Antioxidant Activity and Other Characteristics of Lactic Acid Bacteria Isolated from Korean Traditional Sweet Potato Stalk Kimchi
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing Sweet Potato Stalk Kimchi
2.2. Sampling, pH, and Total Acidity
2.3. Sample Collection and Bacteria Isolation
2.4. Antimicrobial Properties of Bacteria Isolated from Sweet Potato Kimchi
2.5. Acid Tolerance of Selected Strains
2.5/viable cell counts in MRS broth at a pH of 6) × 100%
2.6. Bile Salt Tolerance of Selected Strains
2.7. Heat Resistance of Selected Strains
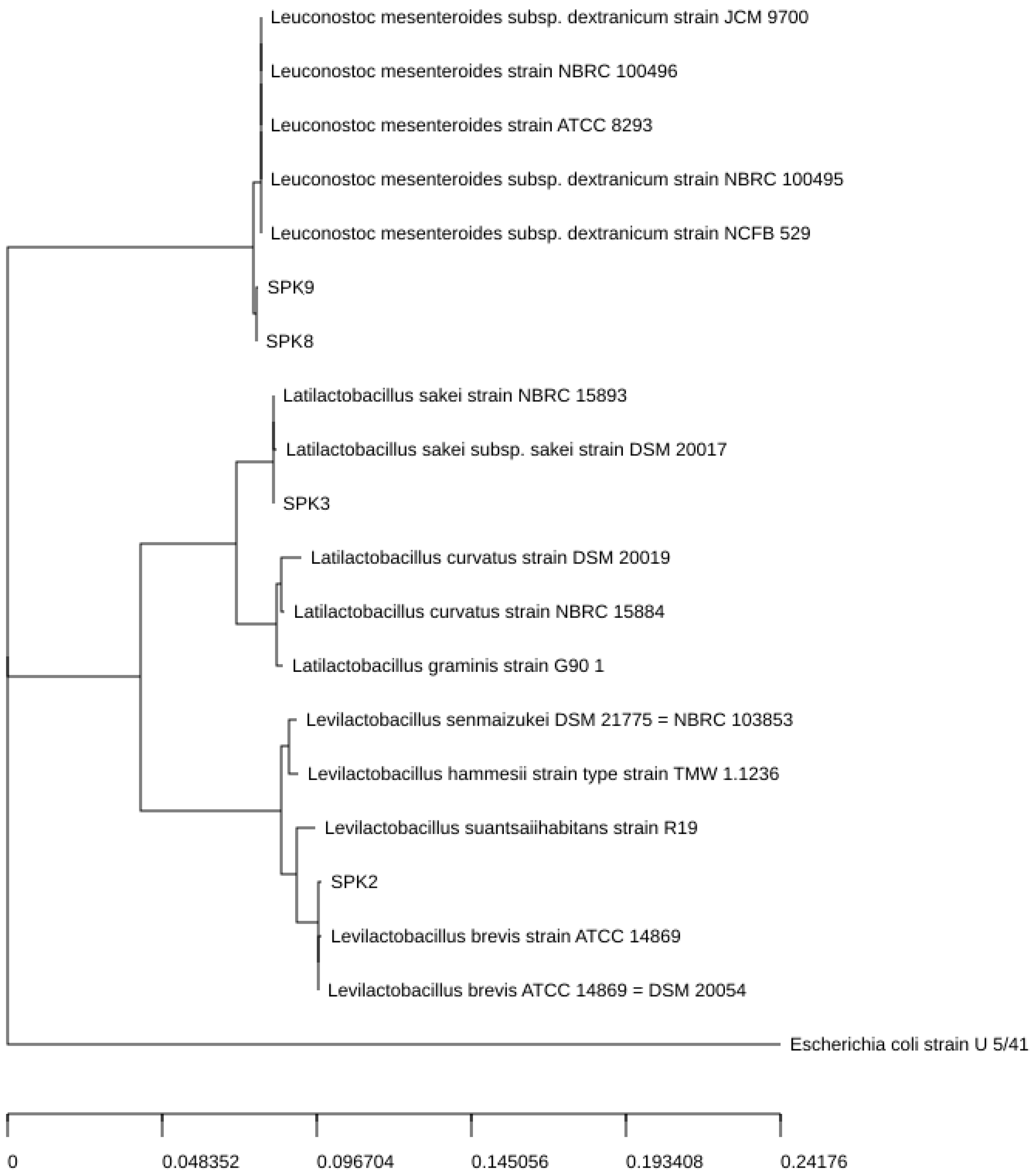
2.8. Molecular Identification of LAB and Phylogenetic Tree Construction of Selected Strains
2.9. Library Construction and Sequencing of Sweet Potato Kimchi
2.10. Data Processing and Amplicon Sequence Variant (ASV) Analysis
2.11. Antioxidant Activity of Sweet Potato Stalk Kimchi
2.11.1. Preparation of Sample
2.11.2. Measuring 2,2-Diphenyl-1-Picrylhydrazyl Radical Scavenging Activity
2.11.3. Determination of Total Polyphenols
