Abstract
Biological decontamination strategies using microorganisms to adsorb aflatoxins have shown promising results for reducing the dietary exposure to these contaminants. In this study, the ability of inactivated biomasses of Lacticaseibacillus rhamnosus (LRB) and Saccharomyces cerevisiae (SCB) incorporated alone or in combination into functional yogurts (FY) at 0.5–4.0% (w/w) to adsorb aflatoxin B1 (AFB1) was evaluated in vitro. Higher adsorption percentages (86.9–91.2%) were observed in FY containing 1.0% LR + SC or 2.0% SC (w/w). The survival of mouse embryonic fibroblasts increased after exposure to yogurts containing LC + SC at 1.0–4.0% (w/w). No significant differences were noted in the physicochemical and sensory characteristics between aflatoxin-free FY and control yogurts (no biomass) after 30 days of storage. The incorporation of combined LRB and SCB into yogurts as vehicles for these inactivated biomasses is a promising alternative for reducing the exposure to dietary AFB1. The results of this trial support further studies to develop practical applications aiming at the scalability of using the biomasses evaluated in functional foods to mitigate aflatoxin exposure.
1. Introduction
Aflatoxins are toxic secondary metabolites produced by certain fungi species belonging to the genus Aspergillus, which develop on crops, foods, and feeds [1,2]. To date, more than 20 types of aflatoxins have been identified, while aflatoxin B1 (AFB1) is considered the major toxic metabolite produced by fungi that can contaminate foods and feeds [3]. AFB1 is classified as a group 1 carcinogen by the International Agency for Research on Cancer [4]. Consistent with the type, dose, and time of exposure through contaminated food, AFB1 can affect the liver as the main target organ and cause immunosuppression, carcinogenic, mutagenic, and teratogenic effects [5].
Because of the high resistance of aflatoxins to food processing techniques, prevention of fungi infection and development on food materials is the main strategy to avoid aflatoxin contamination [3]. However, decontamination can be partially achieved using some physical, chemical, or biological approaches aiming at inactivating, degrading, or sequestrating the aflatoxin in the food matrix [6]. In this context, biological methods using microorganisms to adsorb aflatoxins have shown promising results for reducing these toxins in foods and feeds [7,8]. When microrganisms such as lactic acid bacteria (LAB) and some yeast species are added to a fermented food, they may act like “enterosorbents” and bind to the mycotoxins, thereby alleviating their bioavailability in the gastrointestinal tract [3]. Regarding the reduction or removal of AFB1, both Lacticaseibacillus rhamnosus and Saccharomyces cerevisiae have exhibited excellent performance [7,8]. Although the mechanisms behind the adsorption are not completely understood, there is evidence of the formation of a reversible, stable complex of mycotoxin on the surface of subjected microorganisms, without any chemical modifications, with similar adsorption capacities shown by viable or inactivated microorganisms [8].
Information and consumers’ awareness regarding health has led to the search for a healthier lifestyle, a fact that has increased the demand for functional foods containing probiotics. Probiotics are defined as live microorganisms that are intended to have health benefits when consumed or applied to the body [9]. Probioticity is a strain-specific characteristic and most probiotics belong to the metabolic group of lactic fermentation bacteria such as many Lactobacillus species [10], although recent investigations have presented some yeast species with probiotic properties, including Saccharomyces boulardii (reviewed by Souza et al. [11]). The use of probiotics is valuable in reducing several health risks through its incorporation into functional foods, as they can vitally improve public health, especially in critical age groups [9]. The choice of a suitable food product for delivering probiotics depends on the type of probiotic strain, the food’s ability to protect the probiotics, and consumer preferences. In this context, yogurt is one of the most common probiotic-enriched foods, mainly because its matrix acts as a buffer against stomach acid, helping probiotics reach the gut alive [10]. However, probiotic supplementation for fermented products like yogurt can potentially alter its sensory properties [12]. This stresses the demand for examining the sensory features of functional products containing probiotics, aiming at increasing its acceptability among consumers [13].
A possible alternative for adding beneficial microorganisms to food products is the use of paraprobiotics, defined as inactivated microbial cells (non-viable) that provide a health benefit to the consumer [14]. There is evidence on the anti-inflammatory effect and positive immune responses of paraprobiotics in animals and humans, with some advantages if compared with probiotics since non-viable microbial cells may exhibit enhanced safety and technological advantages, such as longer shelf life and low interference with formulations of the food matrix [15]. These features are in line with the scientific advances on the use of microbial products as a promising approach in the decontamination of mycotoxins [16]. In these products, the use of paraprobiotics and inactivated cells of other, non-probiotic microorganisms is an efficient approach to adsorb toxins in a target matrix of a food or beverage. Abdel-Salam et al. [17] demonstrated the efficacy of a functional yogurt containing viable cells of Lactobacillus acidophilus, Streptococcus thermophilus, and Bifidobacterium bifidum to protect against AFB1 toxicity in rats. However, to the authors’ knowledge, there is no information available on the protective effects of functional yogurts containing inactivated biomasses of microorganisms against the cytotoxic effects of AFB1, or on the potential influence of the incorporation of these products on the quality parameters of yogurts. Therefore, this study aimed to evaluate the efficacy of inactivated biomasses of the probiotic LAB L. rhamnosus and S. cerevisiae, a non-probiotic yeast, incorporated alone or in combination into plain, non-flavored yogurts [18] as vehicles for these inactivated microorganisms, to adsorb AFB1 in vitro and reduce its toxicity to mouse embryonic fibroblasts (MEF-1). The effect of the incorporation of biomasses on the overall quality of the experimental yogurts was also assessed.
