Antibacterial Effects of Thermosonication Technology on Salmonella typhimurium Strains Identified from Swine Food Chain: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Ultrasound Treatments
2.3. Microbiological Analysis
2.4. Statistical Analysis
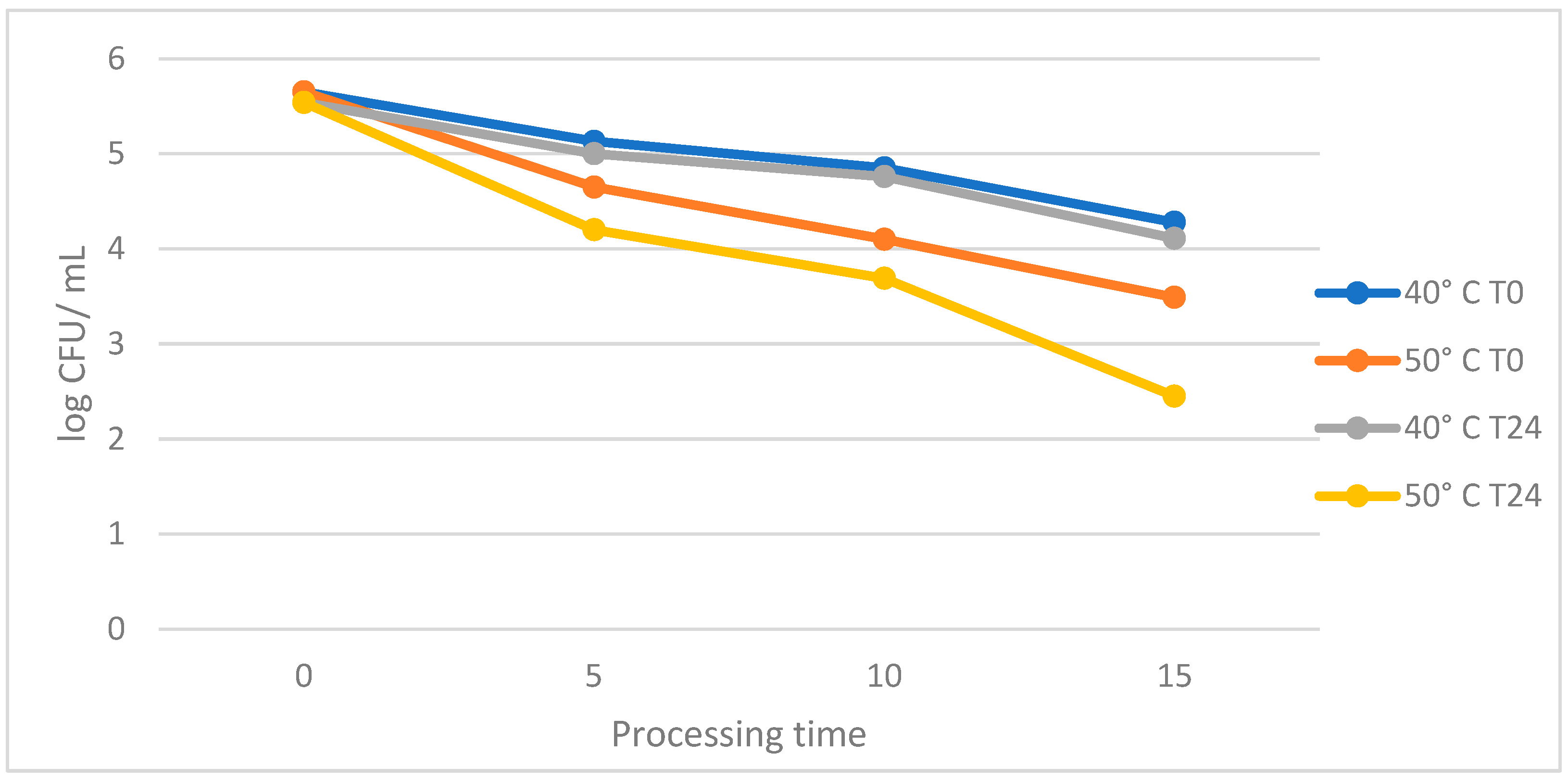
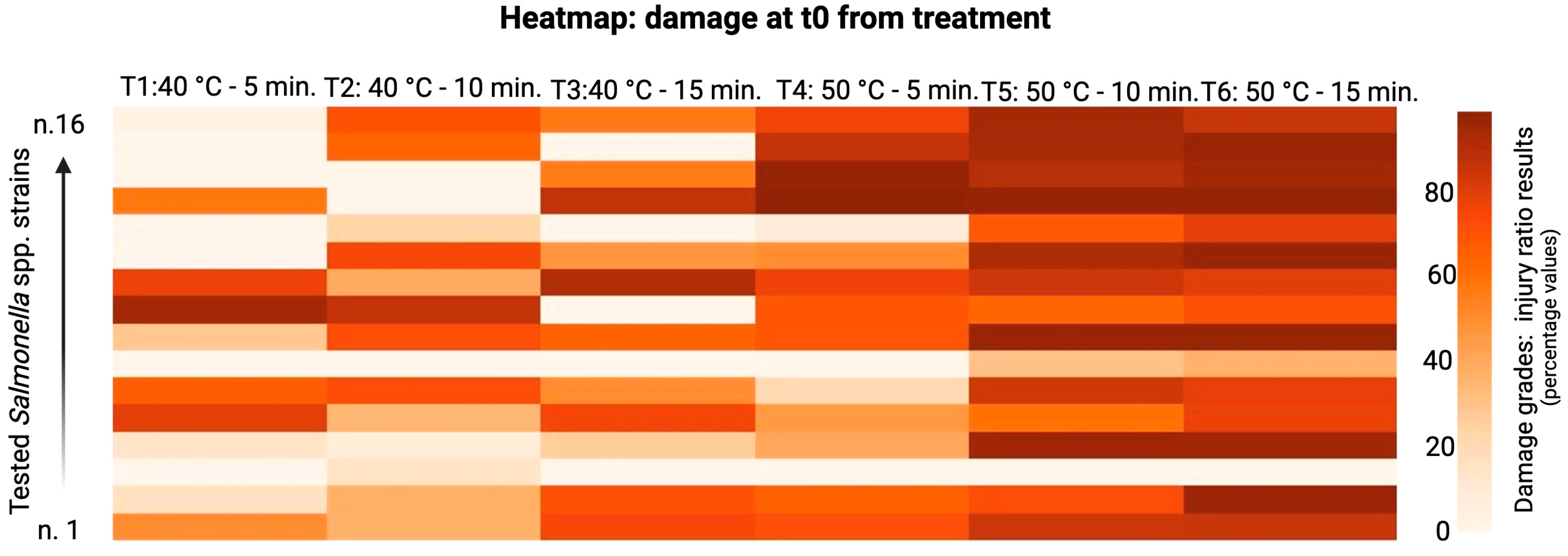
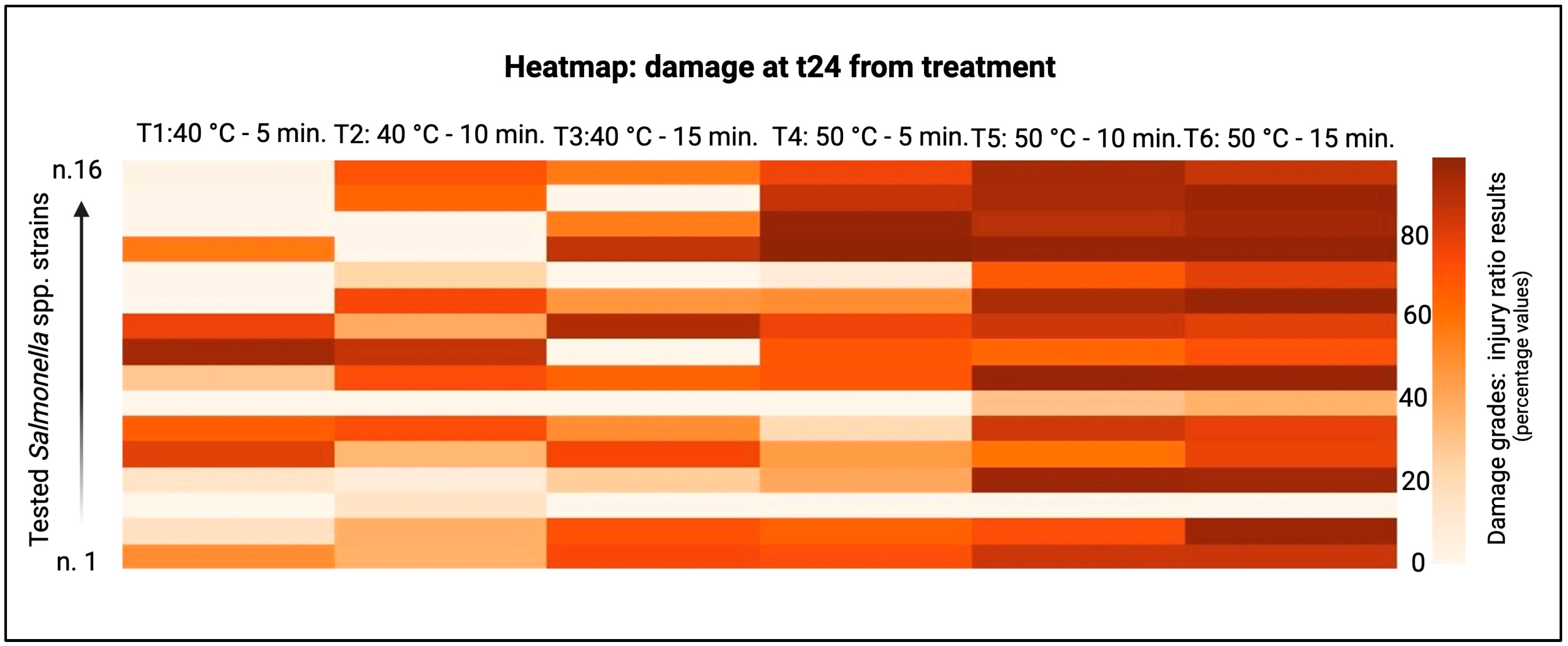
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cebrián, G.; Mañas, P.; Condón, S. Comparative Resistance of Bacterial Foodborne Pathogens to Non-thermal Technologies for Food Preservation. Front. Microbiol. 2016, 7, 734. [Google Scholar] [CrossRef]
- European Regulation No. 2073/2005. Microbiological Criteria for Foodstuffs. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601 (accessed on 12 February 2024).
- Taha, A.; Mehany, T.; Pandiselvam, R.; Anusha Siddiqui, S.; Mir, N.A.; Malik, M.A.; Sujayasree, O.J.; Alamuru, K.C.; Khanashyam, A.C.; Casanova, F.; et al. Sonoprocessing: Mechanisms and recent applications of power ultrasound in food. Crit. Rev. Food Sci. Nutr. 2024, 64, 6016–6054. [Google Scholar] [CrossRef]
