Comparative Evaluation of Conventional and Emerging Maceration Techniques for Enhancing Bioactive Compounds in Aronia Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mash Maceration
- Cold maceration (CM)—the mash was stored at 4 °C for 12 h, slightly modifying the method described in Vagiri and Jensen (2017) [27]. Cold maceration is commonly used in processes like red winemaking, as it has practical benefits such as ease of implementation and low installation and maintenance costs [28]. Additionally, in this process, heat-sensitive compounds in the treated matrix remain largely unaltered due to the absence of heat [29]. Therefore, cold maceration was chosen as the control treatment because of its non-invasive nature and minimal processing impact on the properties of the aronia berries under study.
- Thermomaceration (TM) was carried out by heating the mash at 50 °C for 60 min, according to the method described in Lima et al. (2015) [30].
- Enzymatic maceration (EM) was performed by heating the mash until its internal temperature reached 50 °C, then pectolytic enzymes (ROHAPECT MC®) were added to achieve a concentration of 200 ppm with a holding time of 60 min at 50 °C, as described in Lima et al. (2015) [30]. The enzyme dosage, maceration time, and temperature were applied according to the manufacturers’ recommendations.
- Ultrasound-assisted maceration (US) was performed in an ultrasonic bath with a 250 W power and 37 kHz frequency at 60 °C for 15 min, as described in Lieu and Le (2010) [31].
- Microwave-assisted maceration (MW) was carried out according to the method described in Guler (2023) [9] by heating the fruit mash at a 600 W power for three cycles, with 2 min per cycle.
2.2. Determination of pH and Soluble Solids
2.3. Rheology
2.4. Colour Measurements
2.5. Determination and Quantification of Sugars and Organic Acids
2.6. Determination and Quantification of Phenolic Compounds
2.7. Analysis of Ascorbic Acid Content
2.8. Statistical Analysis
3. Results
3.1. PH and Soluble Solids
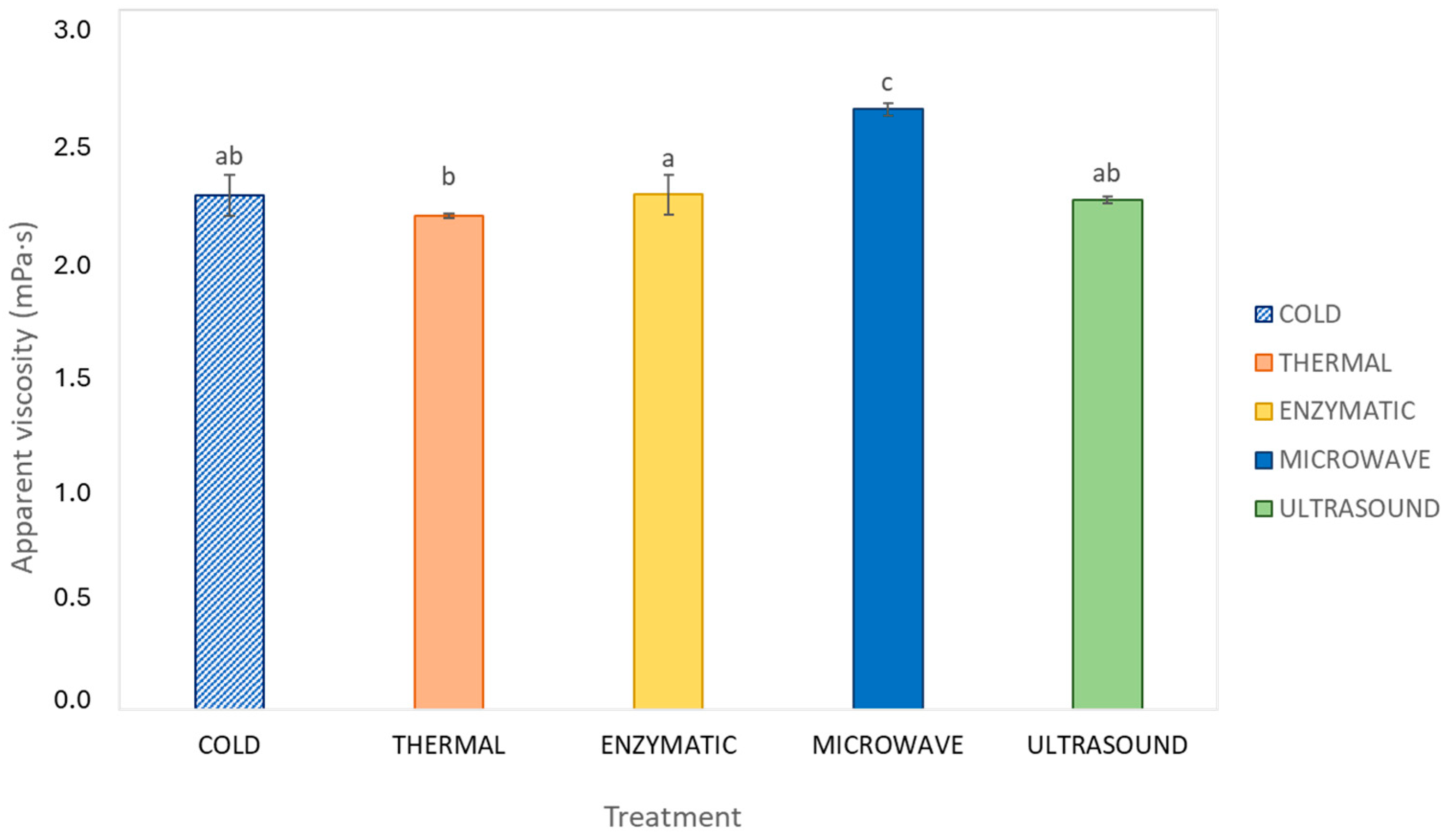
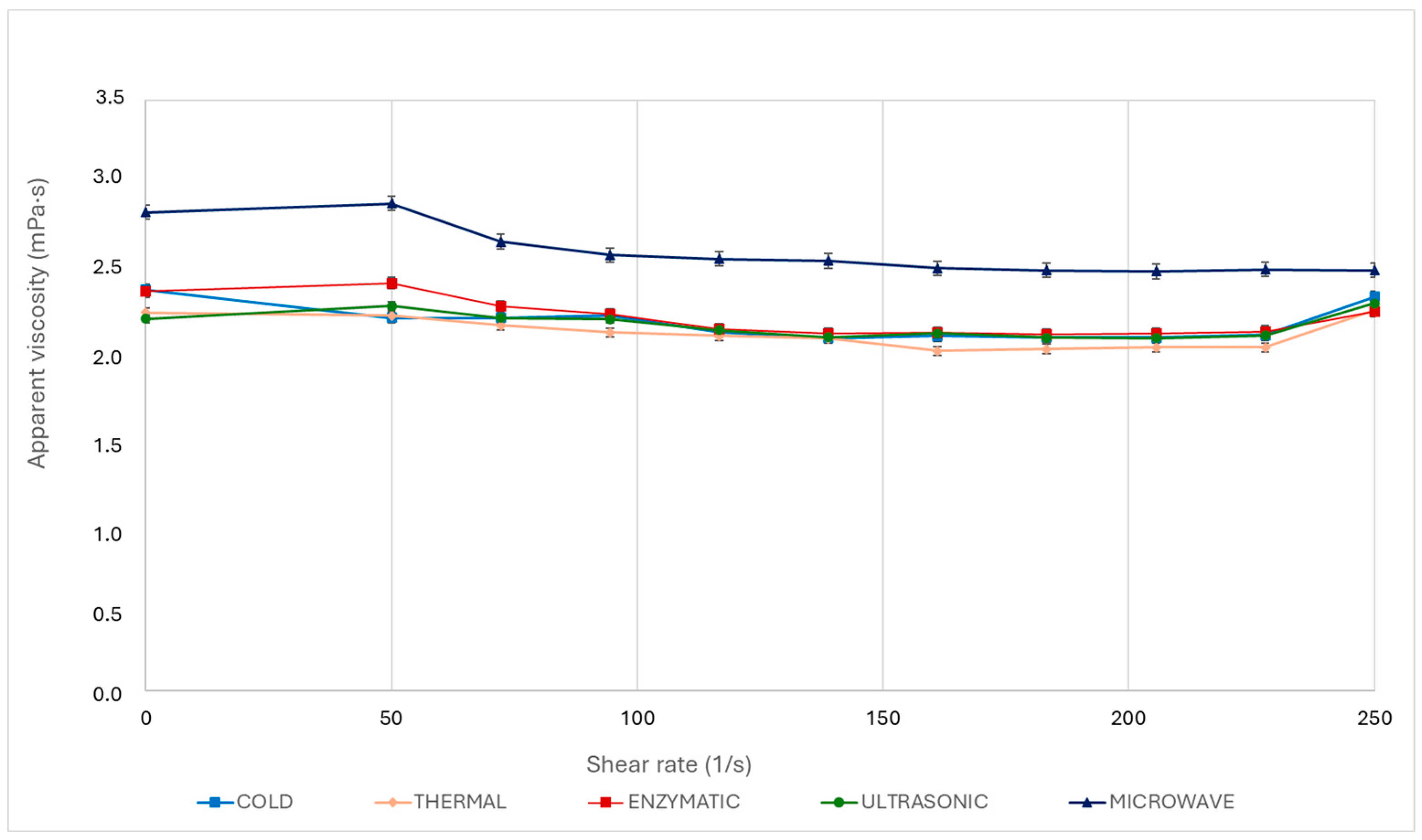
3.2. Rheological Behaviour
3.3. Colour
3.4. Sugars and Organic Acids
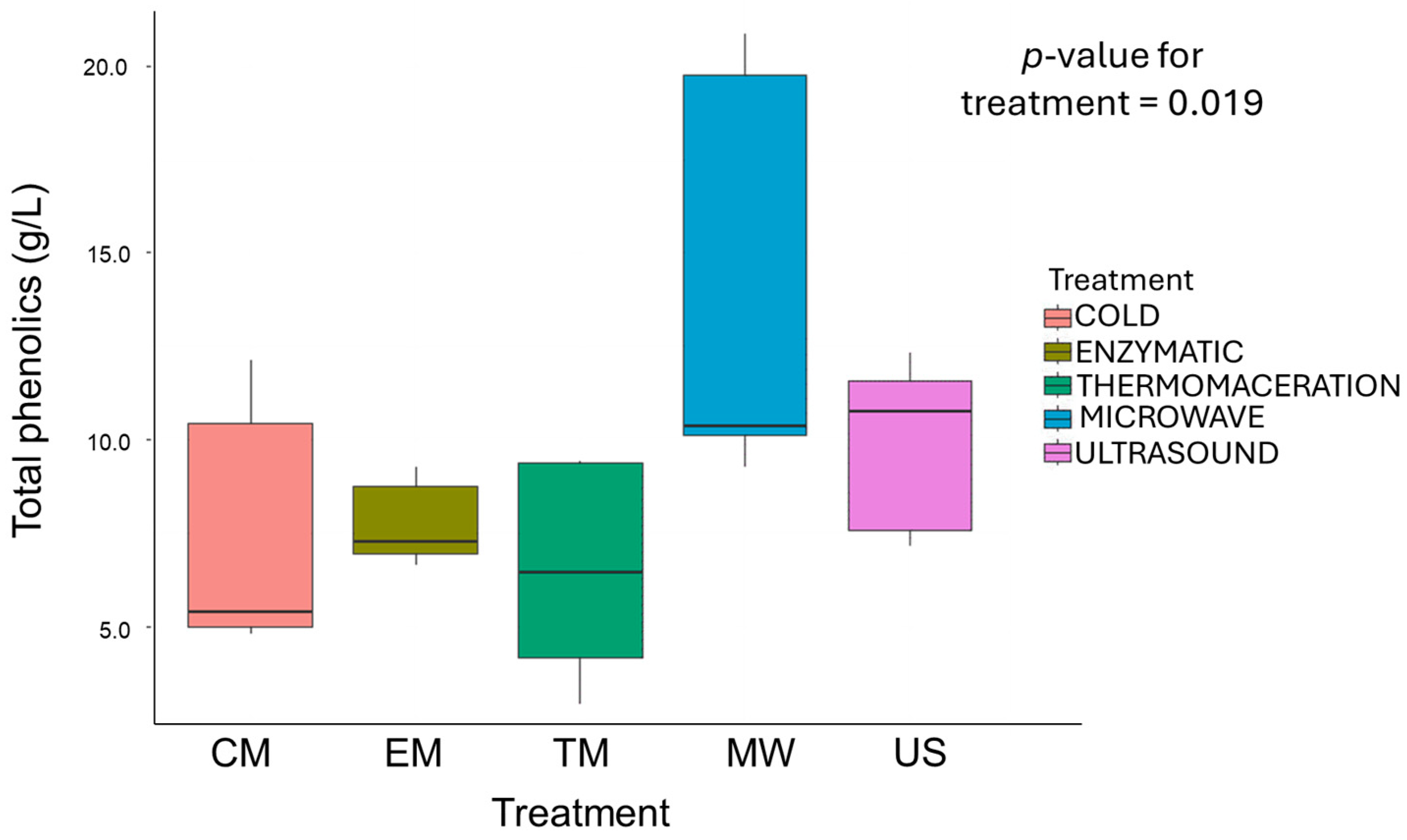
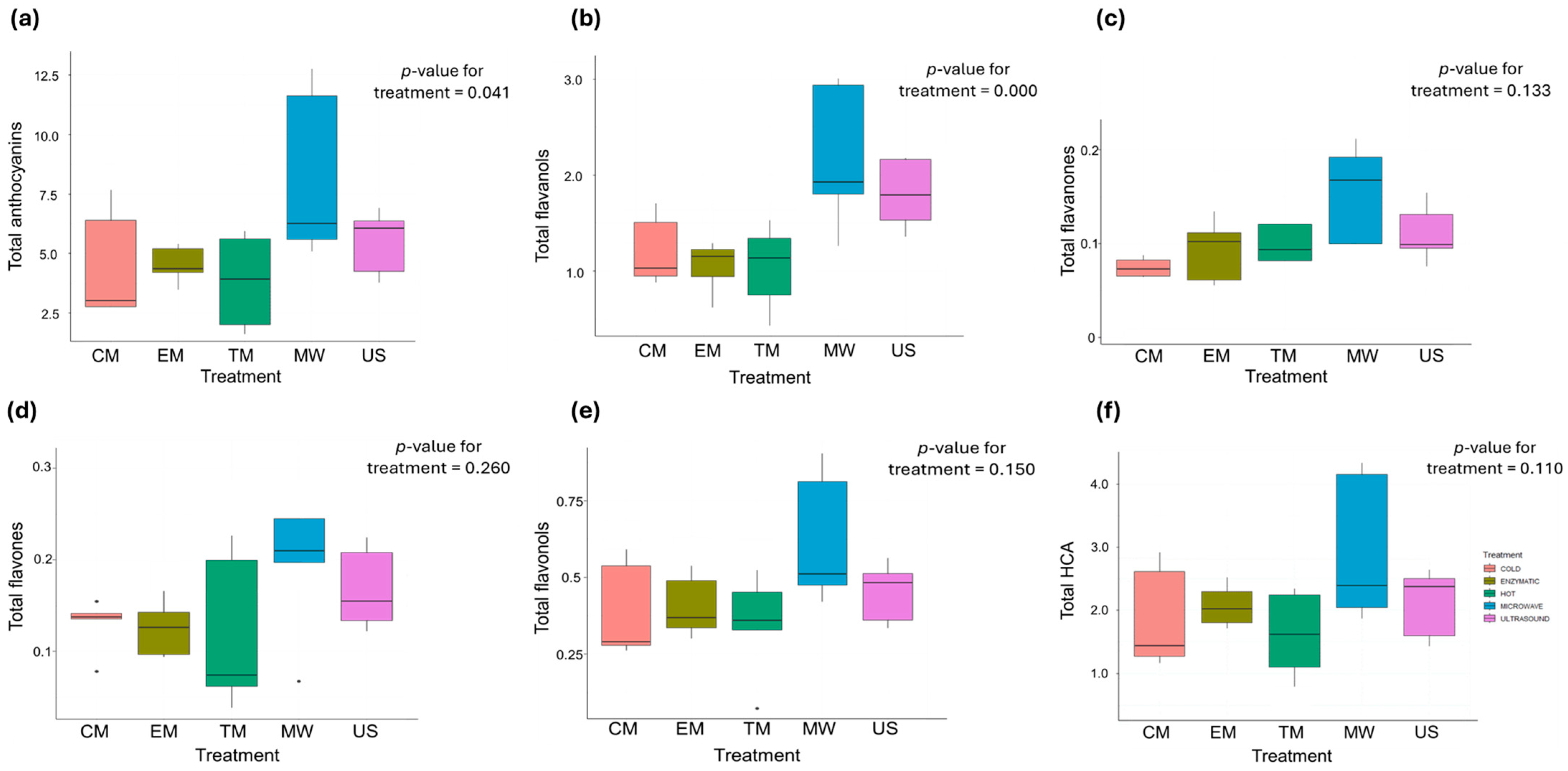
3.5. Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Błaszczak, W.; Amarowicz, R.; Górecki, A.R. Antioxidant Capacity, Phenolic Composition and Microbial Stability of Aronia Juice Subjected to High Hydrostatic Pressure Processing. Innov. Food Sci. Emerg. Technol. 2017, 39, 141–147. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia Melanocarpa Phenolics and Their Antioxidant Activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia Melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Betoret, E.; Taccari, A.; Dalla Rosa, M.; Bordoni, A. Impact of Processing on the Nutritional and Functional Value of Mandarin Juice. J. Sci. Food Agric. 2020, 100, 4558–4564. [Google Scholar] [CrossRef]
