Fluorescent Light
2.4. pH-Dependent Change in the Color of the Anth Solution and GA/Gelatin/Anth/PVA
2.5. Determination of the Limit of Detection (LOD) and Limit of Quantification (LOQ) Regarding Volatile Amines for GA/Gelatin/Anth/PVA
2.6. Storage of GA/Gelatin/Anth/PVA in Controlled pH, RH, and Temperature Conditions
2.7. Film Characterization
2.8. Freshness Monitoring of Beef and Squid Using GA/Gelatin/Anth/PVA
2.9. Statistical Analyses
3. Results
3.1. Color Stability of pH Indicator Films Against Fluorescent Light
3.2. pH-Dependent Color Change of the Anth Solution and GA/Gelatin/Anth/PVA
3.3. Determination of LOD and LOQ of the GA/Gelatin/Anth/PVA Indicator Film
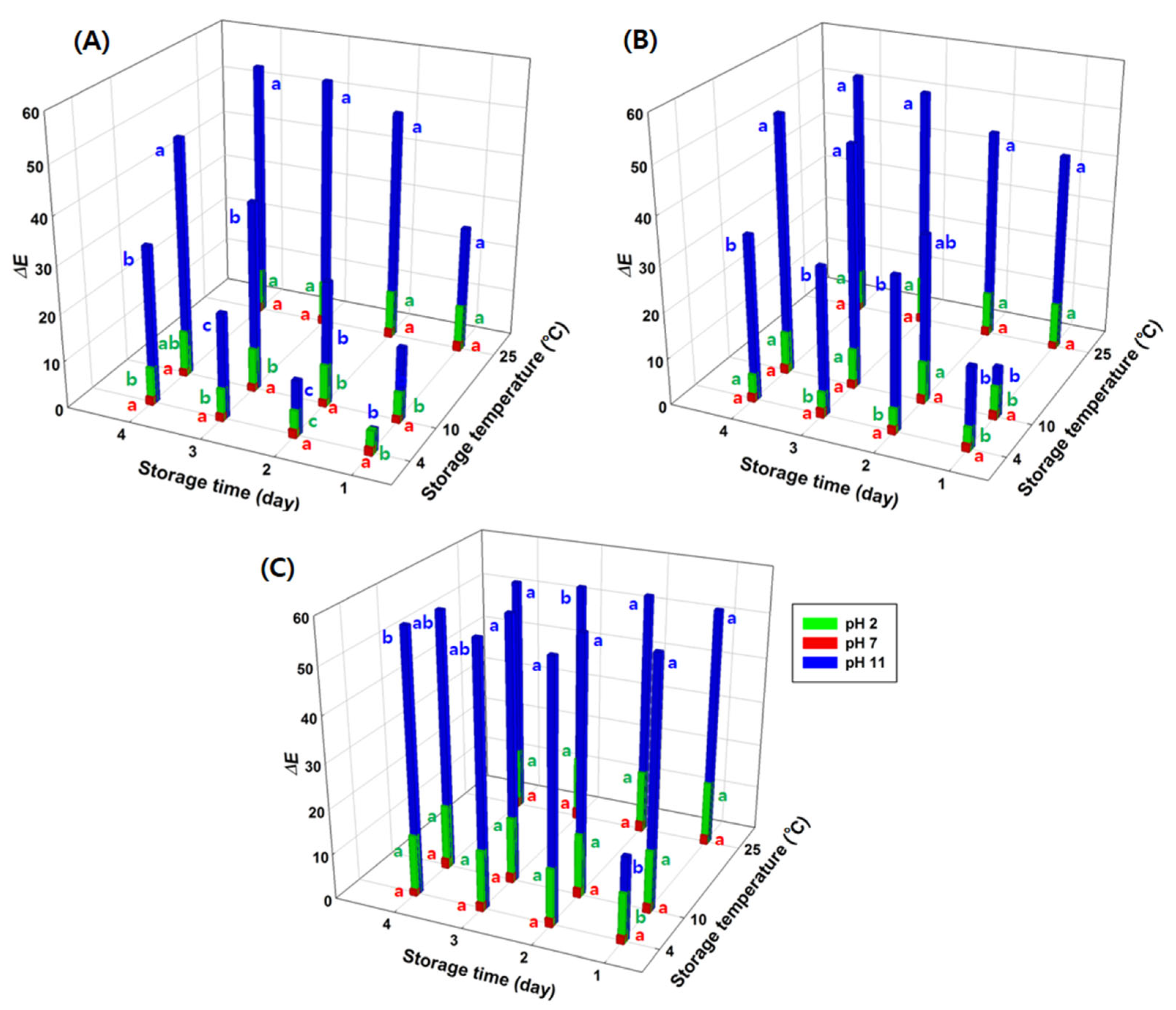
3.4. Effects of Environmental Variables on GA/Gelatin/Anth/PVA
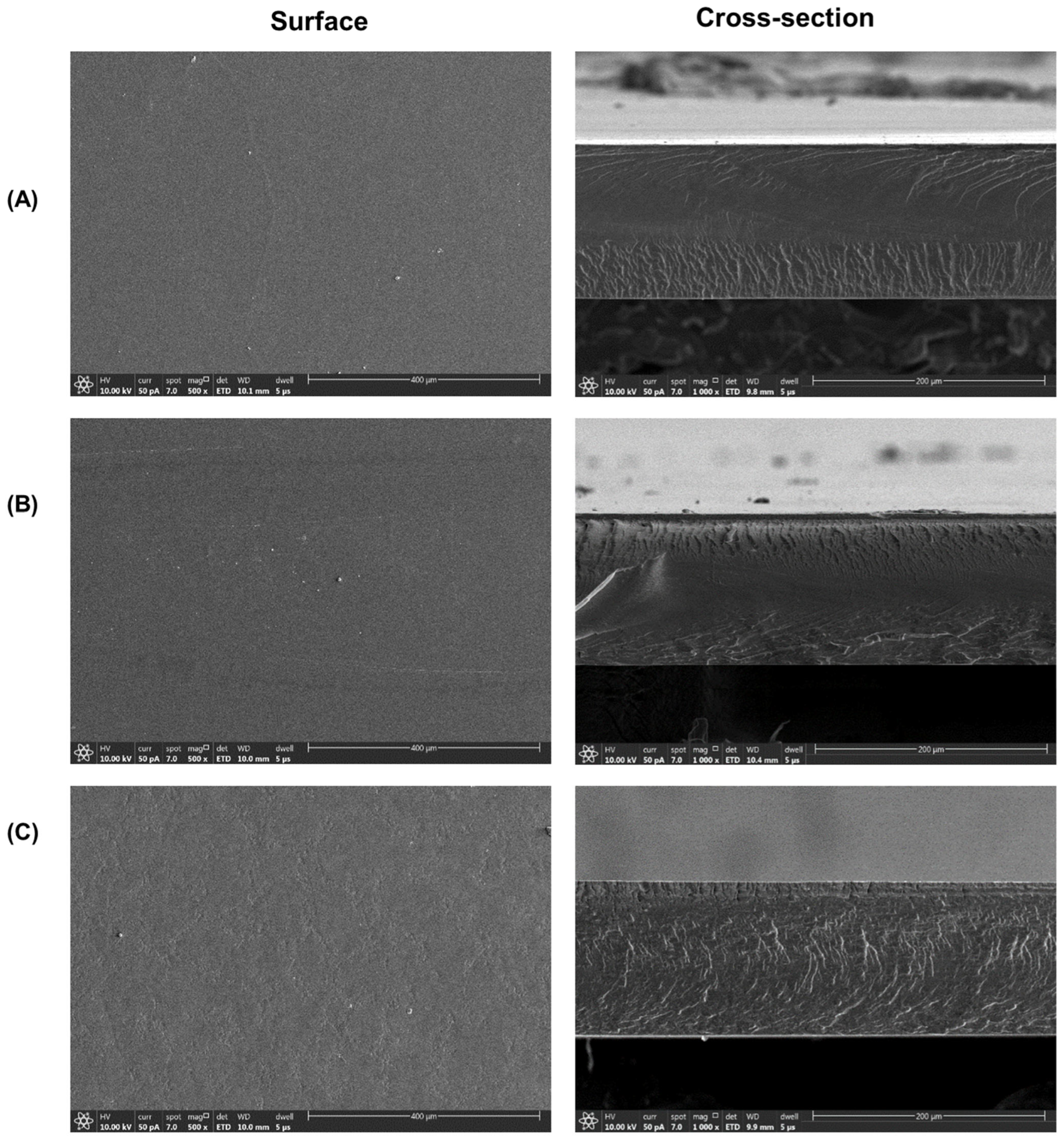
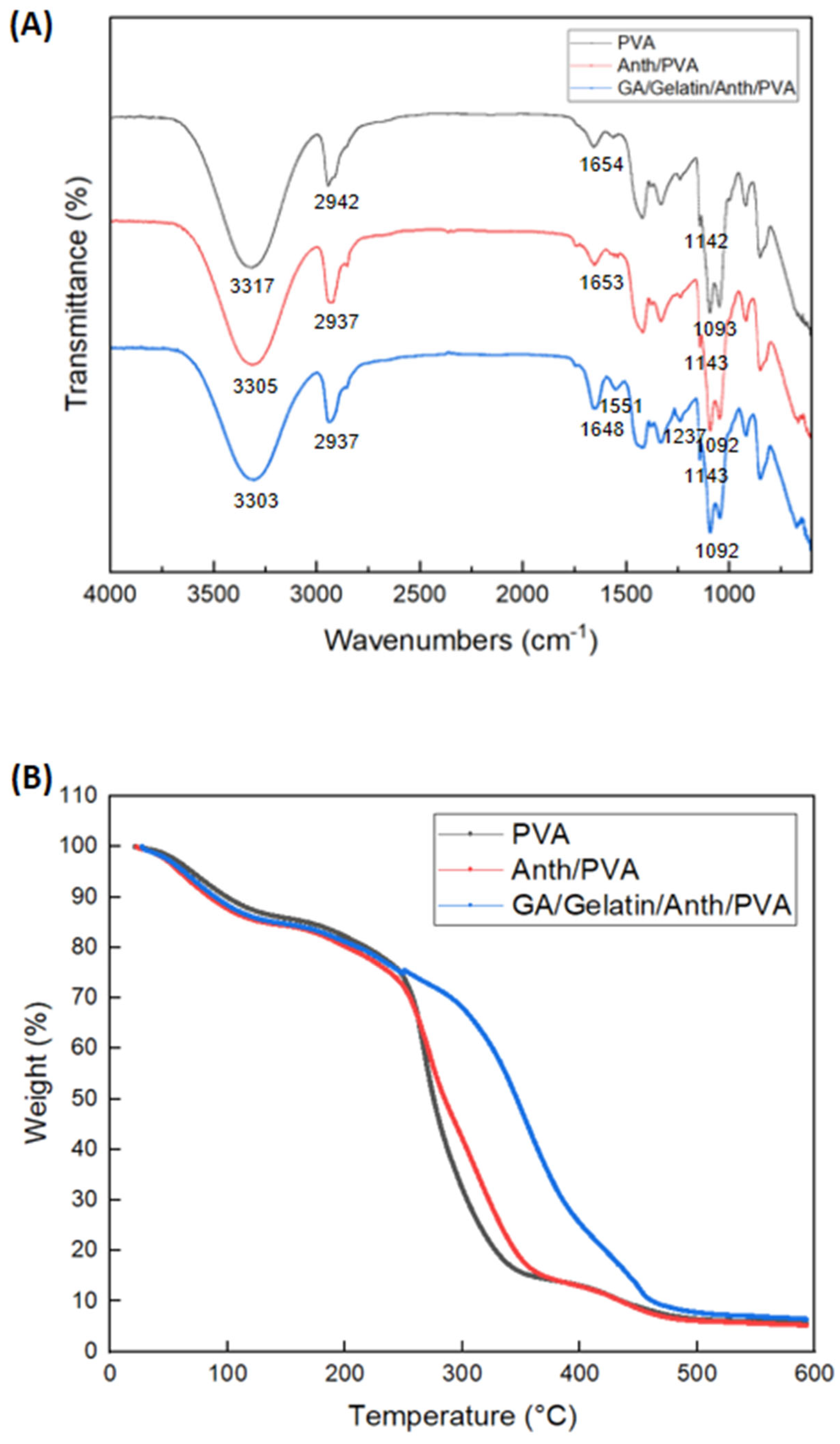
3.5. Film Characterization
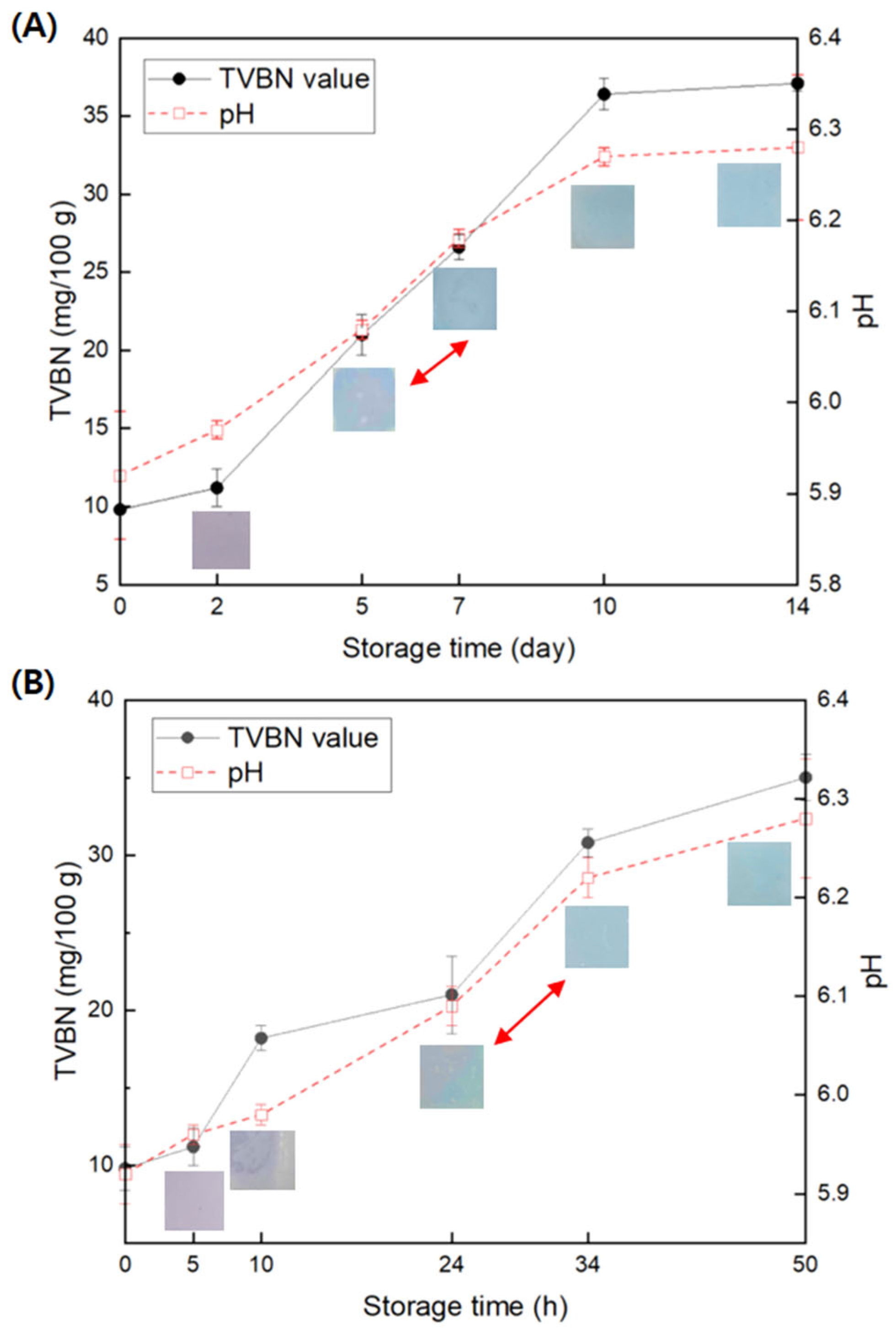
3.6. Freshness Monitoring of Beef and Squid Using GA/Gelatin/Anth/PVA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.M.E. Sustainable food waste management model for Bangladesh. Sustain. Prod. Consum. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Barone, A.M.; Aschemann-Witzel, J. Food handling practices and expiration dates: Consumers’ perception of smart labels. Food Control 2022, 133, 108615. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, A.; Sameen, D.E.; Ahmed, S.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Recent advances in the fabrication of pH-sensitive indicators films and their application for food quality evaluation. Crit. Rev. Food Sci. Nutr. 2023, 63, 1102–1118. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.F.; de Sousa Picciani, P.H.; Calado, V.; Tonon, R.V. Electrical gas sensors for meat freshness assessment and quality monitoring: A review. Trends Food Sci. Technol. 2021, 118, 36–44. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Itkor, P.; Sirieawphikul, C.; Lee, Y.S. Characterization of natural anthocyanin indicator based on cellulose bio-composite film for monitoring the freshness of chicken tenderloin. Molecules 2022, 27, 2752. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitoring. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Huang, X.; Du, L.; Li, Z.; Xue, J.; Shi, J.; Tahir, H.E.; Zou, X. A visual bi-layer indicator based on mulberry anthocyanins with high stability for monitoring Chinese mitten crab freshness. Food Chem. 2023, 411, 135497. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Farag, M.A.; Shao, P. Intelligent starch/chitosan-based film incorporated by anthocyanin-encapsulated amylopectin nanoparticles with high stability for food freshness monitoring. Food Control 2023, 151, 109798. [Google Scholar] [CrossRef]
- Tan, C.; Dadmohammadi, Y.; Lee, M.C.; Abbaspourrad, A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3164–3191. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Paula, D.; Ramos, A.M.; de Oliveira, E.B.; Martins, E.M.F.; de Barros, F.A.R.; Vidigal, M.C.T.R.; de Almeida Costa, N.; da Rocha, C.T. Increased thermal stability of anthocyanins at pH 4.0 by guar gum in aqueous dispersions and in double emulsions W/O/W. Int. J. Biol. Macromol. 2018, 117, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Saito, Y.; Al Riza, D.F.; Kondo, N.; Yang, X.; Han, D. Rapid evaluation of quality deterioration and freshness of beef during low temperature storage using three-dimensional fluorescence spectroscopy. Food Chem. 2019, 287, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Yue, X.; Wu, G.; Yue, P.; Gao, X. Gallic acid as a copigment enhances anthocyanin stabilities and color characteristics in blueberry juice. J. Food Sci. Technol. 2020, 57, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Effect of whey protein isolate and phenolic copigments in the thermal stability of mulberry anthocyanin extract at an acidic pH. Food Chem. 2022, 377, 132005. [Google Scholar] [CrossRef]