2.12. Statistical Analysis
3. Result and Discussion
3.1. Antimicrobial Activity from Sweet Potato Stalk Kimchi
3.2. pH Tolerance
3.3. Bile Salt Reference
3.4. Heat Resistance
3.5. Identification of Isolated Bacteria
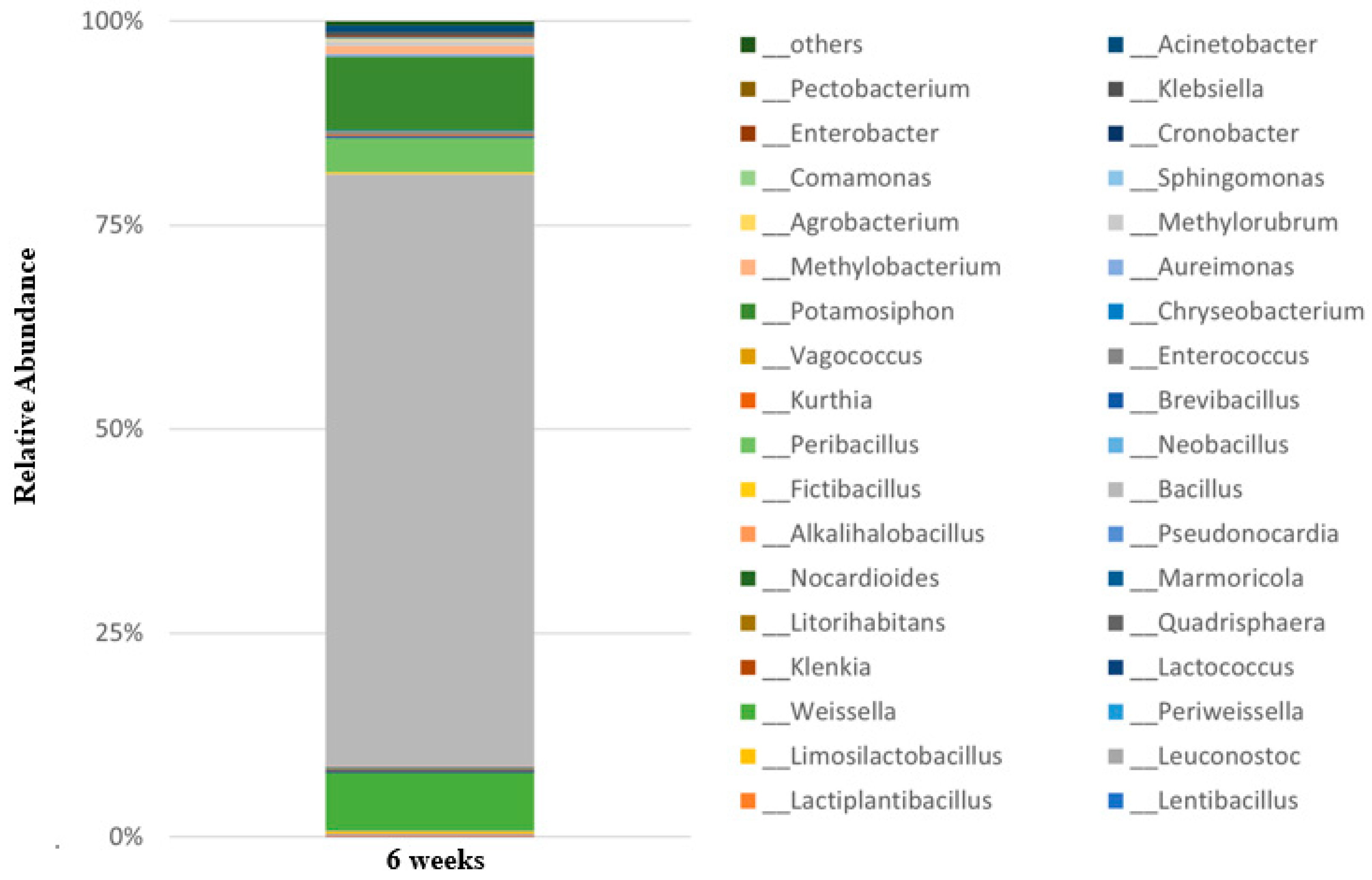
3.6. Microbial Community Analysis
3.7. DPPH Radical Scavenging
3.8. Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behera, S.; Chauhan, V.B.S.; Pati, K.; Bansode, V.; Nedunchezhiyan, M.; Verma, A.K.; Monalisa, K.; Naik, P.K.; Naik, S.K. Biology and biotechnological aspect of sweet potato (Ipomoea batatas L.): A commercially important tuber crop. Planta. 2022, 256, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, H.N.; Zhang, M.; Mu, T.-H.; Khan, N.M.; Ahmad, S.; Validov, S.Z. Fungal communities, nutritional, physiological and sensory characteristics of sweet potato under three Chinese representative storages. Postharvest Biol. Technol. 2023, 201, 112366. [Google Scholar] [CrossRef]
- FAOSTAT. Statistics Division of Food and Agriculture Organization of the United Nations. 2020. Available online: https://www.fao.org/faostat/zh/#data/QC (accessed on 10 June 2024).
- Alam, Z.; Akter, S.; Khan, A.H.; Alam, S.; Sultana, S.; Akhter, S.; Rahman, M.; Islam, M. Yield performance and trait correlation of BARI released sweet potato varieties studied under several districts of Bangladesh. Heliyon 2023, 9, e18203. [Google Scholar] [CrossRef] [PubMed]
- Mussoline, W.A.; Wilkie, A.C. Feed and fuel: The dual-purpose advantage of an industrial sweet potato. J. Sci. Food Agric. 2017, 97, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Endrias, D.; Rerra, N.; Desse, G. Comparison of three sweet potato (Ipomoea batatas (L.) Lam) varieties on nutritional and anti-nutritional factors. Glob. J. Sci. Front. Res. D Agric. Vet. 2016, 16, 63–72. [Google Scholar]
- Johnson, T.; Wilson, N.; Worosz, M.R.; Fields, D.; Bond, J.K. Commodity Highlight: Sweet Potatoes; U.S. Department of Agriculture: Washington, DC, USA, 2015; p. VGS-355-SA1.