- Rajauria, G.; Tiwari, B.K. Chapter 1—Fruit Juices: An Overview. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 3–13. ISBN 978-0-12-802230-6. [Google Scholar]
- Fabjanowicz, M.; Różańska, A.; Abdelwahab, N.S.; Pereira-Coelho, M.; da Silva Haas, I.C.; dos Santos Madureira, L.A.; Płotka-Wasylka, J. An Analytical Approach to Determine the Health Benefits and Health Risks of Consuming Berry Juices. Food Chem. 2024, 432, 137219. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Szwengiel, A.; Ratajkiewicz, H.; Nowak, K. Effect of Ultrasound, Heating and Enzymatic Pre-Treatment on Bioactive Compounds in Juice from Berberis Amurensis Rupr. Ultrason. Sonochem. 2020, 63, 104971. [Google Scholar] [CrossRef]
- Guler, A. Effects of Different Maceration Techniques on the Colour, Polyphenols and Antioxidant Capacity of Grape Juice. Food Chem. 2023, 404, 134603. [Google Scholar] [CrossRef]
- Mathews, A.; Arbal, A.V.; Kaarunya, A.; Jha, P.K.; Le-Bail, A.; Rawson, A. Chapter Five—Conventional vs Modern Extraction Techniques in the Food Industry. In Extraction Processes in the Food Industry; Jafari, S.M., Akhavan-Mahdavi, S., Eds.; Unit Operation and Processing Equipment in the Food Industry; Woodhead Publishing: Sawston, UK, 2024; pp. 97–146. ISBN 978-0-12-819516-1. [Google Scholar]
- Alifakı, Ö.; Şakıyan, Ö.; Isci, A. Extraction of Phenolic Compounds from Cranberrybush (Viburnum opulus L.) Fruit Using Ultrasound, Microwave, and Ultrasound—Microwave Combination Methods. J. Food Meas. Charact. 2022, 16, 4009–4024. [Google Scholar] [CrossRef]
- Đurović, S.; Domínguez-Valencia, R.; Pateiro, M.; Teslić, N.; Lorenzo, J.M.; Pavlic, B. Industrial Hemp Nutraceutical Processing and Technology. In Industrial Hemp; Academic Press: Cambridge, MA, USA, 2022; pp. 191–218. ISBN 978-0-323-90910-5. [Google Scholar]
- Kernou, O.; Belbahi, A.; Kaanin, G.; Adel-Abderrahim, K.; Madani, K. Ultrasound-Microwave Technologies as Alternative Methods for Inactivation Bacterias in Fruit Juice. Int. J. Anal. Appl. Chem. 2023, 8, 31–40. [Google Scholar]
- Albuquerque, B.; Prieto, M.; Vázquez, J.; Barreiro, M.; Barros, L.; Ferreira, I. Recovery of Bioactive Compounds from Arbutus unedo L. Fruits: Comparative Optimization Study of Maceration/Microwave/Ultrasound Extraction Techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Piecko, J.; Mieszczakowska-Frąc, M.; Celejewska, K.; Szwejda-Grzybowska, J. Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit. Foods 2024, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Nemetz, N.J.; Winter, A.R.; Hensen, J.