2. Materials and Methods
2.1. Preparation of Lactic Acid Bacteria and Yeast Biomasses
In this study, a commercially available lyophilized L. rhamnosus (HOWARU® LYO 40 DCU, Danisco Ltd., São Paulo, Brazil) biomass containing 1.0 × 1010 viable cells/g was used. This strain was previously evaluated regarding its ability to bind to AFB1 in phosphate buffer solution (PBS), with a percentage binding of 45.9% at pH 3.0 [19]. The S. cerevisiae strain was a commercially available brewer’s biological dry yeast biomass (Fermentis K-97, SafAle, Bruggeman, Gent, Belgium) containing 1.0 × 1010 viable cells/g. The AFB1 adsorption capacity of the S. cerevisiae strain in PBS was 45.5% to bind to AFB1 in PBS at pH 3.0 [20]. Both the L. rhamnosus strain in the lyophilized starter culture and S. cerevisiae cells were inactivated by autoclaving the material at 121 °C for 10 min. Heat inactivation of microbial cells may increase their aflatoxin binding properties, due to denaturation of membrane proteins, peptidoglycans, and degradation of polysaccharide components of the cell wall, which ultimately change the hydrophobicity of the cell wall and respective binding capacities [19,21]. After this procedure, the inactivated biomasses of the L. rhamnosus (LRB) and S. cerevisiae (SCB) strains were stored at −20 °C, until incorporation into plain yogurts.
2.2. Preparation of the Functional Yogurts
Plain stirred yogurt containing LRB and SCB, alone or in combination, was produced following the procedures described by Soukoulis et al. [19], with some modifications. Briefly, 150 L of unpasteurized skim milk containing <0.1% fat was obtained from a local dairy plant and standardized by adding 5.3 kg of skim milk powder to achieve a total solids content (TS) of 12.5% (w/w). After standardization, duplicate 100 mL samples were collected and analyzed using a Lactoscan ® MCC (Milkotronic, Nova Zagora, Bulgaria) for confirmation of TS and to determine the percentages of fat, total protein, lactose, and minerals. Furthermore, triplicate samples of the standardized milk were analyzed according to Jager et al. [22] and found to have AFB1 or AFM1, the hydroxilated metabolite of AFB1, at levels below the detection limits of the analytical method (0.1 and 0.075 µg/mL, respectively). Next, aliquots of the standardized milk were assigned to 13 yogurt-producing vats (15 L per vat), then incorporated with increasing percentages (w/w) of LRB or SCB biomasses, as follows: Vat 1: no LRB or SCB (control); Vat 2: 0.5% LRB; Vat 3: 0.5% SCB; Vat 4: 0.5% LRB + 0.5% SCB; Vat 5: 1.0% LRB; Vat 6: 1.0% SCB; Vat 7: 1.0% LRB + 1.0% SCB; Vat 8: 2.0% LRB; Vat 9: 2.0% SCB; Vat 10: 2.0% LRB + 2.0% SCB; Vat 11: 4.0% LRB; Vat 12: 4.0% SCB; Vat 13: 4.0% LRB + 4.0% SCB. The contents in all vats were thoroughly mixed using a manual milk mixer during 10 min.
The mixtures were heat-treated at 90 °C for 15 min, then cooled to 42 °C for inoculation of the starter culture (3% inoculum) containing Streptococcus thermophilus and L. delbrueckii ssp. bulgaricus (Yo-Flex, Chr. Hansen, Horsholm, Denmark). The vats were incubated at 42 °C for approximately 3 h, until the products reached a pH of 4.5. The obtained functional yogurts (FY) were cooled rapidly to 10 °C, transferred to 1 L polyethylene bottles, and stored at 5 °C for 30 days.
2.3. Adsorption Assays of Aflatoxin B1 in Functional Yogurts In Vitro
The AFB1 binding assays were conducted using the protocol described by Bovo et al. [19], with some modifications. A working solution of AFB1 at 10.0 µg/mL was prepared in PBS at pH 3.0, and 1 mL aliquots of this solution were transferred to 15 mL test tubes. After this procedure, 10 g of each functional yoghurt containing LRB, SCB, or LRB + SCB at 0.5–4.0% (w/w) and control (yogurt without any biomass incorporation) was added to triplicate tubes and the mixtures were vortexed for 5 min. The concentration of AFB1 in the final mixtures in all tubes was 1.0 µg/g. The tubes were placed on a rotating shaker at 180 rpm for 60 min at room temperature (25 °C). Next, the mixtures were centrifuged at 3100× g for 10 min, and the supernatants were separated for quantification of AFB1.