- Zhou, C.; Okonkwo, C.E.; Inyinbor, A.A.; Yagoub, A.E.A.; Olaniran, A.F. Ultrasound, infrared and its assisted technology, a promising tool in physical food processing: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2023, 63, 1587–1611. [Google Scholar] [CrossRef]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef]
- Beitia, E.; Gkogka, E.; Chanos, P.; Hertel, C.; Heinz, V.; Valdramidis, V.; Aganovic, K. Microbial decontamination assisted by ultrasound-based processing technologies in food and model systems: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2802–2849. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, W.J. Inactivation of Escherichia coli O157: H7 in foods by emerging technologies: A review. Lett. Appl. Microbiol. 2023, 76, ovac007. [Google Scholar] [CrossRef]
- Hurst, A. Bacterial injury: A review. Can. J. Microbiol. 1977, 23, 935–944. [Google Scholar] [CrossRef]
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, Sublethal Injury, Resuscitation, and Virulence of Bacterial Foodborne Pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal Injury and Viable but Non-culturable (VBNC) State in Microorganisms During Preservation of Food and Biological Materials by Non-thermal Processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Wu, D.; Forghani, F.; Banan-Mwine Daliri, E.; Li, J.; Liao, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Microbial response to some nonthermal physical technologies. Trends Food Sci. Technol. 2019, 95, 107–117. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, M.Y.; Ehrenberg, M. Optimal control of gene expression for fast proteome adaptation to environmental change. Proc. Nat. Acad. Sci. USA 2013, 110, 20527–20532. [Google Scholar] [CrossRef] [PubMed]
- Wesche, A.M.; Marks, B.P.; Ryser, E.T. Thermal resistance of heat-, cold-, and starvation-injured Salmonella in irradiated comminuted Turkey. J. Food Prot. 2005, 68, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Obolski, U.; Hadany, L. Implications of stress-induced genetic variation for minimizing multidrug resistance in bacteria. BMC Med. 2012, 10, 89. [Google Scholar] [CrossRef]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of Ultrasound-Induced Damage to Escherichia coli and Staphylococcus aureus by Flow Cytometry and Transmission Electron Microscopy. Appl. Environ. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef]
- Waveco® System (Next Cooking Generation, Milan, Italy)—International Application No.: Patent EP17733039.6.