-P.; Schieber, A.; Weber, F. Toward Gentle Chokeberry Juice Production by Ultrasound-Assisted Enzymatic Maceration. Curr. Res. Food Sci. 2023, 6, 100518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of Temperature-Controlled Ultrasound Treatment on Sensory Properties, Physical Characteristics and Antioxidant Activity of Cloudy Apple Juice. LWT 2021, 142, 111030. [Google Scholar] [CrossRef]
- Demirok, N.T.; Yıkmış, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes 2022, 10, 2100. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of High-Intensity Ultrasound on Bioactive Compounds of Strawberry Juice: Profiles of Ascorbic Acid, Phenolics, Antioxidant Activity and Microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Galvan d’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound Assisted Extraction of Polyphenols from Black Chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and Antioxidant Activity of Anthocyanins from Aronia Melanocarpa Extracted Using an Ultrasonic-Microwave-Assisted Natural Deep Eutectic Solvent Extraction Method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef]
- Simić, V.; Stojičević, S.; Veličković, D.; Nikolić, N.; Lazić, M.; Karabegović, I. RSM Approach for Modeling and Optimization of Microwave-Assisted Extraction of Chokeberry. Adv. Technol. 2018, 7, 11–19. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.V.; González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; F. Barbero, G. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of Polyphenols Extraction from Dried Chokeberry Using Maceration as Traditional Technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Jurendić, T.; Dragović-Uzelac, V. Evaluation of Microwave- and Ultrasound-Assisted Extraction Techniques for Revalorization of Black Chokeberry (Aronia melanocarpa) Fruit Pomace Anthocyanins. Sustainability 2023, 15, 7047. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Aronia Melanocarpa by-Products from Filter-Tea Factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Vagiri, M.; Jensen, M. Influence of Juice Processing Factors on Quality of Black Chokeberry Pomace as a Future Resource for Colour Extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold Maceration Application in Red Wine Production and Its Effects on Phenolic Compounds: A Review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Subramanian, P.; Anandharamakrishnan, C. Chapter Two—Extraction of Bioactive Compounds. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals; Anandharamakrishnan, C., Subramanian, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 45–87. ISBN 978-0-12-824312-1. [Google Scholar]