AFB1 in the supernatants was extracted and purified using immunoaffinity columns (Aflatest WB®, Vicam, Watertown, MA, USA), exactly as described by Jager et al. [22]. For derivatization of AFB1 in samples, 50 µL of the final extract was diluted in 5 mL of milli-Q water (Millipore, Burlington, MA, USA), and 100 µL of the resulting solution was mixed with 900 µL of acetonitrile/water (50:50) in an Eppendorf tube. Then, 500 µL was transferred to a new Eppendorf tube containing 200 µL hexane and 100 µL trifluoroacetic acid, kept at 35 °C for 10 min, evaporated to near-dryness and diluted in 500 µL acetonitrile/water (50:50). Twenty microliters of the final extracts was injected into a Shimadzu 10VP liquid chromatograph (Kyoto, Japan), equipped with a 10 AXL fluorescence detector (excitation at 360 nm and emission above 440 nm). The chromatographic run was achieved using a Kinetex C18 column (Phenomenex, Torrance, CA, USA) 4.6 × 150 mm, 2.6 μm particle size, and the isocratic mobile phase consisted of methanol/water/acetonitrile (61.4:28.1:10.5, v/v/v) with a flow rate of 0.50 mL/min. Five-point calibration curves containing the AFB1 standard diluted in acetonitrile and derivatized as previously described for the samples were prepared at levels of 0.1, 0.25, 0.5, 0.75, and 1.0 μg/mL. Integrated peak areas were linearly correlated with the concentrations. Identification of AFB1 was achieved by comparing the retention time of AFB1 peaks in the samples with the standards in the calibration curves. The limits of detection (LOD) and limits of quantification (LOQ) were calculated at a signal-to-noise ratio of 3 and 10, respectively, being 0.017 and 0.055 μg/kg. The analytical method was previously validated with contaminated yogurt samples at levels of 0.2 and 0.5 μg/kg (n = 3, for each concentration), which resulted in recovery rates from 72 to 93% [22].
The percentage of mycotoxin binding was calculated using Equation (1), in which “a” indicates the percentage of AFB1 adsorbed by LRB and/or SCB, “b” is the concentration of AFB1 added to buffer (1.0 µg/mL), “c” is the concentration of AFB1 in the buffer solution plus yeast and/or LAB inactivated cells after centrifugation, and “d” is the concentration of any interferences in the negative control (buffer solution + LRB/SCB).
a = [b − (c − d)]/b × 100
2.4. Survival of Mouse Embryonic Fibroblasts Exposed to Aflatoxin B1 in Functional Yogurts
Procedures for assessing the cell viability of MEF-1 to AFB1 in FY incorporated with both LRB and SCB were performed as described by Nones et al. [23], with some modifications. MEF-1 cells derived from the American Tissue Culture Collection (ATCC) were obtained from the Cell Bank of Rio de Janeiro (BCRJ) and cultured in Dulbecco’s modified Eagle medium (DMEM) Gibco® (Thermo Fischer Scientific, Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum, penicillin (100 unit/mL), and streptomycin (100 µg/mL). MEF-1 cells were reseeded after trypsination on a weekly basis in a 1:5 split ratio and allowed to grow as monolayers in cell culture flasks (75 cm2) with filter screw caps (Corning®, Corning, MA, USA) until reaching 90–100% confluency. MEF-1 cells from passages between 14 and 18 were maintained at 37 °C in a humidified atmosphere of 5% CO2 and reserved for cell viability experiments.
MEF-1 cell viability was assessed using the 3-{4,5-dimethylthiazol-2-yl} diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO, USA), which measures the cellular metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity [24]. MTT stock solution was prepared in phosphate-buffered saline at 5.0 mg/mL, and the working solution was prepared in DMEM at 0.5 mg/mL. MEF-1 cells were seeded in triplicate in 96-well culture plates at 12,000 cells/cm2 and incubated overnight at 37 °C with 5% CO2 in supplemented DMEM. On the following day, the medium was replaced with 200 µL of one of the following treatment media: (1) DMEM only (control); (2) DMEM + AFB1 at 1.0 µg/mL; (3) DMEM + FY without any LRB or SCB; (4) DMEM + FY without any LRB or SCB + AFB1 (1.0 µg/mL); (5) DMEM + FY with 0.5% (v/v) of LRB and SCB; (6) DMEM + FY with 0.5% (v/v) of LRB and SCB + AFB1 (1.0 µg/mL); (7) DMEM + FY with 1.0% (v/v) of LRB and SCB; (8) DMEM + FY with 1.0% (v/v) of LRB and SCB + AFB1 (1.0 µg/mL); (9) DMEM + FY with 2.0% (v/v) of LRB and SCB; (10) DMEM + FY with 2.0% (v/v) of LRB and SCB + AFB1 (1.0 µg/mL); (11) DMEM + FY with 4.0% (v/v) of LRB and SCB; (12) DMEM + FY with 4.0% (v/v) of LRB and SCB + AFB1 (1.0 µg/mL).