- ISO 6579-1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: London, UK, 2020. Available online: https://www.iso.org/standard/76671.html (accessed on 15 January 2024).
- Han, J.Y.; Song, W.J.; Kang, D.H. Optimization of broth recovery for repair of heat-injured Salmonella enterica serovar Typhimurium and Escherichia coli O157:H7. J. Appl. Microbiol. 2019, 126, 1923–1930. [Google Scholar] [CrossRef]
- Bi, X.; Wang, Y.; Hu, X.; Liao, X. Decreased resistance of sublethally injured Escherichia coli O157:H7 to salt, mild heat, nisin and acids induced by high pressure carbon dioxide. Int. J. Food Microbiol. 2018, 269, 137–143. [Google Scholar] [CrossRef]
- Sienkiewicz, J.J.; Wesołowski, A.; Stankiewicz, W.; Kotowski, R. The influence of ultrasonic treatment on the growth of the strains of Salmonella enterica subs. typhimurium. J. Food Sci. Technol. 2017, 54, 2214–2223. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Magnin, J.P.; Commenges-Bernole, N.; Gondrexon, N.; Willison, J.; Petrier, C. Effectiveness of ultrasound for the destruction of Mycobacterium sp. strain (6PY1). Ultrason. Sonochem. 2010, 17, 106–110. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high-power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020, 60, 104763. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, X.; Liu, Y.; Hu, J.; Yang, C.; Zhao, X. The effects of ultrasound on the growth, nutritional quality and microbiological quality of sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- Valero, M.; Recrosio, N.; Saura, D.; Munoz, N.; Marti, N.; Lizama, V. Effects of ultrasonic treatments in orange juice processing. J. Food Eng. 2007, 80, 509–516. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Chen, Y.; Wang, Y.; Li, R.; Tang, J.; Geng, F. Quantitative proteomic analysis provides insight into the survival mechanism of Salmonella typhimurium under high-intensity ultrasound treatment. Curr. Res. Food Sci. 2022, 5, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Lauteri, C.; Pennisi, L.; Di Clerico, D.; Pennisi, V.; Vergara, A. Low frequency focused thermosonication for Salmonella typhimurium inactivation: In vitro study. Ital. J. Food Saf. 2024, 13, 12217. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.E.; Russell, A.D. The revival of injured microbes. Soc. Appl. 1984, 437, 1–394. [Google Scholar]
- Baumann, A.R.; Martin, S.E.; Feng, H. Power ultrasound treatment of Listeria monocytogenes in apple cider. J. Food Protect. 2005, 68, 2333–2340. [Google Scholar] [CrossRef]
- Pennisi, L.; Di Clerico, D.; Costantini, L.; Festino, A.R.; Vergara, A. Ultrasonic decontamination in smoked salmon experimentally contaminated with Listeria monocytogenes: Preliminary results. Ital. J. Food Saf. 2020, 9, 8398. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020, 105, 211–222. [Google Scholar] [CrossRef]
| Serovar | ID Strain | Origin |
|---|---|---|
| S. typhimurium | 114 | Meat product (sausage) |
| 115 | Meat product (sausage) | |
| 117 | Meat product (sausage) | |
| 118 | Meat product (sausage) | |
| 669 | Cecal sample | |
| 670 | Pig carcass | |
