Exploring the Biological Value of Red Grape Skin: Its Incorporation and Impact on Yogurt Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Total Acidity and pH
2.2.2. Proximate Composition
2.2.3. The Analysis of Polyphenols and Antioxidant Activity
2.2.4. Anthocyanin Profile
2.3. Yogurt-Making Procedure
2.4. Yogurt Proximate Composition
2.5. Color Assessment
2.6. Syneresis Index
2.7. Sensory Analysis
2.7.1. Overall Liking of Yogurt
2.7.2. Consumer Test
2.7.3. Check-All-That-Apply (CATA)
2.8. Statistical Analysis
3. Results and Discussions
3.1. Characteristics of Used Raw Materials
3.2. Biological Value of Grape Skin Powder
3.3. Impact of Grape Skin Powder on Yogurt Quality
3.3.1. Yogurt Physicochemical Characterization
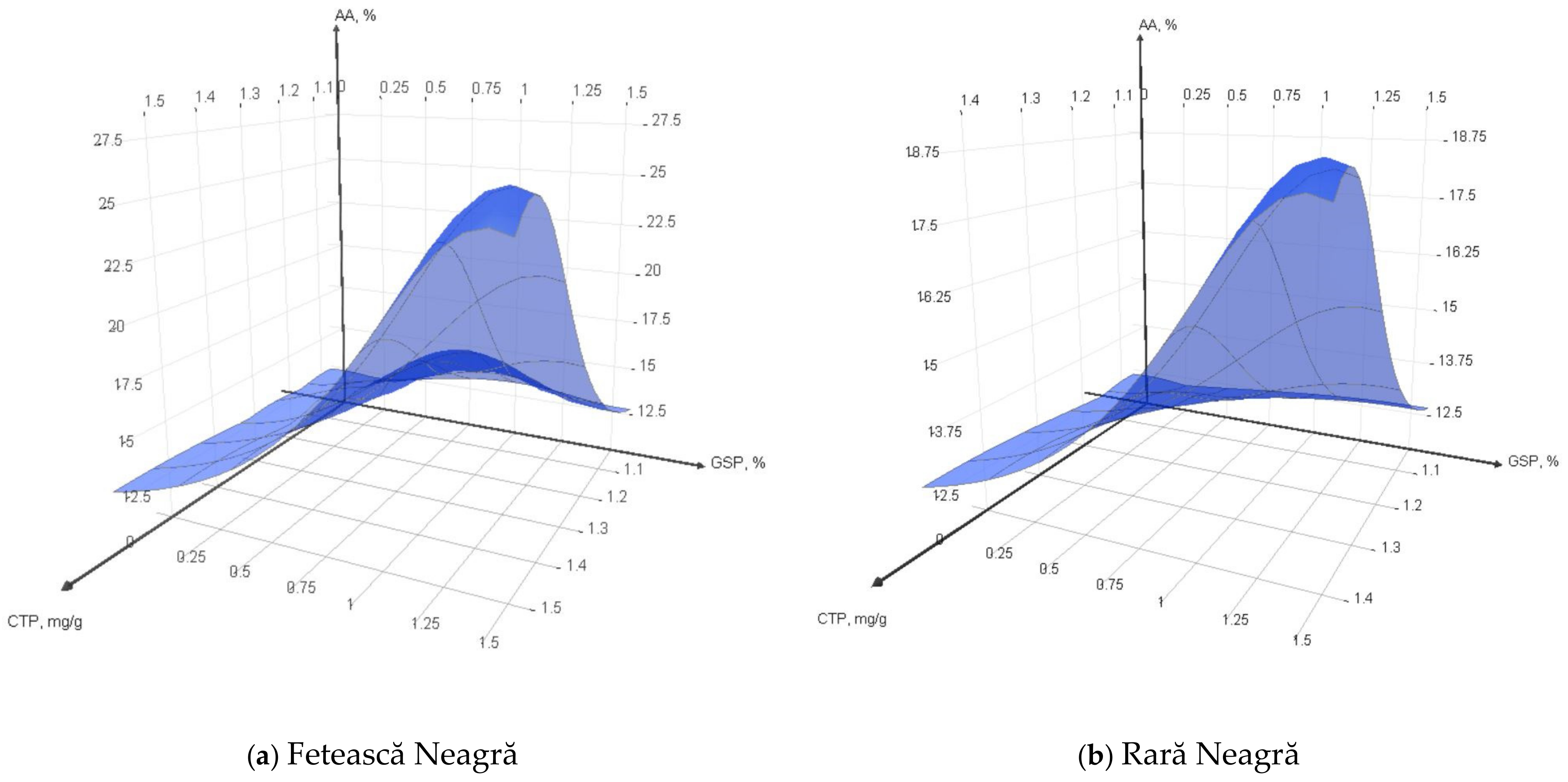
3.3.2. Total Phenols Content and Antioxidant Activity of Yogurt Fortified with GSP
3.3.3. Color Parameter Analysis
3.3.4. The Evolution of Yogurt Syneresis during Storage
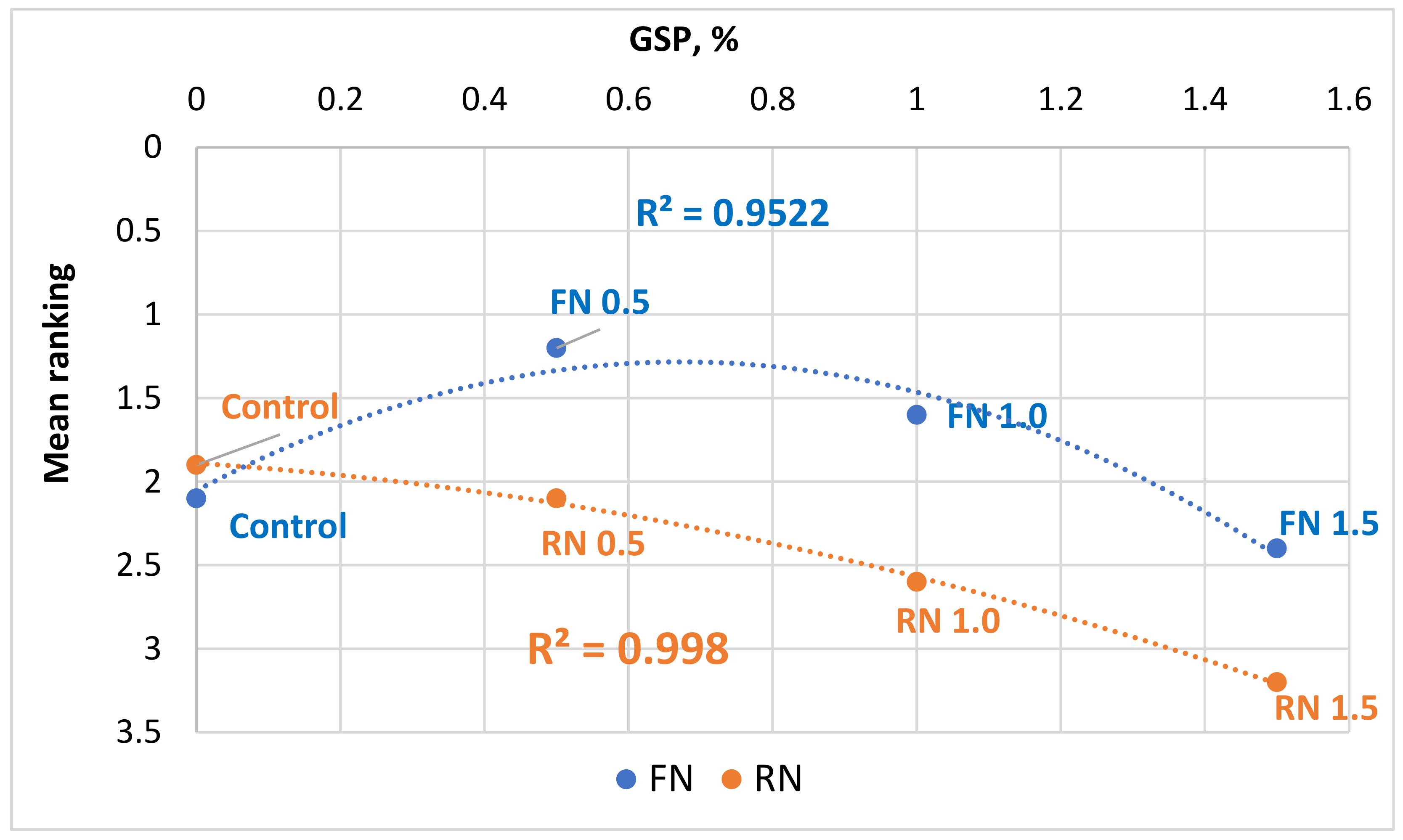
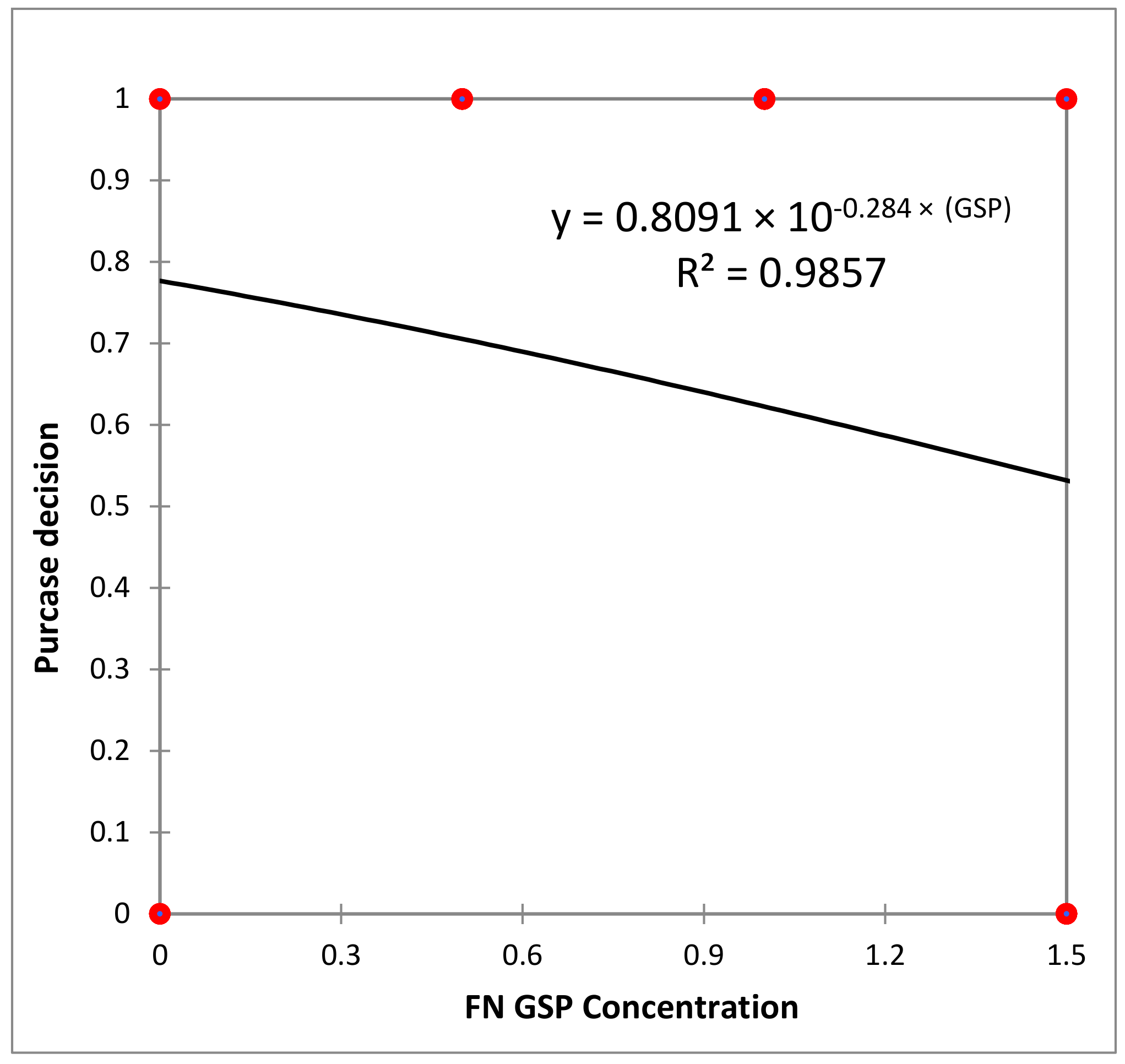
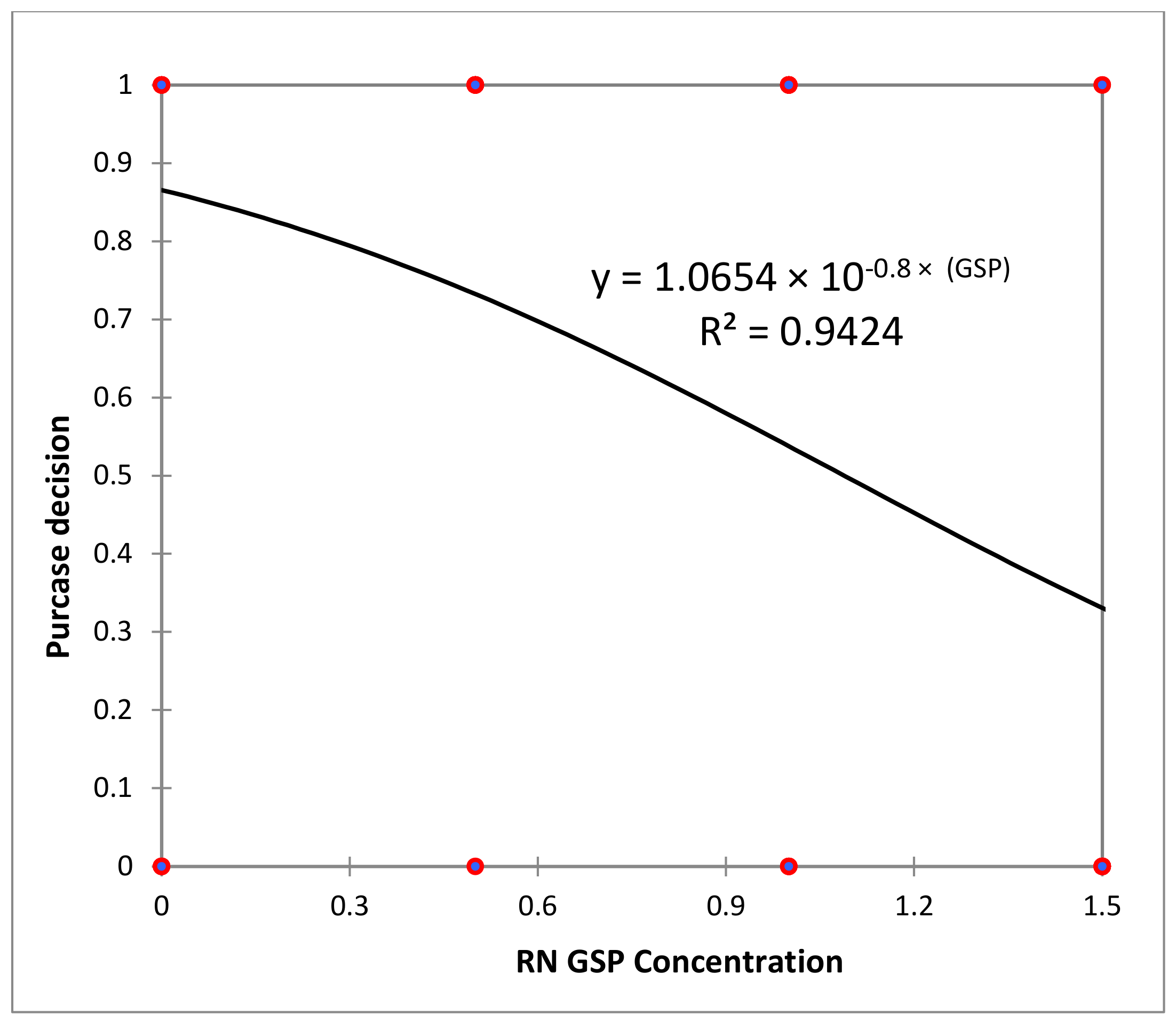
3.4. Consumer Test and Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bírová, K.; Rovný, P.; Palkovič, J.; Vondráček, M. Consumption and Frequency of Wine Drinking in V4 Countries. Ukr. Food J. 2024, 13, 175–191. [Google Scholar] [CrossRef]
- Bodiroga, R.; Banjanin, T.; Vukojević Ateljević, D.; Kerma, S. The Trends in Viticulture and Winemaking in the Context of Wine Tourism Development in Bosnia and Herzegovina. AGS 2024, 64, 33–48. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine Intergovernmental Organisation Annual Assessment of the World Vine and Wine Sector in 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf (accessed on 9 September 2024).