The plates were incubated for 24 h, then the treatment medium was gently replaced with 100 µL fresh medium containing 10% (v/v) of MTT stock solution. After incubation for 4 h at 37 °C, the medium was replaced by dimethylsulphoxide (DMSO) and plates shaken to dissolve the purple formazan product. The absorbance of each well was read at 570 nm using an EZ Read 2000® plate reader (Biochrom, Cambridge, UK). Cell viability was expressed in percentages, according to Equation (2). The experiments were performed in triplicate and each result represents the mean of at least three independent experiments.
Cell viability = [Absorbance570 Treatments/Absorbance570 Control] × 100
2.5. Physicochemical and Sensory Evaluation of Functional Yogurts
FY samples were collected and analyzed on d 30 of storage at 5 °C, to assess the possible effects of LRB and/or SCB on the physicochemical characteristics and sensory grades of the products. Fat, protein, and pH were determined according to the Association of Official Analytical Chemists [25]. For sensory evaluation, samples of FY without any aflatoxin were submitted to a trained panel of 15 individuals (8 men and 7 women, aged 18–25 years), according to Mousavi et al. [26]. This study was approved by the Research Ethics Committee of the FZEA-USP (approval no. 5.742.458). Panelists were trained during 3 sessions, one per week for 3 weeks, to score intensity of appearance, consistency, aroma, and taste, using samples of commercial skim, plain yogurts with TS of 11.5% (w/w) and without any LRB or SCB as reference material. Definitions of sensory attributes related to the typical characteristics of yogurts were based on criteria described by Aktar [27], as follows: appearance: white coagulum, uniform; consistency: moderate viscosity, homogeneous; aroma and taste: pure, typically acid. All attributes were quantified by panelists according to an intensity scale from 1 to 5, in which a rating score of 5 was equivalent to the typical characteristics of yogurt used as reference material and full presence of attributes; 1 was equivalent to a product without typical characteristics and non-detectable attributes.
2.6. Statistical Analysis
The results were subjected to analysis of variance, in accordance with the general linear model (GLM) of SAS® [28], to check for significant differences between mean values. When applicable, the Fisher LSD test (least significant difference) was used for comparison between mean values, considering p < 0.05.
3. Results and Discussion
Table 1 presents the levels of AFB1 and the respective reduction percentages by FY containing LRB, SCB, or their combinations. Lower levels of AFB1 (p < 0.05) were observed in tubes with FY containing the combined biomasses of inactivated cells (LRB + SCB). Consequently, the highest adsorption percentages (p < 0.05) were observed with inclusions of 1.0–4.0% of both biomasses, in which the AFB1 reduction ranged from 86.9 to 91.2%. The second-best response was attained with FY containing 1.0–4.0% of SCB alone, with respective AFB1 reduction values ranging from 81.8 to 84.8%. FY containing only LRB provided AFB1 reduction rates of 11.3 to 42.8%. The mechanisms involved in the adsorption of AFB1 by microbial cells, either viable or inactivated, are not fully understood. However, the adsorption process may involve physical and chemical interactions, such as aflatoxin binding to cell wall components including polysaccharides, proteins, and lipids in LAB cells [29], and β-glucans, mannoproteins, and chitin in yeast cells [30]. β-glucans, in particular, have been shown to adsorb aflatoxins via hydrogen bonding and other interactions [31]. In addition, hydrophobic and electrostatic interactions between the AFB1 molecule and the lipid-rich regions of the microbial cell wall, and weak, non-covalent interactions such as van der Waals forces between AFB1 and the microbial cell surface also contribute to the adsorption process [29,30].

Table 1.
Aflatoxin B1 concentrations and reduction percentages in test tubes with functional yogurts stored at 5 °C for 30 days, containing different percentages of Lacticaseibacillus rhamnosus and Saccharomyces cerevisiae biomasses 1.
Comparing the adsorption results obtained in this work with those described in other studies is a difficult task, due to the absence of previous data on the removal of aflatoxins by combinations of LRB and SCB conveyed in yogurts. However, our findings are consistent with those reported by Zolfaghari et al. [32], who observed that both bacterial and yeast isolates had the capacity to effectively reduce the levels of AFB1. The authors observed that Lactobacillus spp. exhibited a binding capacity ranging from 8.4 to 31.1%, while S. cerevisiae achieved an AFB1 binding rate of 30.5%. Data presented in this work align with previous findings regarding the AFB1 removal capacity of L. rhamnosus [19]. Our results are also in agreement with data reported in previous studies, such as El-Nezami et al. [33] and Luo et al. [34], in which L. rhamnosus demonstrated similar capacities to bind to AFM1 in whole milk, with binding percentages ranging from 36 to 63%. Moreover, Pourmohammadi et al. [35] demonstrated that the combined use of LAB strains enhances the adsorption rate of mycotoxins, when compared with the isolated binding capacities of these strains, which corroborates the improved adsorption results achieved with the LRB and SCB in our study. These findings collectively underscore the potential of both S. cerevisiae and LAB strains for reducing the aflatoxin contamination in foods, holding promise for further exploration and industrial applications.