- dos Santos Lima, M.; da Conceição Prudêncio Dutra, M.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced in Industrial Scale by Different Processes of Maceration. Food Chem. 2015, 188, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lieu, L.N.; Le, V.V.M. Application of Ultrasound in Grape Mash Treatment in Juice Processing. Ultrason. Sonochem. 2010, 17, 273–279. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The Impact of Food Processing on the Phenolic Content in Products Made from Juneberry (Amelanchier Lamarckii) Fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical Profile of Black Currant Fruit Modified by Different Degree of Infection with Black Currant Leaf Spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia Melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.-T.; Krbavčić, I.; Vujevic, P.; Milinović, B.; Landeka, I.; Vahcić, N. Effects of Weather Conditions on Phenolic Content and Antioxidant Capacity in Juice of Chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2016, 67, 1. [Google Scholar] [CrossRef]
- Kobus, Z.; Nadulski, R.; Wilczyński, K.; Kozak, M.; Guz, T.; Rydzak, L. Effect of the Black Chokeberry (Aronia melanocarpa (Michx.) Elliott) Juice Acquisition Method on the Content of Polyphenols and Antioxidant Activity. PLoS ONE 2019, 14, e0219585. [Google Scholar] [CrossRef] [PubMed]
- Anozie, R.C.; Omeje, K.O.; Eze, S.O. Studies on Industrially Processed Fruit Juice and Freshly Prepared Fruit Juice Sold in Enugu State, Nigeria. Bio-Research 2018, 16, 1033–1043. [Google Scholar] [CrossRef]
- Wei, K.; Ma, C.; Sun, K.; Qiang, L.; Zhao, N.; Sun, Y.; Tu, K.; Pan, L. Relationship between Optical Properties and Soluble Sugar Contents of Apple Flesh during Storage. Postharvest Biol. Technol. 2020, 159, 111021. [Google Scholar] [CrossRef]
- Brodie, G.; Harris, G.; Jacob, M.V.; Sheehan, M.; Yin, L. Microwave Modification of Sugar Cane to Enhance Juice Extraction during Milling. J. Microw. Power Electromagn. Energy 2011, 45, 178. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, X.; Zhang, C.; Zhang, J.; Zhang, S.; Xu, J. Ultrasonic and Microwave Treatment Improved Jujube Juice Yield. Food Sci. Nutr. 2020, 8, 4196–4204. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef]
- Al Bittar, S.; Périno-Issartier, S.; Dangles, O.; Chemat, F. An Innovative Grape Juice Enriched in Polyphenols by Microwave-Assisted Extraction. Food Chem. 2013, 141, 3268–3272. [Google Scholar] [CrossRef]