- Enache, P.A. The World Market of the Wine Sector; The Research Institute for Agri-Cultural Economy and Rural Development (ICEADR): Bucharest, Romania, 2023; pp. 245–251. [Google Scholar]
- Covaliov, E.; Ruseva, O.; Resitca, V.; Deseatnicova, O.; Capcanari, T.; Suhodol, N. Harnessing Grape Pomace: Nutritional Aspects, Recovery and Extraction Techniques for Health Benefits. J. Eng. Sci. 2024, 31, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Boix-Fayos, C.; De Vente, J. Challenges and Potential Pathways towards Sustainable Agriculture within the European Green Deal. Agric. Syst. 2023, 207, 103634. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L. Bioactive Compounds from Fruit and Vegetable Waste: Extraction and Possible Utilization. Foods 2024, 13, 775. [Google Scholar] [CrossRef] [PubMed]
- Ghinea, C.; Leahu, A. Valorisation of Apple (Malus Domestica) Wastes. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 325–348. ISBN 978-3-030-84435-6. [Google Scholar]
- Stamatovska, V.; Nakov, G. Management of Apple and Grape Processing By-Products. A Review. Ukr. Food J. 2022, 11, 498–517. [Google Scholar] [CrossRef]
- Capcanari, T.; Covaliov, E.; Negoița, C.; Siminiuc, R.; Chirsanova, A.; Reșitca, V.; Țurcanu, D. Hemp Seed Cake Flour as a Source of Proteins, Minerals and Polyphenols and Its Impact on the Nutritional, Sensorial and Technological Quality of Bread. Foods 2023, 12, 4327. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Radu, O.; Covaliov, E.; Capcanari, T. Technological Properties and Functional Food Potential of Oilseed Cakes. Ukr. Food J. 2024, 13, 287–302. [Google Scholar] [CrossRef]
- Pastushkova, E.; Kryukova, E.; Arisov, A.; Tiunov, V.; Kokoreva, L. Use of Non-Traditional Types of Plant Raw Materials for Food Production within the Framework of Food Security. E3S Web Conf. 2020, 222, 06027. [Google Scholar] [CrossRef]
- Capcanari, T.; Chirsanova, A.; Covaliov, E.; Radu, O.; Siminiuc, R. Pastry Sauce with Carob (Ceratonia siliqua) Powder. Ukr. Food J. 2022, 11, 235–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Zhang, H.; Mai, Q.; Zhang, B.; Li, H.; Deng, Z. The Degradation Rules of Anthocyanins from Eggplant Peel and Antioxidant Capacity in Fortified Model Food System during the Thermal Treatments. Food Biosci. 2020, 38, 100701. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Feng, X.; Astray, G.; Gullón, B.; Lorenzo, J.M. Recent Advances in the Extraction of Polyphenols from Eggplant and Their Application in Foods. LWT 2021, 146, 111381. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Potato Peels as Sources of Functional Compounds for the Food Industry: A Review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Da Silva, D.J.; De Oliveira, M.M.; Wang, S.H.; Carastan, D.J.; Rosa, D.S. Designing Antimicrobial Polypropylene Films with Grape Pomace Extract for Food Packaging. Food Packag. Shelf Life 2022, 34, 100929. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez De La Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of Natural Antioxidants from Grape Seed and Chestnut in Combination with Hydroxytyrosol, as Sodium Nitrite Substitutes in Cinta Senese Dry-Fermented Sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Rebollo-Hernanz, M.; Martin-Cabrejas, M.A.; Moreno-Arribas, M.V.; Bartolomé, B. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From Waste to Health: Sustainable Exploitation of Grape Pomace Seed Extract to Manufacture Antioxidant, Regenerative and Prebiotic Nanovesicles within Circular Economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Štefániková, J.; Miškeje, M.; Tokár, M.; Gabríny, L.; Kowalczewski, P.Ł.; Kačániová, M. Wheat Bread with Grape Seeds Micropowder: Impact on Dough Rheology and Bread Properties. Appl. Rheol. 2020, 30, 138–150. [Google Scholar] [CrossRef]
- Subiria-Cueto, R.; Reyes-Blas, H.; Olivas-Armendáriz, I.; Wall-Medrano, A.; González-Aguilar, G.A.; De La Rosa, L.A.; Martínez-Ruiz, N.D.R.; Alvarez-Parrilla, E. Grape Pomace and Pecan Shell Fortified Bread: The Effect of Dietary Fiber-Phenolic Compounds Interaction on the in Vitro Accessibility of Phenolic Compounds and in Vitro Glycemic Index. Food Chem. 2025, 462, 140925. [Google Scholar] [CrossRef]