- Tan, C.; Sun, Y.; Yao, X.; Zhu, Y.; Jafari, S.M.; Sun, B.; Wang, J. Stabilization of anthocyanins by simultaneous encapsulation-copigmentation via protein-polysaccharide polyelectrolyte complexes. Food Chem. 2023, 416, 135732. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.R.; Zhang, Y.H.; Wang, X.P.; Wang, J.Y.; Ning, Z.; Ruan, Q.; Zhang, Y.S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Zainul, R.; Isara, L.P. Preparation of Dye Sensitized Solar Cell (DSSC) using anthocyanin color dyes from jengkol shell (Pithecellobium lobatum Benth.) by the gallate acid copigmentation. J. Phys. Conf. Ser. 2019, 1185, 012021. [Google Scholar]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Calibrated color measurements of agricultural foods using image analysis. Postharvest Biol. Technol. 2006, 41, 285–290. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Development of a novel colorimetric food package label for monitoring lean pork freshness. LWT 2019, 99, 43–49. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ronte, A.; Chalitangkoon, J.; Foster, E.J.; Monvisade, P. Development of a pH-responsive intelligent label using low molecular weight chitosan grafted with phenol red for food packaging applications. Int. J. Biol. Macromol. 2024, 266, 131212. [Google Scholar] [CrossRef]
- Uhrovčík, J. LOD Strategy for determination and LOQ values–Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lim, L.T. Cinnamil- and quinoxaline-derivative indicator dyes for detecting volatile amines in fish spoilage. Molecules 2019, 24, 3673. [Google Scholar] [CrossRef]
- Sothornvit, R.; Hong, S.I.; An, D.J.; Rhim, J.W. Effect of clay content on the physical and antimicrobial properties of whey protein isolate/organo-clay composite films. LWT 2010, 43, 279–284. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A 1977, 81, 89. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of red cabbage anthocyanins as pH-sensitive pigments in smart food packaging and sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Jiang, K.; Li, J.; Brennan, M.; Brennan, C.; Chen, H.; Qin, Y.; Yuan, M. Smart indicator film based on sodium alginate/polyvinyl alcohol/TiO2 containing purple garlic peel extract for visual monitoring of beef freshness. Polymers 2023, 15, 4308. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Sun, Z.; Liu, F.; Wang, D. A fast-response visual indicator film based on polyvinyl alcohol/methylcellulose/black wolfberry anthocyanin for monitoring chicken and shrimp freshness. Food Packag. Shelf Life 2022, 34, 100939. [Google Scholar] [CrossRef]
- ASTM D882-01; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 1997.