The survival of MEF-1 cells after treatment with AFB1 and/or yogurts incorporated with different percentages of LRB + SCB is presented in Figure 1. The significant increases in cell survival after exposure to yogurts containing ≥1% LRB + SCB indicate a potential protective effect against AFB1-induced cytotoxicity. This suggests that the incorporation of LRB and SCB into yogurt formulations may mitigate the adverse effects of AFB1 exposure, highlighting the potential application of paraprobiotics and other inactivated non-probiotic microorganisms in food safety and health promotion. Although the reversibility of the AFB1 adsorption by LRB or SCB was not assessed in the present experiment, the higher survival of MEF-1 cells indicates a lower bioavailability of AFB1 in yogurts containing ≥1% LRB + SCB after 24 h exposure.
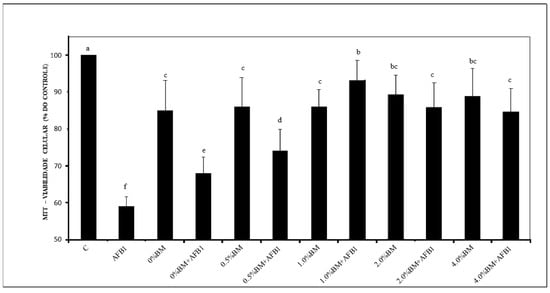
Figure 1.
Survival of mouse embryonic fibroblasts (MEF-1) analyzed by the MTT assay, after exposure to 1.0 µg/mL of aflatoxin B1 (AFB1) and functional yogurts (FY) containing 0, 1.0, 2.0, or 4.0% (w/w) of cell inactivated biomasses (BM) of Lacticaseibacillus rhamnosus (1.0 × 1010 cells/g) and Saccharomyces cerevisiae (1.0 × 1010 cells/g). BM was inactivated by autoclaving at 121 °C for 10 min. Values are expressed as mean ± standard deviation of percentages relative to control (C, no exposure to AFB1 or FY containing BM) of 3 independent experiments with 3 replicates each. a–f Bars with different letters differ significantly (p < 0.05).
Similar protective effects of probiotic microorganisms against aflatoxins were reported by Martinez et al. [36], regarding the adsorption and degradation of AFM1 by selected species of LAB and yeasts in fluid milk. The authors assessed the ability of tested probiotics to detoxify AFM1 using brine shrimp (Artemia salina) toxicity assays and found that probiotics Pediococcus pentosaceus and Kluyveromyces marxianus have the ability to adsorb and degrade AFM1 to less toxic metabolites in milk. Regarding AFB1, its cytotoxicity has been extensively demonstrated in several cell cultures, such as a human liver cancer cell line (HepG2), the human embryonic kidney 293 cell line (HEK293T), and continuous porcine cell lines from alveolar macrophages (3D4/21) [37]. Conversely, cell cultures are practical and viable models for studies on mycotoxin detoxification. The protective effects of a modified bentonite clay against the toxic effects of AFB1 in fibroblasts 3T3 and epithelial colorectal adenocarcinoma cells (Caco-2) were reported by Nones et al. [23].
In this study, the MEF-1 cells were used for the first time to highlight the protective effects of LRB and SCB incorporated into FY against the AFB1-induced cytotoxicity. A notable advantage of using MEF-1 cells is their ease of collection and culture, making them readily accessible for experimentation. Additionally, MEF-1 cells exhibit robust growth and survival characteristics, providing a reliable and reproducible cell model for toxicity assessments [38]. Their ability to support embryonic stem cell (ESC) pluripotency and enhance plating efficiency makes them particularly suitable for studying developmental toxicity and cellular differentiation processes [39]. Furthermore, previous studies have used MEFs as an “embryonic” model for better understanding the mechanism of action for other mycotoxins, such as fumonisin B1 [40].
The physicochemical characteristics of the FY without aflatoxins but containing LRB, SCB, or their combinations at percentage inclusions of 0.5–4.0% (w/w) are presented in Table 2. No significant differences (p > 0.05) were observed in the evaluated physicochemical parameters (fat, protein, and pH) of FY containing cell biomass during 30 d storage. There are no available data on the physicochemical parameters of yogurts incorporated with LRB in combination with SCB. However, our results are consistent with previously reported data [41], indicating that the incorporation of viable probiotics at variable levels did not affect the physicochemical characteristics of the resulting yogurts.

Table 2.
Physicochemical characteristics of yogurts incorporated with different percentages of inactivated biomasses 1 of Lacticaseibacillus rhamnosus and Saccharomyces cerevisiae, on day 30 of storage at 5 °C.