- Boff, J.M.; Strasburg, V.J.; Ferrari, G.T.; De Oliveira Schmidt, H.; Manfroi, V.; De Oliveira, V.R. Chemical, Technological, and Sensory Quality of Pasta and Bakery Products Made with the Addition of Grape Pomace Flour. Foods 2022, 11, 3812. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Characterization of Băbească Neagră Grape Pomace and Incorporation into Jelly Candy: Evaluation of Phytochemical, Sensory, and Textural Properties. Foods 2023, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Altınok, E.; Palabiyik, I.; Gunes, R.; Toker, O.S.; Konar, N.; Kurultay, S. Valorisation of Grape By-Products as a Bulking Agent in Soft Candies: Effect of Particle Size. LWT 2020, 118, 108776. [Google Scholar] [CrossRef]
- Kandylis, P.; Dimitrellou, D.; Moschakis, T. Recent Applications of Grapes and Their Derivatives in Dairy Products. Trends Food Sci. Technol. 2021, 114, 696–711. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Bellocci, M.; Grotta, L.; Sacchetti, G.; Martino, G. Influence of Dietary Grape Pomace Supplementation on Chemical and Sensory Properties of Ewes’ Cheese. Int. Dairy J. 2023, 143, 105671. [Google Scholar] [CrossRef]
- Silva, M.E.D.S.; Grisi, C.V.B.; Silva, S.P.D.; Madruga, M.S.; Silva, F.A.P.D. The Technological Potential of Agro-Industrial Residue from Grape Pulping (Vitis spp.) for Application in Meat Products: A Review. Food Biosci. 2022, 49, 101877. [Google Scholar] [CrossRef]
- Capcanari, T.; Chirsanova, A.; Covaliov, E.; Siminiuc, R. Development of Lactose Free Yogurt Technology for Personalized Nutrition. FNS 2021, 12, 1116–1135. [Google Scholar] [CrossRef]
- Morelli, L. Yogurt, Living Cultures, and Gut Health. Am. J. Clin. Nutr. 2014, 99, 1248S–1250S. [Google Scholar] [CrossRef] [PubMed]
- Covaliov, E.; Grosu, C.; Popovici, V.; Capcanari, T.; Siminiuc, R.; Resitca, V. Impact of Sea Buckthorn Berries (Hippophae rhamnoides) on Yoghurt Biological Value and Quality. Ann. UDJG Food Technol. 2021, 45, 62–76. [Google Scholar] [CrossRef]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T.; et al. New Concept of Fortified Yogurt Formulation with Encapsulated Carrot Waste Extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Atwaa, E.; Abou Sayed-Ahmed, E.; Hassan, M.A.A. Physicochemical, Microbiological and Sensory Properties of Low Fat Probiotic Yoghurt Fortified with Mango Pulp Fiber Waste as Source of Dietary Fiber. J. Food Dairy Sci. 2020, 11, 271–276. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Jattar-Santiago, K.Y.; Avila-Sosa, R.; Pérez-Xochipa, I.; Guerrero-Beltrán, J.A.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Antioxidant Fortification of Yogurt with Red Cactus Pear Peel and Its Mucilage. CyTA J. Food 2019, 17, 824–833. [Google Scholar] [CrossRef]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of Encapsulated Natural Bioactive Compounds from Red Pepper Waste in Yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.-J. Microencapsulated Saffron Floral Waste Extracts as Functional Ingredients for Antioxidant Fortification of Yogurt: Stability during the Storage. LWT 2023, 184, 114976. [Google Scholar] [CrossRef]
- Ahmad, I.; Hao, M.; Li, Y.; Zhang, J.; Ding, Y.; Lyu, F. Fortification of Yogurt with Bioactive Functional Foods and Ingredients and Associated Challenges—A Review. Trends Food Sci. Technol. 2022, 129, 558–580. [Google Scholar] [CrossRef]
- AOAC 947.05-1947; Acidity of Milk. Titrimetric Method. JOAC: Rockville, MD, USA, 1947.
- Ścibisz, I.; Ziarno, M.; Mitek, M. Color Stability of Fruit Yogurt during Storage. J. Food Sci. Technol. 2019, 56, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Decision of the Government of the Republic of Moldova No. 611 of 05.07.2010. Regarding the Approval of the Technical Regulations “Milk and Dairy Products” 2010. Available online: https://www.legis.md/cautare/getResults?doc_id=22246&lang=ro (accessed on 10 August 2024).
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2019; Volume 3, ISBN 978-0-935584-89-9. [Google Scholar]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Softcover Repr.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-90-481-6428-8. [Google Scholar]
- Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae 2023, 9, 1164. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of Quercetin in the Physicochemical Properties, Antioxidant and Antiglycation Activities of Bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- OIV (2021) Official Methods for the Analysis of Musts and Wines of the International Organization of Vine and Wine (OIV) (OIV Official Methods for the Analysis of Musts and Wines of the International Organization of Vine and Wine (OIV). Methods of Analysis of Wines and Musts. (OIV-MA-AS315-11: R2007). Available online: http://www.oiv.int/oiv/info/enmethodesinternationalesvin (accessed on 2 August 2024).