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Dirpan, A.; Djalal, M.; Kamaruddin, I. Application of an intelligent sensor and active packaging system based on the bacterial cellulose of Acetobacter xylinum to meat products. Sensors 2022, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, X.; Shen, Z.; Dong, J. Prediction of total volatile basic nitrogen (TVB-N) content of chilled beef for freshness evaluation by using viscoelasticity based on airflow and laser technique. Food Chem. 2019, 287, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tantasuttikul, A.; Kijroongrojana, K.; Benjakul, S. Quality indices of squid (Photololigo duvaucelii) and cuttlefish (Sepia aculeata) stored in ice. J. Aquat. Food Prod. Technol. 2011, 20, 129–147. [Google Scholar] [CrossRef]
- Malle, P.; Tao, S.H. Rapid quantitative determination of trimethylamine using steam distillation. J. Food Prot. 1987, 50, 756–760. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Rosales-Murillo, S.S.; Sánchez-Bodón, J.; Hernández Olmos, S.L.; Ibarra-Vázquez, M.F.; Guerrero-Ramírez, L.G.; Pérez-Álvarez, L.; Vilas-Vilela, J.L. Anthocyanin-loaded polymers as promising nature-based, responsive, and bioactive materials. Polymers 2024, 16, 163. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Sun, B.; Yang, Y.; Wang, S.; Feng, Z.; Li, J. The structure of anthocyanins and the copigmentation by common micromolecular copigments: A review. Food Res. Int. 2023, 176, 113837. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, R.; He, F.; Zhou, P.P.; Duan, C.Q. Copigmentation of malvidin-3-O-glucoside with five hydroxybenzoic acids in red wine model solutions: Experimental and theoretical investigations. Food Chem. 2015, 170, 226–233. [Google Scholar] [CrossRef]
- Zhang, B.; He, F.; Zhou, P.P.; Liu, Y.; Duan, C.Q. The color expression of copigmentation between malvidin-3-O-glucoside and three phenolic aldehydes in model solutions: The effects of pH and molar ratio. Food Chem. 2016, 199, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Fenger, J.A.; Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M.; Dangles, O. Acylated anthocyanins from red cabbage and purple sweet potato can bind metal ions and produce stable blue colors. Int. J. Mol. Sci. 2021, 22, 4551. [Google Scholar] [CrossRef]
- Fenger, J.A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Xia, M.; Zeng, Q.; Cai, Z. Intelligent colorimetric film incorporated with anthocyanins-loaded ovalbumin-propylene glycol alginate nanocomplexes as a stable pH indicator of monitoring pork freshness. Food Chem. 2022, 368, 130825. [Google Scholar] [CrossRef]
- Bouftou, A.; Aghmih, K.; Belfadil, D.; Lakhdar, F.; Gmouh, S.; Majid, S. Intelligent and active films with thymol and red cabbage anthocyanin for advanced fish packaging. Food Sci. Biotechnol. 2024, 1–12. [Google Scholar] [CrossRef]
- Haq, S.U.; Aghajamali, M.; Hassanzadeh, H. Cost-effective and sensitive anthocyanin-based paper sensors for rapid ammonia detection in aqueous solutions. RSC Adv. 2021, 11, 24387–24397. [Google Scholar] [CrossRef]
