Abstract
Antioxidant capacity is one of the most important biological activities in fruits and vegetables and is closely related to human health. In this study, ‘Eureka’ lemons were used as experimental materials and stored at 7–8 °C MT (melatonin, 200 μmol, soaked for 15 min) and CA (controlled atmosphere, 2–3% O2 + 15–16% CO2) individually or in combination for 30 d. The changes in lemon fruits’ basic physicochemical properties, enzyme activities, and antioxidant capacities were studied. Comparing the combined treatment to the control, the outcomes demonstrated a significant reduction in weight loss, firmness, stomatal opening, and inhibition of polyphenol oxidase (PPO) and peroxidase (POD) activities. Additionally, the combined treatment maintained high levels of titratable acidity (TA), vitamin C (VC), total phenolic content (TPC), and antioxidant capacity and preserved the lemon aroma. Meanwhile, the correlation between fruit color, aroma compounds, and antioxidant capacity was revealed, providing valuable insights into the postharvest preservation of lemons. In conclusion, the combined treatment (MT + CA) was effective in maintaining the quality and antioxidant capacity of lemons.
1. Introduction
Lemons are high in dietary fiber, vitamin C, and minerals, which possess strong antioxidant properties and confer benefits to human health [1]. However, lemons are highly susceptible to discoloration, softening, dry wrinkling, and rotting during storage due to mechanical damage, improper storage conditions, and other factors, resulting in tissue aging and a decline in organoleptic quality and causing significant economic losses [2,3]. The main methods reported to improve the storage quality of lemons such as melatonin, beeswax, controlled atmosphere, chitosan, 1-MCP, and methyl jasmone treatment [4,5,6,7,8,9].
Controlled atmosphere (CA) treatment has been widely used to preserve a variety of fruits and vegetables, such as lentils, blueberries, avocados, plums, and apples [10,11,12,13,14]. It is thought to be an effective way to postpone postharvest fruit ripening and senescence. The postharvest respiration of lemons was inhibited by CA, and transpiration was also hindered by it, which delayed the degradation of lemon chlorophyll and delayed the aging process of lemons [15]. CA used in the preservation of fruits has the unique advantages of being non-residual, effective, energy-saving, and environmentally friendly. However, long-term CA storage promotes anaerobic respiration, leading to elevated levels of acetaldehyde and ethanol in fruits. This process not only results in undesirable odors but also increases the production of reactive oxygen species (ROS) [16,17]. Therefore, additional treatments in combination with CA are required to reduce unwanted odor. By chance, melatonin (N-acetyl-5-methoxytryptamine, MT), an indoleamine, serves as a multi-regulatory compound in living organisms. It significantly contributes to the regulation of ROS, reactive nitrogen species (RNS), and other free radicals, alongside harmful oxidative molecules found in plant cells. [18,19,20]. MT, as a naturally occurring antioxidant, has the potential to meet the developmental requirements for the safe and healthy preservation of fruit, particularly in the context of green and organic practices. Its versatility lends itself to a multitude of prospective applications. Recently, the role of MT in the postharvest preservation of fruits and vegetables has gradually attracted attention, and it has been described that MT application can maintain higher antioxidant activity, delay fruit senescence in sweet cherries, blackberries, and jujubes [21,22,23], and reduce the degradation of chlorophyll in fruits and vegetables, such as bell peppers, passion fruits, and cucumbers, to maintain their good color [24,25,26]. In addition, MT-treated lemons had an increased total phenolic content and better organoleptic qualities [4]. Meanwhile, the postharvest disease incidence and decay rate of blueberries, grapes, tomatoes, and other fruits can be effectively reduced by MT [27,28,29,30], which prolongs the storage time of fruits and vegetables.
To the best of our knowledge, many previous studies have concentrated on the individual impacts of MT or CA on lemon preservation quality. However, there is a lack of studies that investigate the combined effects of MT and CA. In this study, a combination of MT and CA treatments was exogenously applied to postharvest lemons to analyze the effects of MT and CA treatments on lemon physiological characteristics, oxidative defense enzyme activities, and antioxidants and to enhance the shelf life and quality of lemons.
2. Materials and Methods
2.1. Materials and Treatments
The ‘Eureka’ lemons analyzed in this research were cultivated and picked at peak commercial maturity in October 2023, in the Wanzhou District of Chongqing, China (30°24′25” N, 107°55′22” E). These lemons were placed in commercial-grade plastic stacking containers and shipped to Tianjin University of Science and Technology within 24 h using cold chain logistics, insulated with cold packs, and covered with blankets. Following a 24 h pre-cooling period at 7–8 °C and relative humidity (RH) of 90–95% in a refrigerated environment, lemons of uniform size (80–100 g per fruit), ripeness, and free of defects and diseases were selected. The lemons were then divided into four groups. Each group underwent a thorough rinse with water and subsequently dried using sterile gauze. Samples stored at 7–8 °C and 90–95% RH for 30 d were set up as the Control group. Treatment group 1 was placed directly in the gas conditioning bottle (2–3% O2, 15–16% CO2), recorded as CA; Treatment group 2 was immersed in a 200 μmol melatonin solution for 15 min, recorded as MT; and Treatment group 3 was immersed in a 200 μmol melatonin solution for 15 min before being placed in a gas conditioning bottle, recorded as MT + CA. All treated fruits were stored at 7–8 °C and 90–95% RH for 30 d. Physiological changes were measured and recorded in random samples taken at 5-day intervals (0, 5, 10, 15, 20, 25, and 30 d).
2.2. Color Measurement and Browning Index
The lemon of each treatment group was measured using a colorimeter (HP-200, Hanpu Photoelectric Technology Co., Ltd., Shanghai, China), and three different positions were selected at the lemon equator. The L*, a*, and b* values of each point were recorded. The chroma C* is calculated with the Formula (1) [31]. The chlorophyll content was determined by weighing 1 g of the sample into a mortar, adding 2–3 mL of 80% acetone to study into a homogenate until the tissue turned white, leaving it to extract for 3–6 min, and determining the absorbance at 652 nm.
2.3. Fruit Quality
Weight loss was calculated by subtracting the final weight from the initial weight on the storage day, and the results were presented as % (weight in weight). Lemon hardness was determined using a portable digital fruit hardness tester (GY-4, Zhejiang Top Instrument Co., Ltd., Hangzhou, China) with a 3.5 mm cylindrical probe and expressed as N [13]. The soluble solids content (SSC) was measured using a digital refractometer (PAL-1, Atago Co., Tokyo, Japan), and the results were presented as %. The titratable acidity (TA) content of lemon was assessed by dropping 10 mL of lemon juice to reach PH 8.2 using the 0.1 mol L−1 NaOH solution, and the results were expressed as citric acid in %. The vitamin C (VC) was determined by titrating the 2,6-dichlorophenol-indophenol solution until a slight red color appeared and did not fade away for 15 s after extracting the extracts with a 20 g L−1 oxalic acid solution [32].