- Jung, D.W.; Park, Y.K.; Nam, S.S.; Han, S.-K. Effect of hot-air drying temperature on nutritional components and rehydration rate of sweetpotato leaves. Korean J. Food Preserv. 2015, 22, 498–504. [Google Scholar] [CrossRef]
- Tang, C.; Han, J.; Chen, D.; Zong, S.; Liu, J.; Kan, J.; Qian, C.; Jin, C. Recent advances on the biological activities of purple sweet potato anthocyanins. Food Biosci. 2023, 53, 102670. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.H.; Sun, H.; Chen, J. Optimization of the formula and processing of a sweet potato leaf powder-based beverage. Food Sci. Nutr. 2020, 8, 2680–2691. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, N.K.; Paik, H.D. Health Benefits of Lactic Acid Bacteria Isolated from Kimchi, with Respect to Immunomodulatory Effects. Food Sci. Biotechnol. 2015, 24, 783–789. [Google Scholar] [CrossRef]
- Song, E.H.; Ang, L.; Lee, H.W.; Kim, M.-S.; Kim, Y.J.; Jang, D.; Lee, M.S. Effects of kimchi on human health: A scoping review of randomized controlled trials. J. Ethn. Foods 2023, 10, 7. [Google Scholar] [CrossRef]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kim, J.H.; Kwun, S.Y.; Lee, H.Y.; Park, E.H.; Kim, M.D. Review on the Physiochemical Characteristics and Functionality of Kimchi Mixed with Additives. J. Agric. Life Environ. Sci. 2018, 30, 111–120. [Google Scholar]
- Park, J.M.; Zhang, B.Z.; An, B.K. Effect of Fermentation Duration on the Quality Changes of Godulbaegi Kimchi. Foods 2022, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Sun, L.B.; Li, C.B.; Li, Z.-Z.; Zhang, Z.; Wen, X.-B.; Hu, Z.; Zhang, Y.-L.; Li, S.-K. Enhancement of the immune response and protection against Vibrio parahaemolyticus by indigenous probiotic Bacillus strains in mud crab (Scylla paramamosain). Fish Shellfish Immunol. 2014, 41, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluation antimicrobial activity. A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Archer, A.C.; Halami, P.M. Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl. Microbiol. Biotechnol. 2015, 99, 8113–8123. [Google Scholar] [CrossRef]
- Chen, Z.; Leng, X.; Zhou, F.; Shen, W.; Zhang, H.; Yu, Q.; Meng, X.; Fan, H.; Qin, M. Screening and identification of probiotic Lactobacilli from the infant gut microbiota to alleviate lead toxicity. Probiot. Antimicrob. Proteins 2022, 21, 9895. [Google Scholar] [CrossRef]
- Feng, Y.; Qiao, L.; Liu, R.; Yao, H.; Gao, C. Potential probiotic properties of lactic acid bacteria isolated from the intestinal mucosa of healthy piglets. Ann. Microbiol. 2017, 67, 239–253. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chang, F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity or red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef]
- Oun, A.A.; Roy, S.; Shin, G.H.; Yoo, S.; Kim, J.T. pH sensitive smart indicators based on cellulose and different natural pigments for tracing kimchi ripening stages. Int. J. Biol. Macromol. 2023, 242, 1–24905. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin-Cicocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Choi, S.J.; Yang, S.Y.; Yoon, K.S. Lactic acid bacteria starter in combination with sodium chloride controls pathogenic Escherichia coli (EPEC, ETEC, and EHEC) in kimchi. Food Microbiol. 2021, 100, 103868. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Nicolaides, L. Review of the sensitivity of different foodborne pathogens to fermentation. Food Cont. 1997, 8, 227–239. [Google Scholar] [CrossRef]
- Lee, C.H. Lactic acid fermented foods and their benefit in Asia. Food Cont. 1997, 8, 259–269. [Google Scholar] [CrossRef]