- Nguyen, C.L.; Nguyen, H.V.H. Ultrasonic Effects on the Quality of Mulberry Juice. Beverages 2018, 4, 56. [Google Scholar] [CrossRef]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest Date Affects Aronia Juice Polyphenols, Sugars, and Antioxidant Activity, but Not Anthocyanin Stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I.; Denev, P.; Kratchanova, M.; Petrova, I.; et al. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Lepaus, B.M.; Valiati, B.S.; Machado, B.G.; Domingos, M.M.; Silva, M.N.; Faria-Silva, L.; Bernardes, P.C.; da Silva Oliveira, D.; de São José, J.F.B. Impact of Ultrasound Processing on the Nutritional Components of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2023, 138, 752–765. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication Enhances Polyphenolic Compounds, Sugars, Carotenoids and Mineral Elements of Apple Juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Kuzmina, N.; Krasilnikova, A.; Terentyev, K.; Novozhilov, E. The Application of Pectinase in the Lingonberry-Juice Production: The Impact on the Yield and Composition of Biological Valuable Components. Обществo Ограниченнoй Ответственнoстью Стеф92 Технoлoджи 2018, 18, 275–282. [Google Scholar]
- Varghese, T.; Pare, A. Effect of Microwave Assisted Extraction on Yield and Protein Characteristics of Soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Putro, A.W.; Fathimah, R.N.; Kurnia, K.A.; Darmawan, N.; Yulianto, B.; Jiwanti, P.K.; Carrera, C.A.; Palma, M. A Microwave-Based Extraction Method for the Determination of Sugar and Polyols: Application to the Characterization of Regular and Peaberry Coffees. Arab. J. Chem. 2022, 15, 103660. [Google Scholar] [CrossRef]
- Zuluaga, A.M.; Mena-García, A.; Soria Monzón, A.C.; Rada-Mendoza, M.; Chito, D.M.; Ruiz-Matute, A.I.; Sanz, M.L. Microwave Assisted Extraction of Inositols for the Valorization of Legume by-Products. LWT 2020, 133, 109971. [Google Scholar] [CrossRef]
- Tsubaki, S.; Onda, A.; Hiraoka, M.; Fujii, S.; Azuma, J.; Wada, Y. Chapter 7—Microwave-Assisted Water Extraction of Carbohydrates From Unutilized Biomass. In Water Extraction of Bioactive Compounds; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–219. ISBN 978-0-12-809380-1. [Google Scholar]
- Tsubaki, S.; Oono, K.; Onda, A.; Yanagisawa, K.; Azuma, J. Comparative Decomposition Kinetics of Neutral Monosaccharides by Microwave and Induction Heating Treatments. Carbohydr. Res. 2013, 375, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Uzhel, A.S.; Borodina, A.N.; Gorbovskaya, A.V.; Shpigun, O.A.; Zatirakha, A.V. Determination of Full Organic Acid Profiles in Fruit Juices and Alcoholic Beverages Using Novel Chemically Derivatized Hyperbranched Anion Exchanger. J. Food Compos. Anal. 2021, 95, 103674. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Kim, I.H. Protected Organic Acids Improved Growth Performance, Nutrient Digestibility, and Decreased Gas Emission in Broilers. Animals 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, F.; Kang, J.; Li, Q.; Warusawitharana, H.K.; Li, B. Quality Chemistry, Physiological Functions, and Health Benefits of Organic Acids from Tea (Camellia sinensis). Molecules 2023, 28, 2339. [Google Scholar] [CrossRef]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative Phytochemical Analysis of Aronia melanocarpa L. Fruit Juices on Bulgarian Market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z.; Redzynia, M.; Opęchowska, M.; Koziołkiewicz, M. Comparison of in Vitro Anti-Lipase and Antioxidant Activities, and Composition of Commercial Chokeberry Juices. Eur. Food Res. Technol. 2016, 242, 505–515. [Google Scholar] [CrossRef]
- Mutlu, M.; Sarioǧlu, K.; Demir, N.; Ercan, M.T.; Acar, J. The Use of Commercial Pectinase in Fruit Juice Industry. Part I: Viscosimetric Determination of Enzyme Activity. J. Food Eng. 1999, 41, 147–150. [Google Scholar] [CrossRef]
- Singh Jadaun, J. Pectinase: A Useful Tool in Fruit Processing Industries. NFSIJ 2018, 5, 555673. [Google Scholar] [CrossRef]
- Trenka, M.; Nawirska-Olszańska, A.; Oziembłowski, M. Analysis of Selected Properties of Fruits of Black Chokeberry (Aronia melanocarpa L.) from Organic and Conventional Cultivation. Appl. Sci. 2020, 10, 9096. [Google Scholar] [CrossRef]
- Lalou, S.; Ordoudi, S.A.; Mantzouridou, F.T. On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue. Foods 2021, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Lim, S.W.; Tan, S.S.; Tan, S.T. Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh. Horticulturae 2023, 9, 92. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Le, V. Application of Ultrasound to Pineapple Mash Treatment in Juice Processing. Int. Food Res. J. 2012, 19, 547–552. [Google Scholar]
- Sparr Eskilsson, C.; Björklund, E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Loureiro, L.; Machado, L.; Geada, P.; Vasconcelos, V.; Vicente, A.A. Evaluation of Efficiency of Disruption Methods for Coelastrella Sp. in Order to Obtain High Yields of Biochemical Compounds Release. Algal Res. 2023, 73, 103158. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak Jovanović, I. Anthocyanin Content and Antioxidant Activity of Various Red Fruit Juices. Dtsch. Lebensm. Rundsch. Z. Für Leb. Und Leb. 2007, 103, 58–64. [Google Scholar]
- Guiné, R.; Barroca, M. Influence of Processing and Storage on Fruit Juices Phenolic Compounds. Int. J. Med. Biol. Front. 2014, 20, 45–58. [Google Scholar]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The Link between the Phenolic Composition and the Antioxidant Activity in Different Small Berries: A Metabolomic Approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, G.; Poiana, M.-A. An Overview of Effects Induced by Pasteurization and High-Power Ultrasound Treatment on the Quality of Red Grape Juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef]