- Chen, D.; Shen, Y.; Wang, J.; Gao, Y.; Gao, H.; Yao, X. Mapping gaseous dimethylamine, trimethylamine, ammonia, and their particulate counterparts in marine atmospheres of China’s marginal seas–Part 1: Differentiating marine emission from continental transport. Atmos. Chem. Phys. 2021, 21, 16413–16425. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Bakhshayeshi, M.A.; Khayami, M.; Heidari, R.; Jamei, R. The effects of light, storage temperature, pH and variety on stability of anthocyanin pigments in four Malus varieties. Pak. J. Biol. Sci. 2006, 9, 428–433. [Google Scholar] [CrossRef]
- Chirife, J.; Fontan, C.F. Water activity of fresh foods. J. Food Sci. 1982, 47, 661–663. [Google Scholar] [CrossRef]
- Pham, V.T.; Le, D.A. Moisture desorption isotherms of squids. Asia Pac. J. Sustain. Agric. Food Energy 2018, 6, 7–12. [Google Scholar]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Zavadlav, S.; Lacković, I.; Bursać Kovačević, D.; Greiner, R.; Putnik, P.; Vidaček Filipec, S. Utilizing impedance for quality assessment of European squid (Loligo vulgaris) during chilled storage. Foods 2019, 8, 624. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, Q.; Lin, L.; Yang, J.; Liu, Z.; Yang, X.; Zhang, X. Facile fabrication of patterned polymeric films via phase separation-induced surface segregation. Appl. Surf. Sci. 2023, 641, 158537. [Google Scholar] [CrossRef]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K.; Letourneur, D.; Le Visage, C. Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1549–1559. [Google Scholar] [CrossRef]
- Labus, K.; Radosinski, L.; Kotowski, P. Functional properties of two-component hydrogel systems based on gelatin and polyvinyl alcohol—Experimental studies supported by computational analysis. Int. J. Mol. Sci. 2021, 22, 9909. [Google Scholar] [CrossRef] [PubMed]
- Pawde, S.M.; Deshmukh, K.; Parab, S. Preparation and characterization of poly (vinylalcohol) and gelatin blend films. J. Appl. Polym. Sci. 2008, 109, 1328–1337. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Yao, L.; Li, Y.; Weng, Y.; Qiu, D. Fabrication and characterization of gelatin/polyvinyl alcohol composite scaffold. Polymers 2022, 14, 1400. [Google Scholar] [CrossRef]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, X.; Chen, Y.; Zhang, J.; Jiao, C.; Wang, H. Solid-phase esterification between poly (vinyl alcohol) and malonic acid and its function in toughening hydrogels. Polym. Chem. 2020, 11, 4787–4797. [Google Scholar] [CrossRef]
- Li, J.; Bao, Y.; Jiang, Q.; Wen, B.; Wang, L.; He, Y.; Si, X.; Li, B. Indicator-enhanced starch-based intelligent film for nondestructive monitoring of beef freshness: Different structural phenolic acids copigment anthocyanin. J. Food Eng. 2024, 383, 112241. [Google Scholar] [CrossRef]
- Gao, X.; Tang, K.; Liu, J.; Zheng, X.; Zhang, Y. Compatibility and properties of biodegradable blend films with gelatin and poly (vinyl alcohol). J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 351–356. [Google Scholar] [CrossRef]