2.4. Enzyme Activity
The enzymatic activities of polyphenol oxidase (PPO) and peroxidase (POD) were assessed utilizing the methodology outlined by Jiang et al. [33]. Briefly, 3 g of lemon was weighed and added to 3 mL of 100 mM, PH 5.5 phosphate-buffered solution (PBS, 4% polyvinylpyrrolidone, and 1% Triton X-100). This mixture underwent vigorous mixing in an ice water bath. Following that, centrifugation occurred at 10,000× g for 30 min at 4 °C to collect the supernatant as the enzyme extract for subsequent analysis. To evaluate PPO activity, 60 μL of the enzyme extract was reacted with 240 μL of a 50 mM acetic acid-sodium acetate buffer (PH 5.5) and 60 μL of 50 mM catechol, with the absorbance measured at 420 nm. For POD activity assessment, 30 µL of the enzyme extract was combined with 180 µL of 25 mM guaiacol and 30 µL of 500 mM H2O2, followed by absorbance evaluation at 470 nm.
The Phenylalanine Ammonia-Lyase (PAL) assay was conducted by extracting the sample with 40 g L−1 PVP, 2 mmol L−1 EDTA, and 5 mmol L−1 β-mercaptoethanol at a low temperature, centrifuging at 12,000× g for 30 min, and then combining 50 mmol L−1 boric acid buffer and 20 mmol L−1 L-phenylalanine, and the mixture was held for 10 min to determine the initial value at 290 nm. Subsequently, the mixture was held for 60 min to determine the termination value. The initial value was determined at 290 nm after the 10 min holding period, and the termination value was determined after an additional 60 min of holding [34].
Malondialdehyde (MDA) was determined based on prior work [35]. Then, 1 g of lemon was ground with 5 mL of trichloroacetic acid (TCA, 100 g L−1) and centrifuged for 20 min at 10,000× g. Next, 2 mL of the supernatant was combined with 2 mL of thiobarbituric acid (TBA, 6.7 g L−1), and then placed in a boiling water bath for 20 min. After cooling, the mixture was centrifuged once more, and the absorbance of the supernatant was read at 450 nm, 532 nm, and 600 nm. The MDA was measured in µmol kg−1.
2.5. Antioxidant Capacity
The total phenolic content (TPC) was assessed using the Folin–Ciocalteu method, with minor modifications as previously described [36]. Then, 5 g of the freeze-dried lemon sample was mixed with 25 mL of methanol, sonicated for 30 min, and centrifuged at 10,000× g for 10 min, and the supernatant was used for TPC determination. In brief, a 96-well plate was used, containing 25 μL of supernatant and 125 μL of Folin reagent (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) per well. The plate was allowed to incubate for 10 min at room temperature. Subsequently, 125 μL of a 10% Na2CO3 solution was added, and the reaction proceeded for 30 min. A spectrometer (Spectra Max 190, Molecular Devices Corporation, California, USA) was used to measure the absorbance at 765 nm using methanol as a blank. Based on gallic acid, a standard curve was constructed, and the results were expressed in g kg−1 units of a gallic acid equivalent.
The DPPH radical scavenging activity was assessed using a previously reported method [37]. Briefly, 25 μL phenolic extract and 200 μL DPPH solution (350 μM methanol) were added to a 96-well plate, allowing it to remain in darkness for 4 h at room temperature. Absorbance was read at 517 nm. The DPPH scavenging activity was determined from a standard curve of Trolox equivalents and reported in mmol kg−1.
A hydrogen peroxide detection kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used to detect the H2O2 content, and the results were expressed as µmol kg−1.
The catalase (CAT) activity was determined as described by Li et al. [35]. Briefly, 5 g of powder was extracted with 5 mL of PBS (100 mM, pH 7.5) in an ice bath. After centrifugation at 4 °C for 30 min at 10,000× g, the supernatant (0.5 mL) was mixed with 2.9 mL of H2O2 (20 mmol), and the absorbance was measured at 240 nm every 30 s for 6 min.
2.6. Scanning Electron Microscopy (SEM)
The ultrastructure of lemon was observed by the scanning electron microscopy method previously described [7], with slight modifications. Lemons (approximately 10.0 × 10.0 × 1.0 mm3) were immersed in a 2.5% formaldehyde solution at 4 °C for 12 h. They then received three rinses with 0.2 M PBS before fixation in a 2% osmium tetroxide solution for 1 h. Following this, the samples underwent another three rinses with PBS. Subsequently, dehydration occurred through a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 95% v/v), after which they were dried and placed in a vacuum desiccator overnight. Finally, the sample was gold-plated for 2 min with a thickness of 2 nm using a sputtering coating machine. Then, the scanning electron microscope (SU8010, Hitachi Company, Tokyo, Japan) was used for observation.
2.7. Aroma Analysis by E-Nose
Lemon volatiles were analyzed for different storage times utilizing a PEN E-nose instrument (Winmuster Airsense Analytics Inc., Schwerin, Germany) [38]. Ten metal oxide semiconductor sensors detected various compounds and produced corresponding response values. An amount of 3 g of grated lemon was combined with 3 mL of saturated sodium chloride solution in a sealed 20 mL glass vial and warmed for 20 min in a 40 °C water bath; then, we conducted electronic nose detection. Headspace gas was continuously injected into the detector for 100 s until the signal line stabilized. Response values were recorded over time and were processed using WinMuster electronic nose software (WinMuster PEN v 1.6.2.18). Three replicates of each sample were analyzed using the electronic nose.
2.8. Statistical Analysis
The experiment was randomized, and each treatment was replicated three times. Statistical analysis employed IBM SPSS Statistics 22.0 software (IBM Corp., Armonk, NY, USA). The mean values of the various treatments underwent comparison through a multiple-range test (Tukey test), establishing the significance level threshold at p < 0.05.
3. Results and Discussion
3.1. Effect on Color and Chlorophyll Content of Lemon Fruit
The peel color is typically linked to the intrinsic qualities of the fruit, such as flavor and texture, which can impact consumer purchasing decisions [39]. Green lemons undergo bio-enzymatic oxidation during storage, resulting in a gradual increase in the degree of yellow coloration and a concomitant yellowing of the peel, which may even progress to browning in some areas [40]. Research indicates that the shift in lemon fruit color from green to yellow results from changes in the levels of chlorophyll and carotenoids. Several studies have demonstrated that melatonin markedly attenuates chlorophyll degradation in broccoli, tomatoes, and cabbage, while also exerting a beneficial influence on chlorophyll-like and carotenoid levels and photosynthesis [41,42,43,44]. In addition, the postharvest MEL treatment of ‘Fino’ lemon fruits was recently reported to have a positive effect on the reduction in fruit color change, weight loss, and total acidity loss [4]. Our results demonstrated that melatonin had a comparable impact, with the postharvest association of MT and CA, as illustrated in Figure 1, markedly decelerating the fruit degreening process. The use of melatonin diminished the growth rate of the C* value and chlorophyll levels in lemon peels (Table 1). The combined treatment maintained the green color for a longer period than the Control group.