- Zoran, H. Effects of the High Power Ultrasound on Microorganisms in Fruit Juices. MOJFPT 2016, 2, 176–177. [Google Scholar] [CrossRef][Green Version]
- Shiratake, K.; Martinoia, E. Transporters in Fruit Vacuoles. Plant Biotechnol. 2007, 24, 127–133. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of Microwaves and Ultrasound on Bioactive Compounds and Microbiological Quality of Blackberry Juice. LWT 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Dent, M.; Dragovi, V.; Peni, M.; Brni, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Gullón Estévez, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food by-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Gao, N.; Shu, C.; Wang, Y.; Tian, J.; Lang, Y.; Jin, C.; Cui, X.; Jiang, H.; Liu, S.; Li, Z.; et al. Polyphenol Components in Black Chokeberry (Aronia melanocarpa) as Clinically Proven Diseases Control Factors—An Overview. Food Sci. Hum. Wellness 2024, 13, 1152–1167. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak Jovanović, I. Antioxidant Activity and Polyphenols of Aronia in Comparison to Other Berry Species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
| Treatment | pH | SS | ||||
|---|---|---|---|---|---|---|
| Mean | ±SE | Sign. | Mean | ±SE | Sign. | |
| Cold | 3.48 | 0.01 | a | 14.90 | 0.76 | a |
| Enzymatic | 3.48 | 0.01 | a | 15.90 | 0.17 | a |
| Thermal | 3.48 | 0.01 | a | 15.73 | 0.09 | a |
| Microwave | 3.47 | 0.01 | a | 17.93 | 0.13 | b |
| Ultrasound | 3.48 | 0.01 | a | 16.17 | 0.37 | ab |
| p value | 0.785 | 0.004 | ||||
| Treatment | k | n | R2 | ||||
|---|---|---|---|---|---|---|---|
| Mean | ±SE | Sign. | Mean | ±SE | Sign. | ||
| Cold | 0.0023 | 0.0001 | a | 0.9859 | 0.0150 | b | 0.9861 |
| Enzymatic | 0.0030 | 0.0001 | a | 0.9376 | 0.0046 | b | 0.9927 |
| Thermal | 0.0025 | 0.0001 | a | 0.9684 | 0.0066 | b | 0.9855 |
| Microwave | 0.0038 | 0.0001 | b | 0.9204 | 0.0096 | a | 0.9989 |
| Ultrasound | 0.0025 | 0.0001 | a | 0.9704 | 0.0094 | b | 0.9892 |
| p value | 0.000 | 0.005 | |||||
| Treatment | L* | A* | B* | ΔE* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | |
| Cold | 21.70 | 0.24 | a | 1.50 | 0.04 | a | −3.10 | 0.04 | a | 0.00 | 0.00 | a |
| Enzymatic | 21.35 | 0.09 | a | 1.55 | 0.03 | a | −3.30 | 0.15 | a | 0.71 | 0.09 | ab |
| Thermal | 21.15 | 0.13 | a | 1.45 | 0.03 | a | −3.07 | 0.05 | a | 0.80 | 0.10 | ab |
| Microwave | 20.13 | 0.15 | b | 1.70 | 0.03 | b | −3.20 | 0.07 | a | 1.59 | 0.09 | b |
| Ultrasound | 20.28 | 0.29 | b | 1.59 | 0.04 | ab | −3.30 | 0.07 | a | 1.45 | 0.11 | b |
| p value | 0.000 | 0.001 | 0.227 | 0.000 | ||||||||