Concerning the sensory evaluation of the FY after 30 days of storage, the results are shown in Table 3. As observed for physicochemical parameters (Table 3), no differences (p > 0.05) were found in the mean values scored for intensity of appearance, consistency, aroma, and flavor among yogurts containing 0.5–4.0% LRB, SCB, or LRB + SCB inclusions, when compared with controls (no cell biomass incorporated). It is noteworthy that the addition of certain ingredients, such as rice bran and fiber, has been shown to decrease the sensory scores of yogurts [42,43,44], which contrasts with the findings described in this work on the incorporation of inactivated cell biomasses of L. rhamnosus or S. cerevisiae. Despite the absence of literature data on the possible effects of inactivated biomasses of L. rhamnosus or S. cerevisiae on the sensory attributes of plain stirred yogurts, the incorporation of LRB and/or SCB did not significantly affect the acceptance of the FY produced in our study. Conversely, regarding the incorporation of living cells of probiotics into yogurts, some data indicated significantly higher scores in probiotic-enriched samples, thus suggesting a positive contribution of probiotic bacteria to the sensory attributes of the final product [45].

Table 3.
Sensory characteristics 1 of yogurts incorporated with different percentages of inactivated biomasses 2 of Lacticaseibacillus rhamnosus and Saccharomyces cerevisiae, on day 30 of storage at 5 °C.
L. rhamnosus strains are considered as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) and have the qualified presumption of safety (QPS) status for intentional addition to food and feed granted by the European Food Safety Authority (EFSA) [46]. Due to its long history of safe use and consumption, most strains of S. cerevisiae are also classified as GRAS [47] for use in foods. In addition, the consumption of heat-inactivated S. cerevisiae strains administered in capsules or tablets was reviewed by Almada et al. [48], who highlighted their health benefits when administered with diet. This evidence confirms the safety of using the LRB and SCB biomasses as potential adsorbents for dietary aflatoxins.
In our work, the ability of a combination of inactivated microorganisms conveyed in yogurt to adsorb AFB1 in vitro was evaluated. Most of the studies using yogurt as a vehicle for beneficial microorganisms were performed with live isolated cells [10,17], which may change the physicochemical and sensory characteristics of the products. The utilization of inactivated microbial cells has been studied mainly using single or combined microorganisms in PBS, not incorporated into food matrices. Hence, the results presented here indicate for the first time that the addition of LRB and SCB to yogurts was effective in adsorbing AFB1 and reducing its toxicity to MEF-1 cells. In addition, the inclusion of biomasses in the FY had no influence on the physicochemical and sensory characteristics of the products, which also highlights the novelty of this study.
4. Conclusions
In this trial, LRB and/or SCB incorporated into yogurts effectively adsorbed AFB1 in vitro, with the highest adsorption percentages (86.9 to 91.2%) found with inclusions of 1.0–4.0% of both biomasses. In addition, the cytotoxicity of AFB1 to MEF-1 significantly reduced when the cells were simultaneously exposed to FY containing ≥1.0% (w/w) of LRB + SCB. Compared with controls (no biomass included), FY containing LRB and/or SCB at inclusion percentages of up to 4.0% (w/w) had no significant differences in the physicochemical characteristics and sensory attributes during 30 d of storage. Thus, the incorporation of combined LRB and SCB into yogurts as vehicles for these inactivated biomasses is a promising alternative for reducing the AFB1 toxic effects. However, the main limitations of this study involve the lack of in vivo data on the AFB1 adsorption ability of FY containing LRB and SCB. In particular, the protective effects of LRB and SCB incorporated into FY against the AFB1-induced MEF-1 cytotoxicity do not fully replicate the complex interactions and environments found in living organisms. In addition, in vitro systems do not account for metabolic processes that occur in the body, such as the conversion of substances into active or toxic metabolites in the liver, or cellular responses to toxic substances that may involve multi-step processes, interactions with other cells, or systemic effects. Finally, the concentration of AFB1 used in MEF-1 cell cultures in this study may not represent the usual levels of human exposure to dietary aflatoxins Therefore, to extend this research, future studies should explore in vivo experiments to assess the effectiveness of AFB1 adsorption by the evaluated FY under real conditions of aflatoxin exposure. Follow-up experiments in this direction could use animal models intoxicated with AFB1 to assess the protective effects of the ingested FY in the gastrointestinal tract against the toxic effects of this mycotoxin, as well as to ensure the absence of undesirable interactions of the FY with nutrients in the diet. This would provide further valuable information regarding the potential of the FY to serve as a preventive strategy for dietary exposure to aflatoxins.