- Corona-Escalera, A.F.; Tinajero-Díaz, E.; García-Reyes, R.A.; Luna-Bárcenas, G.; Seyfoddin, A.; Padilla-de la Rosa, J.D.; González-Ávila, M.; García-Carvajal, Z.Y. Enzymatic crosslinked hydrogels of gelatin and poly (vinyl alcohol) loaded with probiotic bacteria as oral delivery system. Pharmaceutics 2022, 14, 2759. [Google Scholar] [CrossRef]
- Binti Che Wan, N.H.; Nafchi, A.M.; Huda, N. Tensile strength, elongation at breaking point and surface color of a biodegradable film based on a duck feet gelatin and polyvinyl alcohol blend. Asia Pac. J. Sustain. Agric. Food Energy 2018, 6, 16–21. [Google Scholar]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Shin, H.S. Development of a freshness indicator for monitoring the quality of beef during storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- EU/EC. Amending regulation (EC) No 2074/2005 as regards the total volatile basic nitrogen (TVB-N) limits. Off. J. Eur. Union 2008, 277, 18–20. [Google Scholar]
- Ezati, P.; Bang, Y.J.; Rhim, J.W. Preparation of a shikonin-based pH-sensitive color indicator for monitoring the freshness of fish and pork. Food Chem. 2021, 337, 127995. [Google Scholar] [CrossRef]

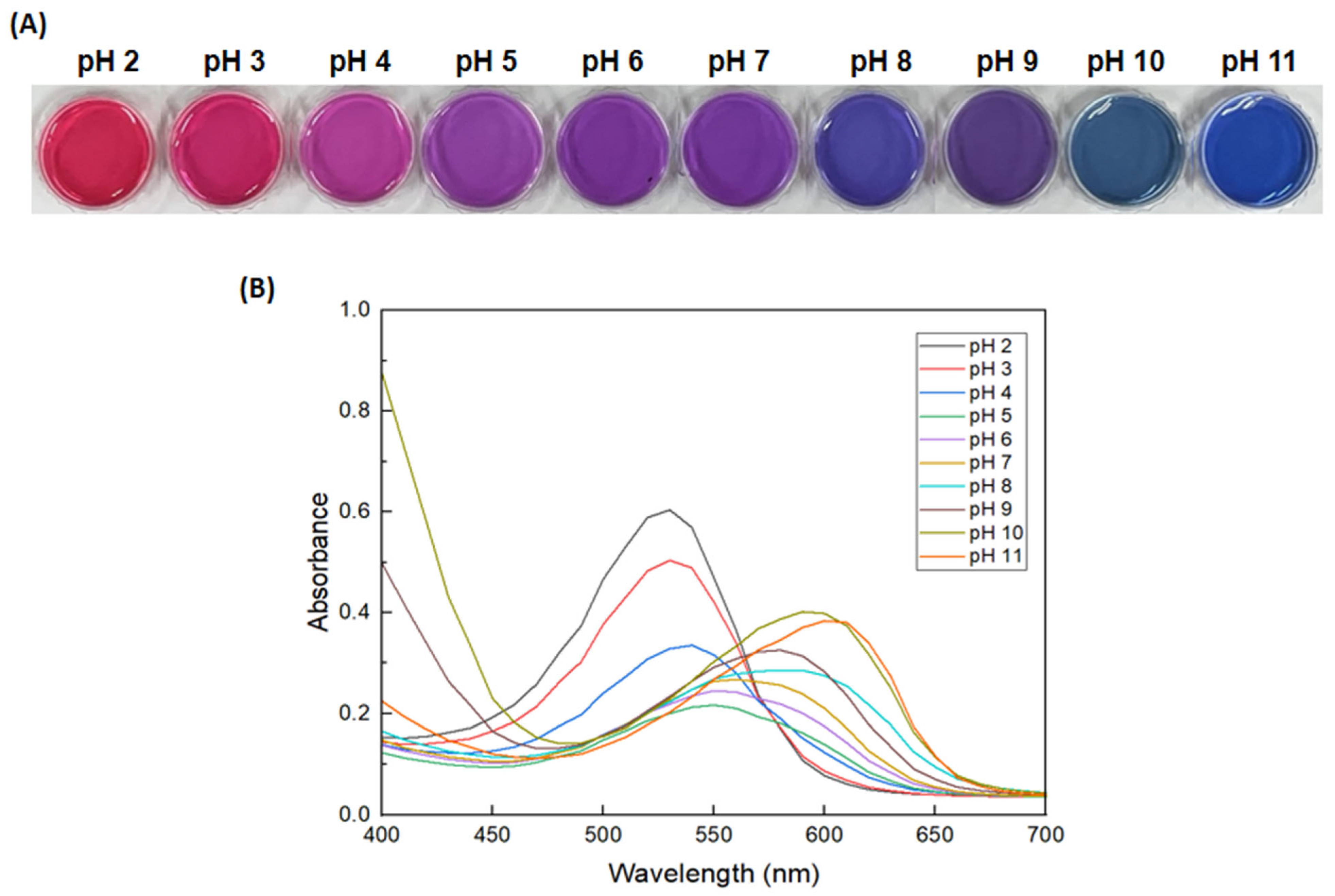

| pH of Buffer Solutions | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| GA/Gelatin/Anth/PVA Images |  |  |  |  |  |  |  |  |  |  |
| L* | 82.21 ± 0.21 d | 84.16 ± 0.34 c | 86.85 ± 0.18 b | 88.06 ± 0.12 a | 87.86 ± 0.33 a | 88.02 ± 0.20 a | 87.71 ± 0.22 a | 87.70 ± 0.11 a | 87.70 ± 0.11 a | 87.70 ± 0.11 a |
| a* | 19.50 ± 0.35 a | 14.76 ± 0.55 b | 7.50 ± 0.13 c | 3.58 ± 0.08 d | 3.15 ± 0.26 e | 1.76 ± 0.29 f | −0.09 ± 0.13 g | −1.68 ± 0.31 h | −2.96 ± 0.17 i | −7.79 ± 0.27 j |
| b* | 1.56 ± 0.05 ef | 1.23 ± 0.10 ef | 1.82 ± 0.03 de | 2.37 ± 0.04 b | 2.24 ± 0.07 bc | 2.18 ± 0.03 bcd | −0.99 ± 0.13 cde | 2.20 ± 0.04 bcd | 1.94 ± 0.06 cde | 7.06 ± 0.55 a |
| Film | PVA Film | Anth/PVA | GA/Gelatin/Anth/PVA |
|---|---|---|---|
| Tensile strength (MPa) | 22.98 ± 2.07 a | 21.21 ± 3.58 a | 20.52 ± 3.30 a |
| Elongation at break (%) | 466.54 ± 0.86 a | 466.31 ± 0.43 a | 466.38 ± 0.16 a |
| Elastic modulus | 55.83 ± 4.20 a | 53.08 ± 2.77 a | 57.24 ± 8.44 a |
| Water solubility (%) | 8.71 ± 2.12 b | 7.78 ± 2.68 b | 13.88 ± 0.90 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.; Min, S.C. Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid. Foods 2024, 13, 3464. https://doi.org/10.3390/foods13213464
Kwak M, Min SC. Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid. Foods. 2024; 13(21):3464. https://doi.org/10.3390/foods13213464
Chicago/Turabian StyleKwak, Minyoung, and Sea C. Min. 2024. "Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid" Foods 13, no. 21: 3464. https://doi.org/10.3390/foods13213464
APA StyleKwak, M., & Min, S. C. (2024). Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid. Foods, 13(21), 3464. https://doi.org/10.3390/foods13213464