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and selection of Lactobacillus sakei strains isolated from traditional dry sausage for their potential use as starter cultures. Food Microbiol. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Cont. 2013, 32, 379–385. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and physical properties of protein films added with cell-free supernatant of Lactobacillus rhamnosus. Food Cont. 2016, 62, 44–51. [Google Scholar] [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum. 2018, 9, 301–306. [Google Scholar]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol against Salmonella Typhi and Salmonella Typhimurium virulence via autoinducer-2 and biofilm interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Kaur, G.; Malik, R.K.; Schillinger, U.; Guigas, C.; Kapila, S. Characterization of intestinal Lactobacillus reuteri strains as potential probiotics. Probiotics Antimicrob. Proteins 2012, 4, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C.; O’mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-R.; Kim, D.-R.; Kim, T.-W.; Park, S.-H.; Kim, H.-J.; Jang, J.-Y.; Han, E.-S. Selection of probiotic bacteria from yulmoo kimchi using a stimulated human intestinal model system. Korean J. Food Nutr. 2012, 41, 396–401. [Google Scholar] [CrossRef]
- Lee, K.W.; Shim, J.M.; Park, S.-K.; Heo, H.-J.; Kim, H.-J.; Ham, K.-S.; Kim, J.H. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT Food Sci. Technol. 2016, 71, 130–137. [Google Scholar] [CrossRef]
- Tulumoglu, S.; Yuksekdag, Z.N.; Beyatli, Y.; Simsek, O.; Cinar, B.; Yaşar, E. Probiotic properties of lactobacilli species isolated from children’s feces. Anaerobe 2013, 24, 36–42. [Google Scholar] [CrossRef]
- Anandharaj, M.; Sivasankari, B. Isolation of potential probiotic Lactobacillus oris HMI68 from mother’s milk with cholesterolreducing property. J. Biosci. Bioeng. 2014, 118, 153–159. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fonden, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Walker, D.K.; Gilliland, S.E. Relationship among bile tolerance, bile salt deconjugation, and assimilation of cholesterol by Lactobacillus acidophilus. J. Dairy. Sci. 1993, 76, 951–961. [Google Scholar] [CrossRef]
- Park, S.-H.; Yang, S.; Lee, J.-H.; Kang, M. Selection of Phytate-degrading Lactic Acid Bacteria from Kimchi and Reaction Properties in Brown Rice. J. Korean Soc. Food Sci. Nutr. 2013, 42, 627–632. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, H.S.; Yoo, J.S.; Cho, Y.A.; Kim, C.-H. Antioxidant activity of lactic acid bacteria isolated from Korean traditional food kimchi. J. Dairy Sci. Biotechnol. 2020, 38, 89–97. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Kim, S.-H.; Choi, Y.-R.; Lee, D.-H.; Lee, C.-Y.; Huh, C.-K. Antibacterial activity and stability of Sophora flavescens and Schisandra chinensis extracts against Streptococcus mutans KCCM 40105. Korean J. Food Preserv. 2022, 29, 494–508. [Google Scholar] [CrossRef]
- Park, E.J.; Han, H.U.; Min, B.H. Isolation of Lactococci inhibiting Listeria monocytogenes from Kimchi habitat and its identification by 16S rDNA analysis. Korean J. Ecol. 1999, 22, 45–50. [Google Scholar]
- Ozogul, F.; Yazgan, H.; Ozogul, Y. Lactic acid bacteria: Lactobacillus acidophilus. In Encyclopedia of Dairy Sciences, 3rd ed.; Mc Sweeney, P.L., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 187–197. [Google Scholar]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef]
- Delcour, J.; Ferain, T.; Hols, P. Advances in the genetics of thermophilic lactic acid bacteria. Curr. Opin. Biotechnol. 2000, 11, 497–504. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Lee, N.-K.; Yang, S.-J.; Kim, W.-S.; Paik, H.-D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Vergel-Suarez, A.H.; García-Martínez, J.B.; López-Barrera, G.L.; Barajas-Solano, A.F.; Zuorro, A. Impact of Biomass Drying Process on the Extraction Efficiency of C-Phycoerythrin. BioTech 2023, 12, 30. [Google Scholar] [CrossRef]