| 685 | Pig carcass | |
| 686 | Pig carcass | |
| 687 | Cecal sample | |
| 689 | Slaughtering environments | |
| 690 | Slaughtering environments | |
| 691 | Slaughtering environments | |
| 693 | Pig carcass | |
| 694 | Pig carcass | |
| 695 | Pig carcass | |
| 785 | Slaughtering environments |
| ID | C-T1 | r | C-T2 | r | C-T3 | r | C-T4 | r | C-T5 | r | C-T6 | r |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 114 | 0.81 | R | 1.93 | I | 1.20 | I | 1.45 | I | 2.05 | S | 2.20 | S |
| 115 | 0.56 | R | 1.39 | I | 1.25 | I | 1.34 | I | 1.92 | I | 4.59 | S |
| 117 | 1.71 | I | 1.90 | I | 2.16 | S | 1.98 | I | 2.06 | S | 3.96 | S |
| 118 | 1.01 | I | 1.81 | I | 2.16 | S | 2.60 | S | 4.61 | S | 3.81 | S |
| 669 | 0.95 | R | 1.62 | I | 0.88 | R | 1.62 | I | 1.92 | I | 2.40 | S |
| 670 | 1.44 | I | 1.15 | I | 0.89 | R | 2.96 | S | 1.45 | I | 3.39 | S |
| 685 | 1.89 | I | 1.65 | I | 1.50 | I | 2.22 | S | 1.89 | I | 2.70 | S |
| 686 | 1.08 | I | 1.69 | I | 1.54 | I | 2.11 | S | 2.23 | S | 2.59 | S |
| 687 | 1.41 | I | 1.07 | I | 0.57 | R | 2.92 | S | 1.30 | I | 3.22 | S |
| 689 | 1.62 | I | 2.07 | S | 1.49 | I | 1.50 | I | 2.15 | S | 2.87 | S |
| 690 | 0.61 | R | 0.99 | R | 1.14 | I | 2.50 | S | 3.64 | S | 2.13 | S |
| 691 | −0.20 | R | 0.63 | R | 0.75 | R | 1.54 | I | 1.96 | I | 3.12 | S |
| 693 | 0.58 | R | 1.52 | I | 1.67 | I | 1.58 | I | 2.14 | S | 2.62 | S |
| 694 | 0.49 | R | 0.56 | R | 2.24 | S | 1.01 | I | 2.54 | S | 2.74 | S |
| 695 | 0.56 | R | 1.23 | I | 1.55 | I | 1.55 | I | 1.66 | I | 3.16 | S |
| 785 | 2.28 | S | 2.89 | S | 1.56 | I | 3.09 | S | 2.21 | S | 3.39 | S |
| ID Strains | Treatments | SI t0 (%) | SI t24 (%) | ID Strains | Treatments | SI t0 (%) | SI t24 (%) | ID Strains | Treatments | SI t0 (%) | SI t24 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 114 | 40 °C–5 min | 50.57 | 71.51 | 685 | 40 °C–5 min | 0.00 | 30.91 | 693 | 40 °C–5 min | 57.23 | 72.96 |
| 40 °C–10 min | 37.16 | 87.05 | 40 °C–10 min | 0.00 | 24.68 | 40 °C–10 min | 0.00 | 50.52 | |||
| 40 °C–15 min | 74.37 | 99.42 | 40 °C–15 min | 0.00 | 2.36 | 40 °C–15 min | 86.29 | 42.59 | |||
| 50 °C–5 min | 72.06 | 0.00 | 50 °C–5 min | 0.00 | 33.33 | 50 °C–5 min | 99.49 | 88.94 | |||
| 50 °C–10 min | 84.36 | 0.00 | 50 °C–10 min | 30.85 | 34.33 | 50 °C–10 min | 98.28 | 95.04 | |||
| 50 °C–15 min | 84.85 | 90.78 | 50 °C–15 min | 0.00 | 96.15 | 50 °C–15 min | 98.86 | 98.67 | |||
| 115 | 40 °C–5 min | 16.33 | 66.67 | 686 | 40 °C–5 min | 28.95 | 0.00 | 694 | 40 °C–5 min | 0.00 | 0.00 |
| 40 °C–10 min | 37.50 | 75.00 | 40 °C–10 min | 72.68 | 0.00 | 40 °C–10 min | 0.00 | 71.43 | |||
| 40 °C–15 min | 71.05 | 48.78 | 40 °C–15 min | 65.38 | 50.00 | 40 °C–15 min | 56.14 | 0.00 | |||
| 50 °C–5 min | 65.71 | 60.42 | 50 °C–5 min | 69.47 | 93.44 | 50 °C–5 min | 98.31 | 77.97 | |||
| 50 °C–10 min | 72.73 | 0.00 | 50 °C–10 min | 97.62 | 85.19 | 50 °C–10 min | 89.47 | 90.00 | |||
| 50 °C–15 min | 97.22 | 75.00 | 50 °C–15 min | 98.11 | 94.12 | 50 °C–15 min | 95.24 | 83.33 | |||
| 117 | 40 °C–5 min | 0.00 | 81.01 | 687 | 40 °C–5 min | 94.68 | 87.00 | 695 | 40 °C–5 min | 0.00 | 58.18 |