Author Contributions
R.C.P.: Conceptualization, Methodology, Investigation, Writing—original draft; J.d.C.C.: Investigation, Writing—original draft; R.E.R.: Methodology, Investigation; R.D.P.: Conceptualization, Data curation; A.M.B.: Investigation, Writing—original draft; S.A.: Investigation, Validation, Writing—review and editing; E.L.d.P.: Visualization, Methodology, Writing—original draft; R.S.: Validation, Data curation, Writing—original draft. T.C.P.: Validation, Writing—review and editing; A.G.d.C.: Validation, Visualization, Writing—review and editing; C.A.F.d.O.: Conceptualization, Validation, Investigation, Supervision, Project administration, Funding acquisition; C.H.C.: Conceptualization, Validation, Visualization, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants # 2019/21603-1, 2022/03952-1, 2022/07439-7, and 2022/15367-6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant no. 304262/2021-8), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eskola, M.; Kos, G.; Elliolutt, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ruan, H.N.; Sun, X.Q.; Luo, J.Y.; Yang, M.H. Contamination status and health risk assessment of 31 mycotoxins in six edible and medicinal plants using a novel green defatting and depigmenting pretreatment coupled with LC-MS/MS. LWT Food Sci. Technol. 2022, 161, 113401. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans; International Agency for Research on Cancer: Lyon, France, 2002; Volume 82, p. 171. Available online: http://monographs.iarc.fr/ENG/Monographs/vol82/mono82.pdf (accessed on 16 May 2024).
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Nunes, V.M.R.; Moosavi, M.; Khaneghah, A.M.; Oliveira, C.A.F. Innovative modifications in food processing to reduce the levels of mycotoxins. Curr. Opin. Food Sci. 2020, 38, 155–161. [Google Scholar] [CrossRef]
- Pires, R.C.; Portinari, M.R.; Moraes, G.Z.; Khaneghah, A.M.; Gonçalves, B.L.; Rosim, R.E.; Oliveira, C.A.F.; Corassin, C.H. Evaluation of anti-aflatoxin M1 effects of heat-killed cells of Saccharomyces cerevisiae in Brazilian commercial yogurts. Qual. Assur. Saf. Crop. Foods 2022, 14, 75–81. [Google Scholar] [CrossRef]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical adsorption of aflatoxin B1 by lactic acid bacteria and Saccharomyces cerevisiae: A theoretical model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Lucatto, J.N.; Silva-Buzanello, R.A.; Mendonça, S.N.T.G.; Lazarotto, T.C.; Sanchez, J.L.; Bona, E.; Drunkler, D.A. Performance of different microbial cultures in potentially probiotic and prebiotic yoghurts from cow and goat milks. Int. J. Dairy Technol. 2020, 73, 144–156. [Google Scholar] [CrossRef]
- Souza, H.F.; Carosia, M.F.; Pinheiro, C.; Carvalho, M.V.; Oliveira, C.A.F.; Kamimura, E.S. On probiotic yeasts in food development: Saccharomyces boulardii, a trend. Food Sci. Technol. 2022, 42, e92321. [Google Scholar] [CrossRef]
- Salehi, F. Quality, physicochemical, and textural properties of dairy products containing fruits and vegetables: A review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar] [CrossRef] [PubMed]
- Mindelo, L.J.; Moraes, J.S.; Glins, B.S.; Pereira, D.R.; Gomes, T.C.; Martins, J.B.; Favacho, C.B.; da Silva Reis, N.C. Sensorial analysis: A tool for the introduction of handicraft yogurt into the market. Braz. J. Dev. 2020, 6, 95795–95801. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, Y.S.; Kang, D.K.; Paik, H.D. Paraprobiotics: Definition, manufacturing methods, and functionality. Food Sci. Biotechnol. 2023, 32, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 8, 1225. [Google Scholar] [CrossRef]
- Oliveira, C.A.F.; Muaz, K.; Møller, C.O.A.; Corassin, C.H.; Rattray, F.P. Probiotics and Mycotoxins. In Probiotics and Prebiotics in Foods: Challenges, Innovations, and Advances; Cruz, A.G., Ranadheera, C.S., Nazzaro, F., Mortazavian, A., Eds.; Academic Press: London, UK, 2021; pp. 309–325. [Google Scholar] [CrossRef]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Bovo, F.; Franco, L.T.; Rosim, R.E.; Trindade, C.S.F.; Oliveira, C.A.F. The ability of Lactobacillus rhamnosus in solution, spray-dried or lyophilized to bind aflatoxin B1. J. Food Res. 2014, 2, 35–42. [Google Scholar] [CrossRef]
- Bovo, F.; Franco, L.T.; Rosim, R.E.; Barbalho, R.; Oliveira, C.A.F. In vitro ability of beer fermentation residue and yeast-based products to bind aflatoxin B1. Braz. J. Microbiol. 2015, 46, 577–581. [Google Scholar] [CrossRef]
- Liew, W.P.; Nurul-Adilah, Z.; Than, L.; Mohd-Redzwan, S. The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front. Microbiol. 2018, 9, 1503. [Google Scholar] [CrossRef]
- Jager, A.V.; Tedesco, M.P.; Souto, P.C.; Oliveira, C.A.F. Assessment of aflatoxin intake in São Paulo, Brazil. Food Control. 2013, 33, 87–92. [Google Scholar] [CrossRef]