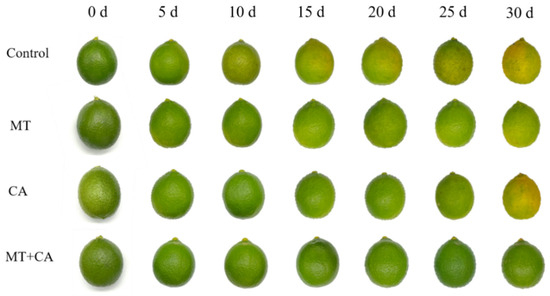
Figure 1.
The visual appearance of lemons treated with MT and/or CA.

Table 1.
Chroma C* and chlorophyll content of lemons treated with MT and/or CA during 30 days of refrigeration at 7–8 °C.
3.2. Effect on Quality Characteristics of Lemon
Weight loss in fruits and vegetables during storage primarily results from water loss through transpiration. This process significantly impacts the quality of lemons, leading to notable degradation [45]. Following harvest, lemons continue to lose water in vapor form through stomata and the surface layer, contributing to reductions in fruit weight [46]. Physiological water loss plays a critical role in determining fruit quality and susceptibility to various postharvest diseases. The effects of various treatments on lemon weight loss can be observed, showing a consistent increase across all groups (Figure 2A). On day 30, the Control group experienced the highest weight loss, reaching 6.64%, while the MT + CA treatment group had the least at 2.88%. Our results and previous work suggest that MT application can delay water loss during cold storage [47]. Wang et al. [21] and Shah et al. [22] demonstrated that the postharvest application of melatonin reduced weight loss in sweet cherries and blackberries. The reduced weight loss in lemons treated with MT may stem from decreased levels of MDA content and H2O2 free radicals. In contrast, the diminished weight loss in CA-stored lemons likely results from lower ripening and respiration rates [48]. Given that all lemons underwent storage under identical temperature and relative humidity conditions, the minimal weight loss observed in the MT + CA treatment can be linked to the reduced respiration rate and limited stomatal opening, as evidenced by SEM observations.
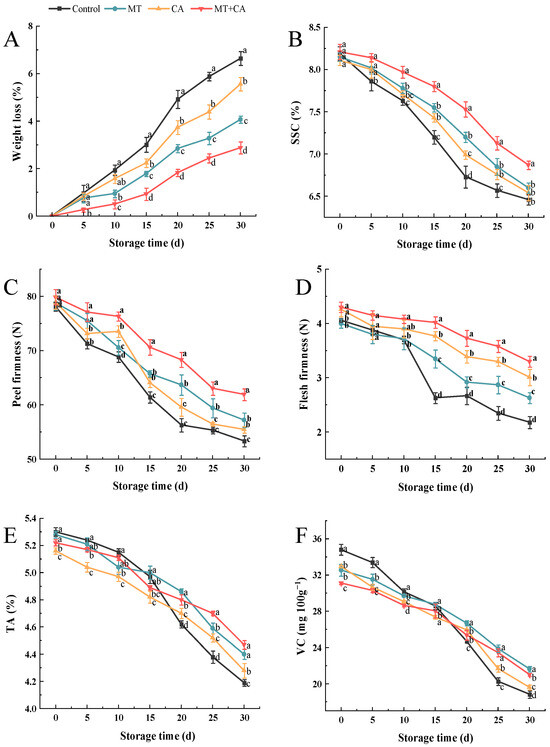
Figure 2.
Basic physicochemical properties of MT or/and CA-treated lemons during 30 d of cold storage at 7–8 °C versus control. (A) Weight loss; (B) SSC; (C) peel hardness; (D) flesh hardness; (E) TA; (F) VC. Values represent means ± SD in triplicate, and different letters denote significant differences compared to the control during the same storage time at the p < 0.05 level.
Softening significantly contributes to quality degradation and reduced shelf life in both menopausal and non-menopausal fruits. It should be noted that all lemons were at the same stage of ripening and the peel firmness diminished over time as storage duration increased, as illustrated in Figure 2C. The peel hardness of the Control group was 78.08 ± 0.78 N at harvest and decreased to 53.36 ± 1.01 N on day 30. After storage, the peel hardness in the MT, CA, and MT + CA groups surpassed that of the Control group by 7.23%, 3.97%, and 16.06%, respectively. The trend in decreasing pulp and peel hardness persisted (Figure 2C), likely due to delayed cell wall degradation [49]. Research indicates that MT treatment positively influences the preservation of fruit firmness and quality, particularly in mangoes, bananas, and pomegranates [50,51,52]. Furthermore, low temperatures, minimal O2 levels, and elevated CO2 concentrations effectively inhibited water migration, thereby maintaining the quality of stored fruits. In our work, MT + CA treatment significantly slowed down the decrease in hardness, especially in lemon peel, and effectively maintained the hardness of the fruit.
The TA, SSC, and VC concentration are key physiological indices for assessing the intrinsic quality of fruits. The SSC can be used to quantify fruit ripening and senescence. As illustrated in Figure 2B, the SSC of all groups exhibited a declining trend. The reduction in SSC may be attributed to the depletion of nutrients through catabolic processes during storage or due to water loss [3,53], indicating that lemons undergo a gradual process of senescence. The SSC of control, MT, CA, and MT + CA exhibited a decline of 1.73%, 1.55%, 1.57%, and 1.34%, respectively, over the course of 30 d of storage. Previous studies have demonstrated that MT maintains higher SSC in blackberry, nectarine, and blueberry [22,54,55]. Similarly, Tian et al. [56] found that SSC was reduced with the increase in storage time and that CA was superior to MAP and RA in preserving fruit SSC. Therefore, the elevated SSC of the combined treatments may be attributed to the delayed onset of senescence in lemons resulting from the application of MT and CA.
Citric acid is the primary organic acid found in lemons. The results are displayed in Figure 2E, where TA gradually decreased, which is consistent with the findings of Serna-Escolano et al. [57]. The difference in the TA change between the MT and CA groups was not significant, but it was significantly different from the other two groups, with only a 0.75% decrease in MT + CA. It has been noted that MT can mitigate the acidity drop in other fruits, including pears, sweet cherries, and navel oranges [49,58,59]. Fruits kept in CA had a high TA level, which was linked to a slower ripening process and a decreased respiration rate [60]. The concentrations of O2 and CO2 were critical in causing the samples to ripen more slowly [61]. The reduced decarboxylation of organic acids, including citric and malic acids, which are frequently utilized as substrates for respiratory enzyme processes, in fruit exposed to high CO2 and low oxygen during storage could also be the cause of this outcome [62].