| Treatment | Total Sugars | Fructose | Glucose | Sorbitol | Sucrose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | |
| Cold | 85.65 | 0.37 | a | 28.66 | 0.21 | a | 23.43 | 0.60 | a | 32.37 | 0.27 | a | 1.19 | 0.05 | a |
| Enzymatic | 125.08 | 0.21 | b | 34.88 | 0.40 | b | 29.85 | 0.53 | b | 58.95 | 0.38 | b | 1.41 | 0.14 | a |
| Thermal | 87.48 | 0.92 | a | 29.53 | 0.49 | a | 23.69 | 0.57 | a | 33.01 | 0.31 | a | 1.25 | 0.06 | a |
| Microwave | 142.39 | 2.36 | c | 41.17 | 0.84 | c | 39.21 | 1.19 | c | 60.35 | 0.71 | b | 1.66 | 0.25 | a |
| Ultrasound | 140.19 | 2.19 | c | 40.57 | 0.78 | c | 37.65 | 1.26 | c | 60.40 | 0.25 | b | 1.57 | 0.17 | a |
| p value | 0.000 | 0.000 | 0.000 | 0.000 | 0.212 | ||||||||||
| Treatment | Total Acids | Citric | Malic | Oxalic | Quinic | Shikimic | Tartaric | Ascorbic | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | Mean | ±SE | Sign. | |
| Cold | 11.64 | 0.09 | a | 1.17 | 0.06 | a | 8.39 | 0.11 | a | 0.39 | 0.07 | a | 1.24 | 0.05 | a | 0.18 | 0.03 | a | 0.17 | 0.01 | a | 0.10 | 0.01 | a |
| Enzymatic | 14.49 | 0.24 | b | 1.49 | 0.15 | ab | 9.14 | 0.13 | a | 0.45 | 0.04 | a | 3.00 | 0.19 | b | 0.15 | 0.02 | a | 0.15 | 0.03 | a | 0.10 | 0.00 | a |
| Thermal | 11.92 | 0.15 | a | 1.14 | 0.08 | a | 8.63 | 0.21 | a | 0.36 | 0.04 | a | 1.35 | 0.14 | a | 0.16 | 0.02 | a | 0.19 | 0.01 | a | 0.11 | 0.00 | a |
| Microwave | 21.31 | 0.62 | d | 2.12 | 0.23 | b | 13.20 | 0.26 | b | 0.70 | 0.08 | b | 4.75 | 0.36 | c | 0.19 | 0.03 | a | 0.21 | 0.01 | a | 0.14 | 0.01 | b |
| Ultrasound | 18.77 | 0.59 | c | 1.94 | 0.17 | b | 12.22 | 0.36 | b | 0.46 | 0.04 | ab | 3.69 | 0.22 | b | 0.16 | 0.03 | a | 0.19 | 0.01 | a | 0.11 | 0.00 | a |
| p value | 0.000 | 0.001 | 0.000 | 0.005 | 0.000 | 0.727 | 0.307 | 0.003 | ||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puzovic, A.; Mikulic-Petkovsek, M. Comparative Evaluation of Conventional and Emerging Maceration Techniques for Enhancing Bioactive Compounds in Aronia Juice. Foods 2024, 13, 3255. https://doi.org/10.3390/foods13203255
Puzovic A, Mikulic-Petkovsek M. Comparative Evaluation of Conventional and Emerging Maceration Techniques for Enhancing Bioactive Compounds in Aronia Juice. Foods. 2024; 13(20):3255. https://doi.org/10.3390/foods13203255
Chicago/Turabian StylePuzovic, Alema, and Maja Mikulic-Petkovsek. 2024. "Comparative Evaluation of Conventional and Emerging Maceration Techniques for Enhancing Bioactive Compounds in Aronia Juice" Foods 13, no. 20: 3255. https://doi.org/10.3390/foods13203255
APA StylePuzovic, A., & Mikulic-Petkovsek, M. (2024). Comparative Evaluation of Conventional and Emerging Maceration Techniques for Enhancing Bioactive Compounds in Aronia Juice. Foods, 13(20), 3255. https://doi.org/10.3390/foods13203255