| 40 °C–10 min | 14.95 | 98.81 | 40 °C–10 min | 86.11 | 58.14 | 40 °C–10 min | 64.35 | 0.00 | |||
| 40 °C–15 min | 0.00 | 6.45 | 40 °C–15 min | 0.00 | 77.22 | 40 °C–15 min | 0.00 | 44.74 | |||
| 50 °C–5 min | 0.00 | 57.58 | 50 °C–5 min | 68.83 | 95.00 | 50 °C–5 min | 86.11 | 90.00 | |||
| 50 °C–10 min | 0.00 | 50.00 | 50 °C–10 min | 63.64 | 95.35 | 50 °C–10 min | 93.75 | 94.12 | |||
| 50 °C–15 min | 0.00 | 0.00 | 50 °C–15 min | 71.43 | 92.86 | 50 °C–15 min | 97.56 | 0.00 | |||
| 118 | 40 °C–5 min | 14.04 | 26.32 | 689 | 40 °C–5 min | 77.17 | 91.67 | 785 | 40 °C–5 min | 2.44 | 48.15 |
| 40 °C–10 min | 8.33 | 84.00 | 40 °C–10 min | 39.47 | 0.00 | 40 °C–10 min | 70.97 | 0.00 | |||
| 40 °C–15 min | 26.47 | 95.45 | 40 °C–15 min | 90.83 | 0.00 | 40 °C–15 min | 57.14 | 0.00 | |||
| 50 °C–5 min | 40.74 | 0.00 | 50 °C–5 min | 76.97 | 96.00 | 50 °C–5 min | 76.27 | 94.68 | |||
| 50 °C–10 min | 96.43 | 0.00 | 50 °C–10 min | 84.17 | 88.00 | 50 °C–10 min | 94.29 | 31.58 | |||
| 50 °C–15 min | 95.24 | 90.91 | 50 °C–15 min | 79.41 | 91.67 | 50 °C–15 min | 85.71 | 66.67 | |||
| 669 | 40 °C–5 min | 79.40 | 98.25 | 690 | 40 °C–5 min | 0.00 | 0.00 | ||||
| 40 °C–10 min | 34.84 | 60.87 | 40 °C–10 min | 74.92 | 0.00 | ||||||
| 40 °C–15 min | 75.44 | 70.83 | 40 °C–15 min | 47.25 | 88.24 | ||||||
| 50 °C–5 min | 45.77 | 0.00 | 50 °C–5 min | 50.24 | 64.09 | ||||||
| 50 °C–10 min | 59.93 | 99.04 | 50 °C–10 min | 92.37 | 97.58 | ||||||
| 50 °C–15 min | 77.50 | 0.00 | 50 °C–15 min | 97.87 | 50.00 | ||||||
| 670 | 40 °C–5 min | 67.09 | 0.00 | 691 | 40 °C–5 min | 0.00 | 0.00 | ||||
| 40 °C–10 min | 72.58 | 71.74 | 40 °C–10 min | 23.08 | 0.00 | ||||||
| 40 °C–15 min | 50.56 | 0.00 | 40 °C–15 min | 0.00 | 82.08 | ||||||
| 50 °C–5 min | 20.00 | 0.00 | 50 °C–5 min | 9.09 | 0.00 | ||||||
| 50 °C–10 min | 83.72 | 94.12 | 50 °C–10 min | 68.66 | 56.72 | ||||||
| 50 °C–15 min | 78.57 | 85.29 | 50 °C–15 min | 79.17 | 98.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennisi, L.; Ferri, G.; Lauteri, C.; Di Clerico, D.; Vergara, A. Antibacterial Effects of Thermosonication Technology on Salmonella typhimurium Strains Identified from Swine Food Chain: An In Vitro Study. Foods 2024, 13, 3259. https://doi.org/10.3390/foods13203259
Pennisi L, Ferri G, Lauteri C, Di Clerico D, Vergara A. Antibacterial Effects of Thermosonication Technology on Salmonella typhimurium Strains Identified from Swine Food Chain: An In Vitro Study. Foods. 2024; 13(20):3259. https://doi.org/10.3390/foods13203259
Chicago/Turabian StylePennisi, Luca, Gianluigi Ferri, Carlotta Lauteri, Daniele Di Clerico, and Alberto Vergara. 2024. "Antibacterial Effects of Thermosonication Technology on Salmonella typhimurium Strains Identified from Swine Food Chain: An In Vitro Study" Foods 13, no. 20: 3259. https://doi.org/10.3390/foods13203259
APA StylePennisi, L., Ferri, G., Lauteri, C., Di Clerico, D., & Vergara, A. (2024). Antibacterial Effects of Thermosonication Technology on Salmonella typhimurium Strains Identified from Swine Food Chain: An In Vitro Study. Foods, 13(20), 3259. https://doi.org/10.3390/foods13203259