A higher VC content in lemons indicates a higher nutritional value and showed a decreasing trend during storage. As shown in Figure 2F, after the experiment, the VC content of the control fruits dropped dramatically from 34.8 mg 100 g−1 to 18.84 mg 100 g−1, while the VC content of the MT + CA-treated fruits only declined to 21.02 mg 100 g−1. After 15 days of storage, it was discovered that the VC content of the treatments drastically dropped. Similarly, Selcuk et al. [63] and Tian et al. [64] reported a trend toward a decline in VC levels in wolfberry and sweet cherry fruits throughout storage. The use of various organic acids during fruit respiration or their potential conversion to sugars may be the cause of the drop in VC under storage conditions [65]. VC, as a major antioxidant, directly scavenges ROS, and the higher VC level indicates the superior antioxidant capacity of MT + CA-treated lemon.
3.3. Synergistic Promotion of TPC and Inhibition of ROS
The phenolic content of lemons is one of the main reasons for their antioxidant and anti-tumor properties. The evolution of TPC in lemons for 30 d is shown in Figure 3A, with a general trend of increasing and then decreasing in all groups. Among them, the MT treatment reached the highest value of 3.2 mmol GAE kg−1 on day 10, consistent with studies by Ma, Q et al. [58] MT also showed an accumulation of TPC during the storage of oranges, thus maintaining the postharvest quality. After 10 d of storage, the TPC started to decrease in all treatments, and the Control group saw the fastest decline; on day 30, there was a noticeable difference. Because of the loss of astringent flavor and changes in enzyme activity, phenolic degradation is responsible for the decrease in TPC [63,66]. Samples kept in CA may have high TPC levels because oxidation is inhibited and membrane leakage is decreased. POD and PPO may eventually bind to the contents due to compromised membrane integrity [65]. The role of MT and CA in the accumulation of TPC has been demonstrated in lemon and lychee, among others [9,58,65]. Lemons treated with MT + CA in the current study had greater TPC during cold storage, which could be related to increased activity of antioxidant enzymes such as CAT, weight loss, and decreased activity of PPO and POD enzymes.
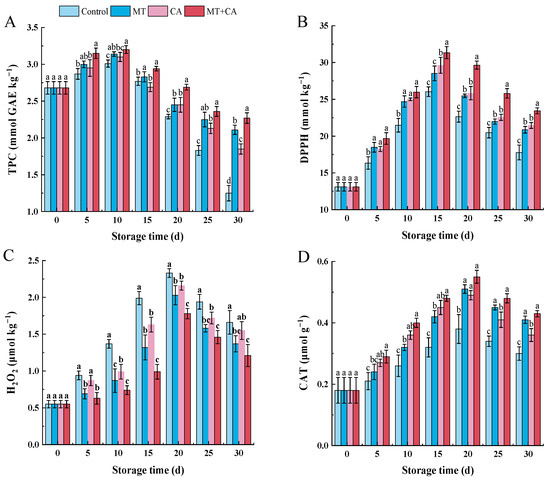
Figure 3.
Effects of MT, CA, or combined treatments on ROS production and membrane peroxidation compared with the control within the same storage time. (A) TPC, (B) DPPH scavenging capacity, (C) H2O2, (D) CAT activity. Values were expressed in the mean ± SD (n = 3), and different letters suggest significant differences within different treatments compared with control at p < 0.05.
The antioxidant activity of lemon fruits has received wide attention from consumers, and DPPH and H2O2 are commonly used indicators for evaluating antioxidant activity in vitro. As shown in Figure 3B,C, DPPH and H2O2 showed an increasing and then decreasing trend during lemon storage. The control fruits showed low DPPH scavenging capacity and high H2O2 accumulation, reaching a maximum value of 2.33 μmol g−1 Trolox after 20 d. In comparison, MT+CA significantly improved the scavenging capacity of DPPH and decreased H2O2 damage to the fruits. The oxidation and degradation of TPC may be the cause of the loss of antioxidant activity in fruit. TPC was a major contributor to the total non-enzyme antioxidant ability, which had a negative correlation with the senescence of fruit [67]. Thus, lemons treated with MT+CA showed an increased ability to scavenge DPPH, potentially linked to increased TPC levels. These results are consistent with several other reports that melatonin application significantly reduced H2O2 in stored peaches and cassava [68,69].
Fruits treated with MT, CA, and co-treatment had significantly higher CAT activity than control fruits during the cold storage period. CAT activity increased in all treatments up to 20 d of chilling, with the highest CAT activity in MT+CA treated fruits. On day 20, CAT activity decreased sharply (Figure 3D). MT mediates communication within and between plants and coordinates plant defense responses, including antioxidant systems. Cellular dezonalisation, accompanied by higher oxidase activity and senescence, improved CAT activity [70]. The slowing of lemon senescence and the retardation of oxidase activity after MT+CA treatment may be responsible for the increased CAT activity. This shows that CAT activity is a key factor in the reduction in oxidative stress by MT+CA treatment by reducing weight loss, H2O2 radicals, and MDA levels.
3.4. Enhancing the Activity of POD, PPO, and PAL and Inhibiting Membrane Peroxidation
Lemons accumulate more POD activity with storage time due to the cold storage environment. The control lemon had the highest activity after 30 d, at 1.976 U g−1 min−1. The POD activity of the lemon fruits treated with MT+CA, on the other hand, was significantly lower than that of the control fruits, at 1.648 U g−1 min−1 (Figure 4A). Comparatively, from day 25 to day 30, PPO activity exhibited an increasing trend followed by a decrease, as seen in Figure 4B. However, the low ethylene content and high citric acid content of all lemon fruits may be the reason for their minimal to negligible PPO activity. Lemons are common non-menopausal fruits that have a high citric acid content and comparatively little ethylene production [71,72]. The notable distinction between PPO and POD activities raises the possibility that distinct mechanisms are causing POD and PPO activities during cold storage [73]. The primary roles of POD and PPO enzymes are in phenolic catabolism and cellular catabolism brought on by weight loss and the buildup of ROS [74]. Thus, it is also possible to argue that the reduced POD and PPO activities in MT+CA-treated lemons might be the result of reduced ROS accumulation, weightlessness, and increased antioxidant capacity influenced by enzymatic and non-enzymatic antioxidants. These findings are in line with those of MT-treated blueberries that were previously discovered [55].
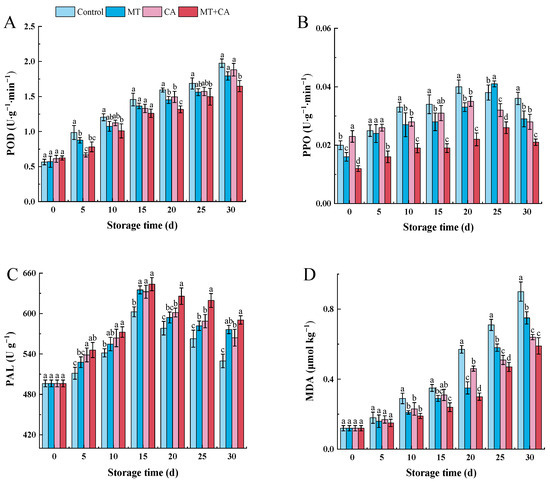
Figure 4.