- Carmona, R.; Murillo, M.C.; Lafarga, T.; Bermejo, R. Assessment of the Potential of Microalgae-Derived Phycoerythrin as a Natural Colorant in Beverages. J. Appl. Phycol. 2022, 34, 3025–3034. [Google Scholar] [CrossRef]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some Leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kandasamy, S.; Kavitake, D.; Shetty, P.H. Probiotic characterization and antioxidant properties of Weissella confusa KR780676, isolated from an Indian fermented food. LWT Food Sci. Technol. 2018, 97, 53–60. [Google Scholar] [CrossRef]
- Fairfax, M.R.; Lephart, P.R.; Salimnia, H. Weissella confusa: Problems with identification of an opportunistic pathogen that has been found in fermented foods and proposed as a probiotic. Front. Microbiol. 2014, 5, 00254. [Google Scholar] [CrossRef]
- Choi, H.J.; Cheigh, C.I.; Kim, S.B.; Lee, J.C.; Lee, D.W.; Choi, S.W.; Park, J.M.; Pyun, Y.R. Weissella kimchii sp. nov., a novel lactic acid bacterium from kimchi. Int. J. Syst. Evol. Microbiol. 2002, 52, 507–511. [Google Scholar] [CrossRef]
- Kim, M.; Chun, J. Bacterial community structure in kimchi, a Korean fermented vegetable food, as revealed by 16S rRNA gene analysis. Int. J. Food Microbiol. 2005, 103, 91–96. [Google Scholar] [CrossRef]
- Dey, D.K.; Koo, B.G.; Sharma, C.; Kang, S.C. Characterization of Weissella confusa DD_A7 isolated from kimchi. LWT - Food Sci. Technol. 2019, 111, 663–672. [Google Scholar] [CrossRef]
- Qiao, N.; Bechtner, J.; Cnockaert, M.; Depoorter, E.; Díaz-Muñoz, C.; Vandamme, P.; De Vuyst, L.; Gänzle, M.G. Comparative genomic analysis of Periweissella and the characterization of novel motile species. Food Microbiol. 2023, 89, e0103423. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Sim, K.; Han, Y. Effect of red pepper seed on kimchi antioxidant activity during fermentation. Food Sci. Biotechnol. 2008, 17, 295–301. [Google Scholar]
- Park, M.J.; Jeon, Y.S.; Han, J.S. Antioxidative activity of mustard leaf Kimchi added green tea and pumpkin powder. J. Korean Soc. Food Sci. Nutr. 2001, 30, 1053–1059. [Google Scholar]
- Borowska, S.; Brzoska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.Y.; Ohmura, R.; Toshima, S.; Park, H.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Changes in polyphenols, anthocyanins, and DPPH radical-scavenging activities in sweetpotato (Ipomoea batatas L.) during tuber growth. Sci. Hortic. 2021, 284, 110100. [Google Scholar] [CrossRef]
- Luo, C.L.; Zhou, Q.; Yang, Z.W.; Wang, R.-D.; Zhang, J.-L. Evaluation of structure and bioprotective activity of key high molecular weight acylated anthocyanin compounds isolated from the purple sweet potato (Ipomoea batatas L. cultivar Eshu No.8). Food Chem. 2018, 241, 23–31. [Google Scholar] [CrossRef]
- Islam, M.S.; Yoshimoto, M.; Ishiguro, K.; Yamakawa, O. Bioactive and functional properties of Ipomoea batatas L. leaves. Acta. Hortic. 2003, 628, 693–699. [Google Scholar] [CrossRef]
- Nakayama, T.; Niimi, T.; Osawa, T.; Kawakishi, S. The protective role of polyphenols in cytotoxicity of hydrogen peroxide. Mutat. Res. Lett. 1992, 281, 77–80. [Google Scholar] [CrossRef]
- Korus, A.; Bernas, E.; Korus, J. Health-Promoting Constituents and Selected Quality Parameters of Different Types of Kimchi: Fermented Plant Products. Int. J. Food Sci. 2021, 9925344. [Google Scholar] [CrossRef]
- Hwang, J.H.; Song, Y.O.; Cheigh, H.S. Fermentation characteristics and antioxidative effect of red mustard leaf kimchi. J. Korean Soc. Food Sci. Nutr. 2000, 29, 1009–1015. [Google Scholar]