- Nones, J.; Solhaug, A.; Eriksen, G.S.; Macuvele, D.L.P.; Polid, A.; Soares, C.; Trentin, A.G.; Riella, H.G.; Nones, J. Bentonite modified with zinc enhances aflatoxin B1 adsorption and increase survival of fibroblasts (3T3) and epithelial colorectal adenocarcinoma cells (Caco-2). J. Hazard. Mater. 2017, 337, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Cunniff, P. Official Methods of Analysis of AOAC International; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT-Food Sci. Technol. 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Aktar, T. Physicochemical and sensory characterisation of different yoghurt production methods. Int. Dairy J. 2022, 125, 105245. [Google Scholar] [CrossRef]
- SAS. SAS/STAT® 9.1 User’s Guide: Statistics. Version 9.1; SAS Institute Incorporation: Cary, NC, USA, 2005. [Google Scholar]
- Corassin, C.H.; Bovo, F.; Rosim, R.E.; Oliveira, C.A.F. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control 2013, 31, 80–83. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Muaz, K.; Coppa, C.F.S.C.; Rosim, R.E.; Kamimura, E.S.; Oliveira, C.A.F.; Corassin, C.H. Aflatoxin M1 absorption by non-viable cells of lactic acid bacteria and Saccharomyces cerevisiae strains in Frescal cheese. Food Res. Int. 2020, 136, 109604. [Google Scholar] [CrossRef] [PubMed]
- Campagnollo, F.B.; Franco, L.T.; Rottinghaus, G.E.; Kobashigawa, E.; Ledoux, D.R.; Daković, A.; Oliveira, C.A. In vitro evaluation of the ability of beer fermentation residue containing Saccharomyces cerevisiae to bind mycotoxins. Food Res. Int. 2015, 77, 643–648. [Google Scholar] [CrossRef]
- Zolfaghari, H.; Khezerlou, A.; Ehsani, A.; Khosroushahi, A.Y. Detoxification of aflatoxin B1 by probiotic yeasts and bacteria isolated from dairy products of Iran. Adv. Pharm. Bull. 2020, 10, 482–487. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Sayadi, M.; Abedi, E.; Mousavifard, M. Determining the adsorption capacity and stability of aflatoxin B1, ochratoxin A, and zearalenone on single and co-culture L. acidophilus and L. rhamnosus surfaces. J. Food Compos. Anal. 2022, 110, 104517. [Google Scholar] [CrossRef]
- Martinez, M.P.; Magnoli, A.P.; Pereyra, M.G.; Cavaglieri, L. Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon 2019, 172, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo evaluation of AFB1 and OTA-toxicity through immunofluorescence and flow cytometry techniques: A systematic review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.J.; Stack, M.E.; Troy, A.L.; Chirtel, S.J. Assessment of the embryotoxic potential of the total hydrolysis product of fumonisin B1 using cultured organogenesis-staged rat embryos. Food Chem. Toxicol. 1997, 35, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Piorczynski, T.B. Use of primary mouse embryonic fibroblasts in developmental toxicity assessments. Methods Mol. Biol. 2019, 1965, 7–17. [Google Scholar] [CrossRef]
- Gardner, N.M.; Riley, R.T.; Showker, J.L.; Voss, K.A.; Sachs, A.J.; Maddox, J.R.; Gelineau-Van Waes, J.B. Elevated nuclear sphingoid base-1-phosphates and decreased histone deacetylase activity after fumonisin B1 treatment in mouse embryonic fibroblasts. Toxicol. Appl. Pharmacol. 2016, 298, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jaman, S.; Islam, M.Z.; Sojib, M.S.; Hasan, M.S.; Khandakar, M.M.; Bari, M.S.; Sarker, M.A.; Habib, R.; Siddiki, M.S.; Islam, M.A.; et al. Physicochemical characteristics, sensory profile, probiotic, and starter culture viability of synbiotic yogurt. J. Adv. Vet. Anim. Res. 2022, 9, 694. [Google Scholar] [CrossRef]
- Azari-Anpar, M.; Payeinmahali, H.; Garmakhany, A.D.; Mahounak, A.S. Physicochemical, microbial, antioxidant, and sensory properties of probiotic stirred yoghurt enriched with Aloe vera foliar gel. J. Food Process. Preserv. 2017, 41, 13209. [Google Scholar] [CrossRef]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef]
- Hasani, S.; Khodadadi, I.; Heshmati, A. Viability of Lactobacillus acidophilus in rice bran-enriched stirred yoghurt and the physicochemical and sensory characteristics of product during refrigerated storage. Int. J. Food Sci. Technol. 2016, 51, 2485–2492. [Google Scholar] [CrossRef]
- Altuntaş, S.; Korukluoglu, M. The impact of different commercial probiotic cultures with starters on technological, physicochemical and sensorial properties of a traditional yogurt-based appetizer “Cacik”. Mljekarstvo 2019, 69, 193–205. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, F.; Yang, M.; Lin, Y.; Han, S. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of valuable chemicals. Crit. Rev. Biotechnol. 2023, 44, 163–190. [Google Scholar] [CrossRef] [PubMed]
- De Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).