Changes in enzyme activities and membrane peroxidation in MT or/and CA-treated lemons during 30 d of cold storage at 7–8 °C versus control. (A) POD; (B) PPO; (C) PAL; (D) MDA. Values represent means ± SD in triplicate, and different letters indicate significant differences at the p < 0.05 level compared with the control during the same storage time.
When stresses like low temperature are present, the PAL is essential to the metabolism of both the primary (mangiferic acid pathway) and secondary (phenylpropanoid pathway) types. There is substantial evidence that the phenylpropanoid pathway and PAL activity are connected to defense mechanisms in plants [75,76]. PAL activity increased significantly in the MT+CA treatment group, followed by the MT treatment group (Figure 4C). The results indicated that postharvest treatment MT+CA increased PAL activity, which was consistent with the increase in the cold tolerance of lemon fruits. It may be hypothesized that the enhancement of PAL activity is a defense mechanism against cold, which is related to PAL gene expression, and that MT might play a role in this process. Generally speaking, PAL, POD, and PPO are all involved in the expression of CI symptoms and are critical for phenolic metabolism [77].
MDA is frequently employed as a direct indicator of cellular oxidative damage and damage to membranes. The MDA content of lemon fruits increased during cold storage, and after the storage period, the MT+CA treatment dramatically reduced the MDA content of lemon fruits (Figure 4D). The MDA content of MT+CA-treated fruits on day 30 was 34.4% lower than that of control fruits. Mditshwa et al. [78] revealed that increased lipid peroxidation led to an increase in MDA content in all of the apples that were stored for a longer period. According to these results, membrane deterioration is caused by increased ROS-induced lipid peroxidation, which, in turn, causes an increase in MDA content, a lipid peroxidation product that indicates the true degree of ROS-induced membrane lipid peroxidation [67]. It is possible to link the decreased generation of MDA in MT+CA-stored fruits to a decrease in ROS accumulation and the inhibition of membrane peroxidation.
3.5. Regulation of Volatile Substances by MT and CA
Fruit flavor and consumer preference are largely influenced by volatile compounds. The lemon flavor is the result of a combination of volatile (primarily olefins, ketones, alcohols, aldehydes, and esters) and non-volatile (primarily soluble sugars, amino acids, and organic acids) aroma compounds. In this study, the electronic nose volatile profiles of lemons were recorded and analyzed throughout storage as shown in Figure 5, and the perceptual responses of W3S, W6S, W1S, and W2W did not differ significantly between groups and did not vary much over storage time. The remaining sensors responded to each treatment to varying degrees, with sensor W1W being the most responsive, followed by W2S, W1C, and W5C. MT+CA showed higher responses than the control. According to the study of Zhong S. et al. [79], benzene and terpenoids were the major bound volatiles in Eureka lemon fruit. Aldehydes are thought to be among the most significant groups of volatile substances that affect the aroma of lemons [80]. Esters are a major source of flavor compounds in fruit and can give an indication of the flavor quality of the fruit; alcohols serve as solvents or carriers for the synthesis of aroma compounds [81]. It is noteworthy that the response of sensor W5S to the MT treatment decreased abruptly from day 5 to day 15.
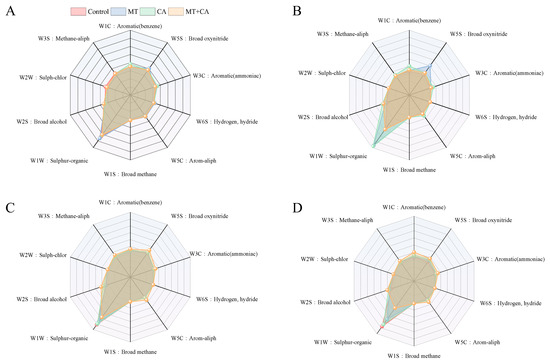
Figure 5.
E-nose sensing on volatile organic compounds from MT, CA-treated, or mixed-treated lemons during storage at 7–8 °C compared to control. (A) E-nose sensing profiles on day 0, (B) day 5, (C) day 15, and (D) day 30. W1C: Aromatic, benzene; W5S: Broad range, oxynitride; W3C: Aromatic, ammoniac compounds; W6S: Hydrogen, hydride; W5C: Arom-aliph; W1S: Broad-methane; W1W: Sulfur-organic; W2S: Broad-alcohol; W2W: Sulph-chlor; W3S: Methane-aliph.
3.6. Lemon Microstructure
The stomata are the passages through which the water and air permeability of the lemons are invaded, and the opening and closing of the stomata in the guard cells are involved in the regulation of carbon assimilation, respiration, and transpiration [7]. SEM images of lemon microstructure showed no difference in stomata between the different treatment groups on day 0 (Figure 6). With the extension of storage time, the stomatal opening of the Control group became larger and larger, while the stomatal opening of the MT+CA treatment group was significantly smaller than that of the Control group. The results showed that MT+CA could reduce the size of subcutaneous thin-walled cells and delay stomatal expansion, thus inhibiting the reduction in water loss and delaying fruit senescence.
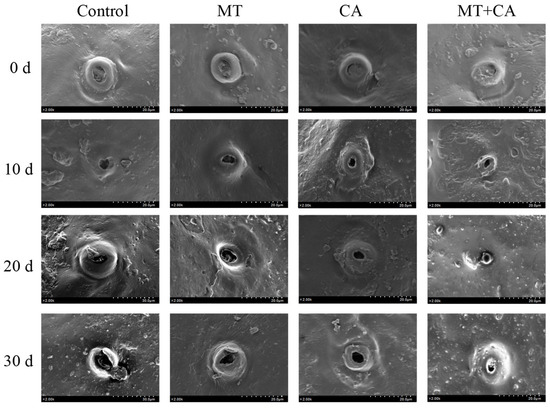
Figure 6.
SEM images of MT and/or CA-treated lemon fruits stored at 7–8 °C for 30 d stomata compared to control. The SEM used an analytical mode of secondary electrons with a magnification of 2.00 k, a spot size of 2.00 k × 20.0 μm–30.0 μm, and a working distance of 9.5 mm.
3.7. Correlation Analysis
Correlations between textural, antioxidant, membrane peroxidation, and volatiles were analyzed using Pearson analysis in the study by Jiang et al. [33]. Therefore, in this study, Pearson correlation analysis was used to find potential connections between the physical quality characteristics, enzyme activities, and antioxidant activities of lemons during postharvest storage (Figure 7). Chlorophyll content was positively correlated with SSC, peel hardness, flesh hardness, TA, VC, and TPC (r2 ≥ 0.88) and negatively correlated with weight loss (r2 = −0.80). TPC was positively correlated with chroma c*, chlorophyll, SSC, peel hardness, flesh hardness, TA, and VC (0.78 < r2 < 0.88), and negatively correlated with POD (r2 = −0.73), PPO (r2 = −0.50), and MDA (r2 = −0.89) were negatively correlated. POD and PPO were both associated with PAL, MDA, DPPH, and H2O2, and CAT were positively correlated.