- Skorbanova, E.; Taran, N.; Ponomariova, I.; Degteari, N.; Rinda, P.; Efremov, E. Studiul compoziției antocienilor monomerici în vinurile roşii din soiurile de struguri autohtone și de selecţie nouă prin metoda HPLC. PVV 2024, 1, 58–62. [Google Scholar] [CrossRef]
- Macdougall, D.B. Colour Measurement of Food: Principles and Practice. In Colour Measurement; Elsevier: Amsterdam, The Netherlands, 2010; pp. 312–342. ISBN 978-1-84569-559-0. [Google Scholar]
- Vásquez-Mazo, P.; Loredo, A.G.; Ferrario, M.; Guerrero, S. Development of a Novel Milk Processing to Produce Yogurt with Improved Quality. Food Bio. Process. Technol. 2019, 12, 964–975. [Google Scholar] [CrossRef]
- ISO 8586:2023(E); Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023.
- Biró, B.; Sipos, M.A.; Kovács, A.; Badak-Kerti, K.; Pásztor-Huszár, K.; Gere, A. Cricket-Enriched Oat Biscuit: Technological Analysis and Sensory Evaluation. Foods 2020, 9, 1561. [Google Scholar] [CrossRef]
- Abdi-Moghadam, Z.; Darroudi, M.; Mahmoudzadeh, M.; Mohtashami, M.; Jamal, A.M.; Shamloo, E.; Rezaei, Z. Functional Yogurt, Enriched and Probiotic: A Focus on Human Health. Clin. Nutr. ESPEN 2023, 57, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Brito-Oliveira, T.C.; Gonçalves, G.A.; Ferreira, L.S.; Santos, Y.J.S.; Turatti, R.C.; De Oliveira, G.C.; Pinho, S.C.; Callejon, D.R. Production of Yogurts Fortified with Nanoencapsulated Coenzyme Q10: Technological Feasibility and Bioactive’s Release during in Vitro Digestion. Int. J. Dairy. Tech. 2024, 77, 905–915. [Google Scholar] [CrossRef]
- Yeasmin, N.; Sarker, B.R.; Begum, A.; Al Mamun, Z.U.; Rahman, N.; Hossen, M.S.; Motalab, M.; Sathee, R.A. Fortification of Yogurt with Red Dragon Fruit’s (Hylocereus polyrhizus) Peel Powder: Effects on Comprehensive Quality Attributes and Sensory Properties. Turk. J. Agric. Food Sci. Technol. 2023, 11, 2300–2307. [Google Scholar] [CrossRef]
- Herrera, T.; Iriondo-DeHond, M.; Ramos Sanz, A.; Bautista, A.I.; Miguel, E. Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt. Foods 2023, 12, 3332. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.A.S.; Xavier, A.M.R.B.; Evtuguin, D.V.; Lopes, L.P.C. Integrated Utilization of Grape Skins from White Grape Pomaces. Ind. Crop. Prod. 2013, 49, 286–291. [Google Scholar] [CrossRef]
- Abdrabba, S.; Hussein, S. Chemical Composition of Pulp, Seed and Peel of Red Grape from Libya. GJSR J. 2015, 3, 6–11. [Google Scholar]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From Grape to Wine: Changes in Phenolic Composition and Its Influence on Antioxidant Activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Jug, U.; Naumoska, K.; Vovk, I. (−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of Five Greek Red Grape Varieties According to the Anthocyanin and Proanthocyanidin Profiles of Their Skins and Seeds. J. Food Compos. Anal. 2020, 92, 103547. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Ferreira, V.; Dizy, M.; Fernández-Zurbano, P. Characterization of Taste-Active Fractions in Red Wine Combining HPLC Fractionation, Sensory Analysis and Ultra Performance Liquid Chromatography Coupled with Mass Spectrometry Detection. Anal. Chim. Acta 2010, 673, 151–159. [Google Scholar] [CrossRef]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of Grape and Wine Anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin Pattern of Several Red Grape Cultivars and Wines Made from Them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, D.V.; Shevchenko, O.G.; Golubev, D.A.; Platonova, E.Y.; Zemskaya, N.V.; Shoeva, O.Y.; Gordeeva, E.I.; Patov, S.A.; Shaposhnikov, M.V.; Khlestkina, E.K.; et al. Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants 2023, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Wei, Z.; Huang, W.; Yan, Z.; Luo, Z.; Beta, T.; Xu, X. Effects of Four Drying Methods on the Quality, Antioxidant Activity and Anthocyanin Components of Blueberry Pomace. Food Prod. Process. Nutr. 2023, 5, 35. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Gamel, T.H.; Saeed, S.M.G.; Ali, R.; Abdel-Aal, E.-S.M. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods 2023, 12, 1358. [Google Scholar] [CrossRef]
- Wang, Y.; Julian McClements, D.; Chen, L.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Zhi, C.; Ye, L.; Zhao, J.; et al. Progress on Molecular Modification and Functional Applications of Anthocyanins. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Radu, O.; Hubenia, V.; Kovaliov, E.; Capcanari, T.; Popovici, C. Physico-Chemical and Sensory Properties of Functional Confectionery Products with Rosa Canina Powder. Ukr. Food J. 2019, 8, 815–827. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Varnaitė, L.; Keršienė, M.; Šipailienė, A.; Kazernavičiūtė, R.; Venskutonis, P.R.; Leskauskaitė, D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Stoica, F.; Rațu, R.N.; Motrescu, I.; Cara, I.G.; Filip, M.; Țopa, D.; Jităreanu, G. Application of Pomace Powder of Black Carrot as a Natural Food Ingredient in Yoghurt. Foods 2024, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Peixoto Araujo, N.M.; Pereira, G.A.; Pastore, G.M.; Marostica Junior, M.R. Anthocyanins Recovered from Agri-Food By-Products Using Innovative Processes: Trends, Challenges, and Perspectives for Their Application in Food Systems. Molecules 2021, 26, 2632. [Google Scholar] [CrossRef]
- Gomez Mattson, M.; Sozzi, A.; Corfield, R.; Gagneten, M.; Franceschinis, L.; Schebor, C.; Salvatori, D. Colorant and Antioxidant Properties of Freeze-Dried Extracts from Wild Berries: Use of Ultrasound-Assisted Extraction Method and Drivers of Liking of Colored Yogurts. J. Food Sci. Technol. 2022, 59, 944–955. [Google Scholar] [CrossRef]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of Orange Fiber Addition on Yogurt Color during Fermentation and Cold Storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Kumari, T.; Das, A.B.; Deka, S.C. Effect of Enzymatic Modified Pea Peel Dietary Fibre on Syneresis, Texture, Rheology and Microstructural Properties of Yogurt. Biomass Convers. Biorefinery 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Al Juhaimi, F.Y.; Al-Quh, H.; Babiker, E.E. Influence of White Radish (Raphanus sativus L.) Powder on the Quality Characteristics of Set-Type Yogurt during Cold Storage. CyTA J. Food 2024, 22, 2391530. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of Polyphenol−Protein Interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
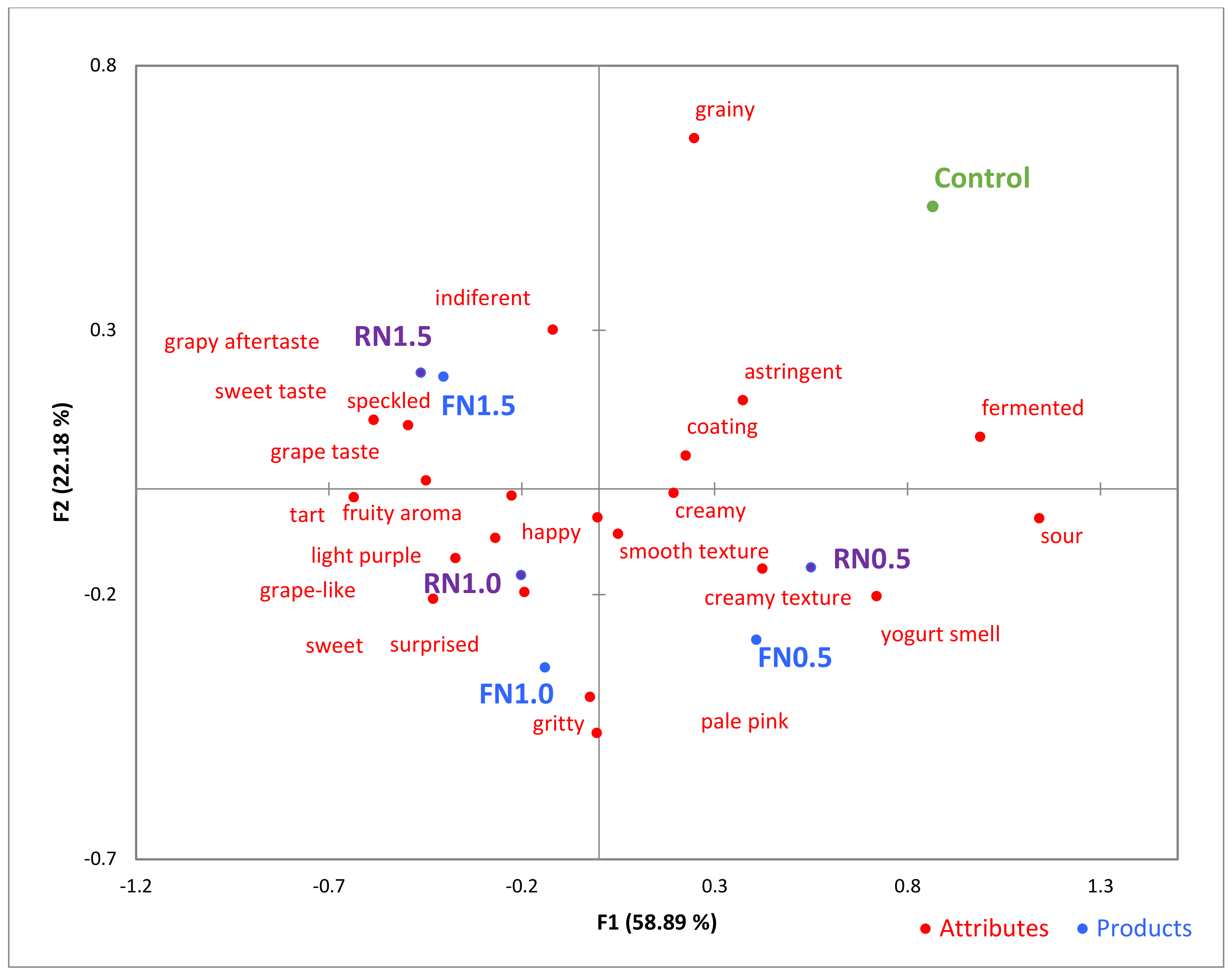
| Product Attribute | CATA Terms |
|---|---|
| Color | pale pink, light purple, creamy white, speckled |
| Texture | creamy texture, smooth texture, gritty, thick, grainy |