- Kim, M.J.; Park, H.S.; Lee, C.I.; Kim, S.H.; Kim, P.N.; Huh, W.; Lee, D.Y.; Son, J.C. Component analysis and antioxidant effects of Youngia sonchifolia max. J. Food Hyg. Saf. 2010, 25, 354–359. [Google Scholar]
- Özer, C.; Yıldırım, H.K. Some special properties of fermented products with cabbage origin: Pickled cabbage, sauerkraut and kimchi. Turk. J. Agric. Food Sci. Technol. 2019, 7, 490–497. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total phenolics and anthocyanins contents and antioxidant activity in four different aerial parts of leafy sweet potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Hassan, S.E.; Fatima, M.; Chaouch, M.; Chaqroune, A.; Taleb, M. Effects of extraction technique and solvent on phytochemicals, antioxidant, and antimicrobial activities of cultivated and wild rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco). Biointerface Res. Appl. Chem. 2021, 12, 8441–8452. [Google Scholar]
- Zeroual, A.; Sakar, E.H.; Eloutassi, N.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A. Wild chamomile [Cladanthus mixtus(L.) Chevall.] collected from central-northern Morocco: Phytochemical profiling, antioxidant, and antimicrobial activities. Biointerface Res. Appl. Chem. 2021, 11, 11440–11457. [Google Scholar]
- Islam, S. Sweetpotato (Ipomoea batatas L.) Leaf: Its Potential Effect on Human Health and Nutrition. J. Food Sci. 2006, 71, 1. [Google Scholar] [CrossRef]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Ulrih, N.P. Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]


| Strains | Negative Control | Species Clear Zone Surrounding the Disc (mm) | |||
|---|---|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Salmonella Typhimurium | Listeria monocytogenes | ||
| 2 | - | +++ | - | + | - |
| 3 | - | ++ | - | ++ | - |
| 8 | - | ++ | - | + | - |
| 9 | - | + | - | ++ | - |
| Strains | Tolerance to Low pH (log CFU/mL) | Tolerance to Bile Salt (log CFU/mL) | ||||
|---|---|---|---|---|---|---|
| Initial Population | After 3 h | Survival Rate (%) | 0% Bile Salt | 0.3% Bile Salt | Survival Rate (%) | |
| 2 | 10.39 ± 0.31 a | 8.40 ± 0.10 a,b | 81.28 a,b | 6.82 ± 0.04 c | 5.74 ± 0.03 a | 84.15 ± 0.52 b |
| 3 | 10.31 ± 0.07 a | 8.52 ± 0.05 a | 82.61 a | 9.67 ± 0.47 a | 6.25 ± 0.60 a | 64.64 ± 5.83 d |
| 8 | 10.47 ± 0.08 a | 8.38 ± 0.08 a,b | 79.99 b,c | 8.72 ± 0.10 b | 6.16 ± 0.24 a | 70.59 ± 2.02 c |
| 9 | 10.51 ± 0.12 a | 8.26 ± 0.05 b | 78.66 c | 6.50 ± 0.06 c | 6.13 ± 0.08 a | 94.23 ± 0.93 a |
| Strains | Total Phenol Content (µg of GAEs/mg Extract) |
|---|---|
| SPK 2 | 44.96 ± 0.29 |
| SPK 3 | 22.53 ± 0.28 |
| SPK 8 | 34.76 ± 1.29 |
| SPK 9 | 29.56 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Moon, J.-W.; Zhang, B.-Z.; An, B.-K. Antioxidant Activity and Other Characteristics of Lactic Acid Bacteria Isolated from Korean Traditional Sweet Potato Stalk Kimchi. Foods 2024, 13, 3261. https://doi.org/10.3390/foods13203261
Park J-M, Moon J-W, Zhang B-Z, An B-K. Antioxidant Activity and Other Characteristics of Lactic Acid Bacteria Isolated from Korean Traditional Sweet Potato Stalk Kimchi. Foods. 2024; 13(20):3261. https://doi.org/10.3390/foods13203261
Chicago/Turabian StylePark, Jung-Min, Ji-Woon Moon, Bo-Zheng Zhang, and Byoung-Ki An. 2024. "Antioxidant Activity and Other Characteristics of Lactic Acid Bacteria Isolated from Korean Traditional Sweet Potato Stalk Kimchi" Foods 13, no. 20: 3261. https://doi.org/10.3390/foods13203261
APA StylePark, J.-M., Moon, J.-W., Zhang, B.-Z., & An, B.-K. (2024). Antioxidant Activity and Other Characteristics of Lactic Acid Bacteria Isolated from Korean Traditional Sweet Potato Stalk Kimchi. Foods, 13(20), 3261. https://doi.org/10.3390/foods13203261