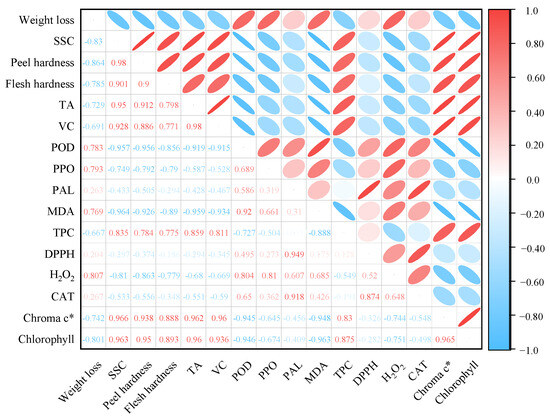
Figure 7.
Pearson correlation coefficients for lemon quality traits, including Chroma c*, Chlorophyll, weight loss, SSC, peel hardness, flesh hardness, TA, VC, POD, PPO, PAL, MDA, TPC, DPPH scavenging capacity, H2O2, CAT, and the following volatile substances: Aromatic (benzene), Broad alcohol, Arom-aliph, Broad oxynitride. Red and blue dots are positive and negative correlations, respectively, and the number shown is the correlation coefficient.
4. Conclusions
In conclusion, the postharvest application of MT+CA treatment maintained the firmness of lemon fruits, kept good color, decreased fruit weight loss and TA, VC, and aroma substance loss, and effectively controlled the senescence process of lemon fruits during cold storage. In addition, the combined treatment inhibited the activities of POD, PPO, and CAT and increased PAL activity, the content of TPC, and DPPH scavenging capacity. The levels of H2O2 and malondialdehyde were reduced, thereby inhibiting the increase in membrane permeability. These effects prolonged the shelf life of lemon fruit, suggesting that MT+CA treatment could be a potential method for improving the quality of lemons under long-term chilling conditions.
Author Contributions
Data curation, M.Y., E.Z. and Z.M.; Funding acquisition, X.L. and Y.J.; Investigation, Z.L., Y.L., S.H. and Y.G.; Resources, X.L.; Writing—original draft, M.Y.; Writing—review and editing, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Programme of Shandong Province (2021CXGC010809), Yinchuan Science and Technology Programme Project (YCLX2024845) and Yinchuan Science and Technology Programme (2023NY07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declared that there are no commercial conflicts of interest.
References
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-Harvest Methyl Jasmonate Treatments Increase Antioxidant Systems in Lemon Fruit without Affecting Yield or Other Fruit Quality Parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Huang, R.; Xia, R.; Hu, L.; Lu, Y.; Wang, M. Antioxidant Activity and Oxygen-Scavenging System in Orange Pulp During Fruit Ripening and Maturation. Sci. Hortic. 2007, 113, 166–172. [Google Scholar] [CrossRef]
- Sun, Y.; Singh, Z.; Tokala, V.Y.; Heather, B. Harvest Maturity Stage and Cold Storage Period Influence Lemon Fruit Quality. Sci. Hortic. 2019, 249, 322–328. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Kaur, J.; Jawandha, S.K.; Gill, P.P.S.; Grewal, S.K.; Singh, H.; Adhikary, T. Beeswax Enriched Gibberellic Acid Coatings Preserve Antioxidant Properties and Quality of Lemon Fruit under Cold Storage Condition. Acta Physiol. Plant. 2023, 45, 93. [Google Scholar] [CrossRef]
- Liao, L.; Li, S.; Li, Y.; Huang, Z.; Li, J.; Xiong, B.; Zhang, M.; Sun, G.; Wang, Z. Pre- or Post-Harvest Treatment with Meja Improves Post-Harvest Storage of Lemon Fruit by Stimulating the Antioxidant System and Alleviating Chilling Injury. Plants 2022, 11, 2840. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Chen, C.; Kowaleguet, M.G.G.M.; Ban, Z.; Fei, L.; Xu, C. Chitosan-Based Layer-by-Layer Assembly: Towards Application on Quality Maintenance of Lemon Fruits. Adv. Polym. Technol. 2020, 2020, 7320137. [Google Scholar] [CrossRef]
- Frempong, K.E.B.; Chen, Y.; Liang, L.; Lin, X. Effect of Calcium Chloride and 1-Methylcyclopropene Combined Treatment on Pectin Degradation and Textural Changes of Eureka Lemon During Postharvest Storage. Curr. Res. Food Sci. 2022, 5, 1412–1421. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Yin, X.; Xing, Y.; Lin, H.; Xu, Q.; Bi, X.; Chen, C. Effects of Controlled Atmosphere on the Storage Quality and Aroma Compounds of Lemon Fruits Using the Designed Automatic Control Apparatus. BioMed Res. Int. 2019, 2019, 6917147. [Google Scholar] [CrossRef]
- Lee, Y.; Chang, C.; Hsu, M.i.; Chung, H.; Liang, Y. Effects of Different Concentrations of Oxygen Used for Storage on the Postharvest Physiology and Quality of Wax Apple (Syzygium samarangense [Blume] Merr. & L. M. Perry Cv. Pink). Sci. Hortic. 2023, 313, 111906. [Google Scholar]
- Olivares, D.; Ulloa, P.A.; Vergara, C.; Hernández, I.; García-Rojas, M.Á.; Campos-Vargas, R.; Pedreschi, R.; Defilippi, B.G. Effects of Delaying the Storage of ‘Hass’ Avocados under a Controlled Atmosphere on Skin Color, Bioactive Compounds and Antioxidant Capacity. Plants 2024, 13, 1455. [Google Scholar] [CrossRef]
- Shirvani, A.; Sadrnia, H.; Rohani, A. Effect of Vacuum and Modified Atmosphere Packaging on the Quality Characteristics and Shelf Life of ‘California’ plum. Erwerbs-Obstbau 2023, 65, 2345–2355. [Google Scholar] [CrossRef]
- Smrke, T.; Cvelbar Weber, N.; Razinger, J.; Medic, A.; Veberic, R.; Hudina, M.; Jakopic, J. Short-Term Storage in a Modified Atmosphere Affects the Chemical Profile of Blueberry (Vaccinium corymbosum L.) Fruit. Horticulturae 2024, 10, 194. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, X.; Su, M.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Controlled Atmosphere Storage Alleviates Internal Browning in Flat Peach Fruit by Regulating Energy and Sugar Metabolisms. Plant Physiol. Biochem. 2022, 186, 107–120. [Google Scholar] [CrossRef]
- Patiño, L.S.; Castellanos, D.A.; Herrera, A.O. Influence of 1-Mcp and Modified Atmosphere Packaging in the Quality and Preservation of Fresh Basil. Postharvest Biol. Technol. 2018, 136, 57–65. [Google Scholar] [CrossRef]
- Van Der Steen, C.; Jacxsens, L.; Devlieghere, F.; Debevere, J. Combining High Oxygen Atmospheres with Low Oxygen Modified Atmosphere Packaging to Improve the Keeping Quality of Strawberries and Raspberries. Postharvest Biol. Technol. 2002, 26, 49–58. [Google Scholar] [CrossRef]
- Lumpkin, C.; Fellman, J.K.; Rudell, D.R.; Mattheis, J. ‘Scarlett Spur Red Delicious’ Apple Volatile Production Accompanying Physiological Disorder Development During Low PO2 Controlled Atmosphere Storage. J. Agric. Food Chem. 2014, 62, 1741–1754. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin Mediates Enhancement of Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S. Exogenous Melatonin Enhances Salt Stress Tolerance in Maize Seedlings by Improving Antioxidant and Photosynthetic Capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous Melatonin Delays Postharvest Fruit Senescence and Maintains the Quality of Sweet Cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Singh, Z.; Hasan, M.U.; Afrifa-Yamoah, E.; Woodward, A. Preharvest Melatonin Application Alleviates Red Drupelet Reversion, Improves Antioxidant Potential and Maintains Postharvest Quality of ‘Elvira’ blackberry. Postharvest Biol. Technol. 2023, 203, 112418. [Google Scholar] [CrossRef]
- Tang, Q.; Li, C.; Ge, Y.; Li, X.; Cheng, Y.; Hou, J.; Li, J. Exogenous Application of Melatonin Maintains Storage Quality of Jujubes by Enhancing Anti-Oxidative Ability and Suppressing the Activity of Cell Wall-Degrading Enzymes. LWT 2020, 127, 109431. [Google Scholar] [CrossRef]
- Ahamad, S.; Asrey, R.; Singh, A.K.; Sethi, S.; Joshi, A.; Vinod, B.R.; Meena, N.K.; Menaka, M.; Choupdar, G.K. Melatonin Treatment Enhances Bioactive Compound Retention, Antioxidant Activity and Shelf-Life of Bell Pepper (Capsicum annuum L.) During Cold Storage. Int. J. Food Sci. Technol. 2024, 59, 7918–7931. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Z.; Wang, J.; Fu, Y.; Zhang, Z.; Khan, M.R.; Cong, X. Effect of Exogenous Melatonin on Postharvest Storage Quality of Passion Fruit through Antioxidant Metabolism. LWT 2024, 194, 115835. [Google Scholar] [CrossRef]
- Liu, Q.; Xin, D.; Xi, L.; Gu, T.; Jia, Z.; Zhang, B.; Kou, L. Novel Applications of Exogenous Melatonin on Cold Stress Mitigation in Postharvest Cucumbers. J. Agric. Food Res. 2022, 10, 100459. [Google Scholar] [CrossRef]
- Magri, A.; Petriccione, M. Melatonin Treatment Reduces Qualitative Decay and Improves Antioxidant System in Highbush Blueberry Fruit During Cold Storage. J. Sci. Food Agric. 2022, 102, 4229–4237. [Google Scholar] [CrossRef]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin May Increase Disease Resistance and Flavonoid Biosynthesis through Effects on DNA Methylation and Gene Expression in Grape Berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin Treatment Inhibits Gray Mold and Induces Disease Resistance in Cherry Tomato Fruit During Postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y. Exogenous Melatonin Improves M Alus Resistance to M Arssonina Apple Blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Z. Postharvest Ethephon Degreening Improves Fruit Color, Flavor Quality and Increases Antioxidant Capacity in ‘Eureka’ Lemon (Citrus limon (L.) Burm. F.). Sci. Hortic. 2019, 248, 70–80. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol Composition, Vitamin C Content and Antioxidant Capacity of Mauritian Citrus Fruit Pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Li, X.; Wang, Z.; Wang, H.; Li, W.; Liu, T.; Li, X.; Jiang, Y.; Tang, Y. Combination of 1-Methylcyclopropene and Phytic Acid Inhibits Surface Browning and Maintains Texture and Aroma of Fresh-Cut Peaches. Postharvest Biol. Technol. 2023, 200, 112328. [Google Scholar] [CrossRef]
- Du, M.; Liu, Z.; Zhang, X.; Li, H.; Liu, Z.; Li, X.; Song, J.; Jia, X.; Wang, L. Effect of Pulsed Controlled Atmosphere with CO2 on the Quality of Watercored Apple During Storage. Sci. Hortic. 2021, 278, 109854. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Ran, Y.; Li, L.; Chen, L.; Lin, Q.; Liang, F.; Li, J.; Li, X.; Tang, Y. Short-Term High Oxygen Pre-Stimulation Inhibits Browning of Fresh-Cut Watercored Fuji Apples. Postharvest Biol. Technol. 2022, 191, 111959. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.; Liu, R.; Tsao, R. Characterisation of Phenolics, Betanins and Antioxidant Activities in Seeds of Three Chenopodium Quinoa Willd. Genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, H.; Wu, Y.; Zhou, F.; Hua, C.; Ba, L.; Shamim, S.; Zhang, W. Characterization of Volatile Compounds and Microstructure in Different Tissues of ‘Eureka’ lemon (Citrus limon). Int. J. Food Prop. 2022, 25, 404–421. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality Parameters of Spanish Lemons with Commercial Interest. Foods 2020, 10, 62. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Duan, M.; Yan, S.; Shi, W.; Zhu, Z.; Zhang, H.; Yue, J.; Xu, R.; Guo, L.; et al. Integrated Transcriptomics and Metabolomics Reveal the Mechanisms of Postharvest Uneven Degreening of Green Lemon. Postharvest Biol. Technol. 2024, 216, 113072. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Zhao, M.; Wang, J.; Wang, Q. Effect of Melatonin Treatment on Visual Quality and Health-Promoting Properties of Broccoli Florets under Room Temperature. Food Chem. 2020, 319, 126498. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N. Melatonin Promotes Carotenoid Biosynthesis in an Ethylene-Dependent Manner in Tomato Fruits. Plant Sci. 2020, 298, 110580. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, Y.; Shan, W.; Kuang, J.; Lu, W.; Su, X.; Tao, N.; Lakshmanan, P.; Chen, J. Melatonin Delays Leaf Senescence of Postharvest Chinese Flowering Cabbage through Ros Homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective Effect of Melatonin against Chlorophyll Degradation During the Senescence of Barley Leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water Loss of Fresh Fruit: Influencing Pre-Harvest, Harvest and Postharvest Factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Sivakumar, D.; Korsten, L. Fruit Quality and Physiological Responses of Litchi Cultivar Mclean’s Red to 1-Methylcyclopropene Pre-Treatment and Controlled Atmosphere Storage Conditions. LWT-Food Sci. Technol. 2010, 43, 942–948. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F. Melatonin Limited Ethylene Production, Softening and Reduced Physiology Disorder in Pear (Pyrus communis L.) Fruit During Senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of Ripening and Softening in ‘Guifei’ mango Fruit by Postharvest Application of Melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Postharvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valverde, J.M.; Valero, D.; Serrano, M. Melatonin Treatment to Pomegranate Trees Enhances Fruit Bioactive Compounds and Quality Traits at Harvest and During Postharvest Storage. Antioxidants 2021, 10, 820. [Google Scholar] [CrossRef]
- Kaur, S.; Jawandha, S.K.; Singh, H. Response of Baramasi Lemon to Various Post-Harvest Treatments. Int. J. Agric. Environ. Biotechnol. 2014, 7, 895–902. [Google Scholar] [CrossRef]
- Bal, E. Effect of Melatonin Treatments on Biochemical Quality and Postharvest Life of Nectarines. J. Food Meas. Charact. 2021, 15, 288–295. [Google Scholar] [CrossRef]
- Shang, F.; Liu, R.; Wu, W.; Han, Y.; Fang, X.; Chen, H.; Gao, H. Effects of Melatonin on the Components, Quality and Antioxidant Activities of Blueberry Fruits. LWT 2021, 147, 111582. [Google Scholar] [CrossRef]
- Tian, S.; Li, B.; Xu, Y. Effects of O2 and CO2 Concentrations on Physiology and Quality of Litchi Fruit in Storage. Food Chem. 2005, 91, 659–663. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing Antioxidant Systems by Preharvest Treatments with Methyl Jasmonate and Salicylic Acid Leads to Maintain Lemon Quality During Cold Storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin Treatment Delays Postharvest Senescence and Maintains the Organoleptic Quality of ‘Newhall’ navel Orange (Citrus sinensis (L.) Osbeck) by Inhibiting Respiration and Enhancing Antioxidant Capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; Del Pozo, T. Melatonin Triggers Metabolic and Gene Expression Changes Leading to Improved Quality Traits of Two Sweet Cherry Cultivars During Cold Storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y. Extending the Shelf Life of Fresh-Cut Lotus Root with Antibrowning Agents, Cinnamon Oil Fumigation and Moderate Vacuum Packaging. J. Food Process Eng. 2012, 35, 505–521. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y.; Gong, L. Effect of Chitosan Coating and Oil Fumigation on the Microbiological and Quality Safety of Fresh-Cut Pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Goswami, T.K. Extended Storage Life of Litchi Fruit Using Controlled Atmosphere and Low Temperature. J. Food Process. Preserv. 2004, 28, 388–403. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. The Effects of Modified and Palliflex Controlled Atmosphere Storage on Postharvest Quality and Composition of ‘Istanbul’medlar Fruit. Postharvest Biol. Technol. 2015, 99, 9–19. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, A.; Xu, Y.; Wang, Y. Responses of Physiology and Quality of Sweet Cherry Fruit to Different Atmospheres in Storage. Food Chem. 2004, 87, 43–49. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of Controlled Atmosphere Storage on Pericarp Browning, Bioactive Compounds and Antioxidant Enzymes of Litchi Fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Harvest Discrimination of Pomegranate Fruit: Postharvest Quality Changes and Relationships between Instrumental and Sensory Attributes During Shelf Life. J. Food Sci. 2013, 78, S1264–S1272. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Chai, H.; Cheng, N.; Yang, Y.; Wang, D.; Yang, T.; Cao, W. Melatonin Treatment Delays Postharvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z. Melatonin Attenuates Postharvest Physiological Deterioration of Cassava Storage Roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Singh, Z.; Hasan, M.U.; Kaur, J.; Afrifa-Yamoah, E.; Woodward, A. Melatonin Application Suppresses Oxidative Stress and Maintains Fruit Quality of Cold Stored ‘Esperanza’raspberries by Regulating Antioxidant System. Postharvest Biol. Technol. 2024, 207, 112597. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: St. Davis, CA, USA, 2002. [Google Scholar]
- Fujii, H.; Shimada, T.; Sugiyama, A.; Nishikawa, F.; Endo, T.; Nakano, M.; Ikoma, Y.; Shimizu, T.; Omura, M. Profiling Ethylene-Responsive Genes in Mature Mandarin Fruit Using a Citrus 22k Oligoarray. Plant Sci. 2007, 173, 340–348. [Google Scholar] [CrossRef]
- Raimbault, A.K.; Marie-Alphonsine, P.A.; Horry, J.P.; Francois-Haugrin, M.; Romuald, K.; Soler, A. Polyphenol Oxidase and Peroxidase Expression in Four Pineapple Varieties (Ananas comosus L.) after a Chilling Injury. J. Agric. Food Chem. 2011, 59, 342–348. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, Y.; Wang, Z.; Qin, L.; Cao, L.; Li, H.; Ma, X.; Yin, J.; Zhao, Z.; Liu, P. Melatonin-Induced Mybs Alleviates Fresh-Cut Lotus Root (Nelumbo nucifera Gaertn.) Browning During Storage by Attenuating Flavonoid Biosynthesis and Reactive Oxygen Species (ROS). J. Sci. Food Agric. 2023, 103, 5452–5461. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tiznado-Hernandez, M.E.; Zavaleta-Gatica, R.; Martınez-Téllez, M.A. Methyl Jasmonate Treatments Reduce Chilling Injury and Activate the Defense Response of Guava Fruits. Biochem. Biophys. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Wang, H. Effect of Salicylic Acid on the Activities of Anti-Oxidant Enzymes and Phenylalanine Ammonia-Lyase in Cucumber Fruit in Relation to Chilling Injury. J. Hortic. Sci. Biotechnol. 2009, 84, 125–130. [Google Scholar] [CrossRef]
- Martínez-Téllez, M.A.; Lafuente, M.T. Chilling-Induced Changes in Phenylalanine Ammonia-Lyase, Peroxidase, and Polyphenol Oxidase Activities in Citrus Flavedo Tissue. Physiol. Basis Postharvest Technol. 1992, 343, 257–263. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Vries, F.; Van Der Merwe, K.; Crouch, E.; Opara, U.L. Impact of Dynamic Controlled Atmospheres on Reactive Oxygen Species, Antioxidant Capacity and Phytochemical Properties of Apple Peel (Cv. Granny Smith). Sci. Hortic. 2017, 216, 169–176. [Google Scholar] [CrossRef]
- Zhong, S.; Ren, J.; Chen, D.; Pan, S.; Wang, K.; Yang, S.; Fan, G. Free and Bound Volatile Compounds in Juice and Peel of Eureka Lemon. Food Sci. Technol. Res. 2014, 20, 167–174. [Google Scholar] [CrossRef]
- He, C.; Ran, Y.; Zeng, L.; Zhang, X.; Zhang, Y.; Wang, C.; Jiao, B. Analysis of Aroma Components from Peels of Different Lemon Varieties by Gc-Ms. Food Sci. 2013, 34, 175–179. [Google Scholar]
- El Hadi, M.A.M.; Zhang, F.; Wu, F.; Zhou, C.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).