| Aroma | fruity aroma, grape-like, yogurt smell, fermented, sweet aroma, earthy |
| Taste | sweet taste, tart, sour, bitter, grape taste, astringent |
| Mouthfeel | creamy, gritty, smooth mouthfeel, dry, coating |
| Aftertaste | sweet, tart, bitter, grapy aftertaste |
| Emotions | surprised, happy, scarred, acceptable, indifferent, disgusted |
| Indicator | Milk (Commercial, Pasteurized) | Fetească Neagră Grape Skin Powder | Rară Neagră Grape Skin Powder |
|---|---|---|---|
| Acidity, °T | 14.9 ± 0.63 | - | - |
| Fat content, % | 3.5 * | ||
| Crude protein content, % | 2.8 * | 9.84 ± 0.32 a | 9.91 ± 0.27 b |
| Glucides, % | 4.7 * | 12.77 ± 0.16 b | 12.43 ± 0.25 a |
| Fiber content | - | 30.52 ± 0.53 a | 31.21 ± 0.34 b |
| Ash | 15.84 ± 0.27 b | 15.23 ± 0.46 a |
| GSP Variety | TPC, mg/g | AA, % |
|---|---|---|
| Rară Neagră | 3.41 ± 0.02 a | 69.01 ± 0,18 a |
| Fetească Neagră | 6.39 ± 0.6 b | 90.24 ± 0.21 b |
| Nr. | Phenolic Compound | Relative Content, mg/100 g | |
|---|---|---|---|
| Rară Neagră | Fetească Neagră | ||
| 1 | Galic acid | 0.13 ± 0.01 a | 0.42 ± 0.01 b |
| 2 | 3,4-Dihydroxybenzoic acid | 0.60 ± 0.02 a | 1.61 ± 0.08 b |
| 3 | 4-Hydroxybenzoic acid | 0.49 ± 0.01 a | 1.05 ± 0.09 b |
| 4 | Vanillic acid | 1.22 ± 0.03 a | 1.60 ± 0.04 b |
| 5 | Catechin | 0.86 ± 0.02 a | 4.75 ± 0.11 b |
| 6 | Chlorogenic acid | 3.06 ± 0.09 a | 4.79 ± 0.17 b |
| 7 | Vanillin | 4.43 ± 0.14 a | 6.24 ± 0.21 b |
| 8 | Syringic acid | 5.56 ± 0.17 a | 8.59 ± 0.23 b |
| 9 | Epicatechin | 0.50 ± 0.01 a | 1.04 ± 0.02 b |
| 10 | Ferulic acid | 0.04 ± 0.01 a | 0.36 ± 0.01 b |
| 11 | Sinapic acid | 0.16 ± 0.01 a | 0.94 ± 0.02 b |
| 12 | Resveratrol | 2.86 ± 0.08 a | 4.37 ± 0.12 b |
| 13 | Rosmarinic acid | 5.04 ± 0.11 a | 18.79 ± 0.31 b |
| 14 | Quercetin | 66.83 ± 0.76 a | 109.82 ± 1.11 b |
| Anthocyanin Compound | Relative Content, % | |
|---|---|---|
| Fetească Neagră | Rară Neagră | |
| Delphinidin-3-glucoside | 5.96 ± 0.11 a | 2.09 ± 0.08 b |
| Cyanidin-3-glucoside | 0.54 ± 0.02 a | 0.89 ± 0.06 b |
| Malvidol diglucoside | 1.98 ± 0.14 a | 0.49 ± 0.05 b |
| Petunidin-3-glucoside | 12.06 ± 0.18 a | 4.19 ± 0.11 b |
| Peonidin-3-glucoside | 5.36 ± 0.09 a | 3.99 ± 0.14 b |
| Malvidin-3-glucoside | 67.63 ± 0.21 a | 63.84 ± 0.08 b |
| Peonidin-3-acetylglucoside | 0.54 ± 0.01 a | 0.71 ± 0.05 b |
| Malvidin-3-acetylglucoside | 2.34 ± 0.09 a | 6.07 ± 0.24 b |
| Peonidin-3-coumaringlucoside | 1.79 ± 0.02 a | 4.35 ± 0.09 b |
| Malvidin-3-coumaringlucoside | 3.75 ± 0.07 a | 13.35 ± 0.11 b |
| Sample | Moisture Content, % | Protein Content, % | Fat Content, % | Acidity, % Lactic Acid | pH (after Fermentation) |
|---|---|---|---|---|---|
| Control | 86.79 ± 1.12 | 3.21 ± 0.01 | 3.31 ± 0.12 | 0.46 ± 0.01 | 4.62 ± 0.03 |
| FN0.5 | 86.75 ± 0.78 | 3.28 ± 0.02 | 3.28 ± 0.09 | 0.51 ± 0.01 | 4.53 ± 0.01 |
| FN1.0 | 85.94 ± 1.05 | 3.33 ± 0.01 | 3.34 ± 0.13 | 0.49 ± 0.02 | 4.51 ± 0.02 |
| FN1.5 | 85.37 ± 1.22 | 3.36 ± 0.03 | 3.30 ± 0.07 | 0.54 ± 0.01 | 4.50 ± 0.02 |
| RN0.5 | 86.87 ± 0.83 | 3.27 ± 0.03 | 3.35 ± 0.11 | 0.48 ± 0.03 | 4.56 ± 0.01 |
| RN1.0 | 86.05 ± 1.17 | 3.36 ± 0.01 | 3.33 ± 0.14 | 0.52 ± 0.01 | 4.48 ± 0.01 |
| RN1.5 | 85.43 ± 1.24 | 3.36 ± 0.04 | 3.36 ± 0.09 | 0.54 ± 0.01 | 4.47 ± 0.03 |
| Sample | L* | a* | b* | ΔE | WI |
|---|---|---|---|---|---|
| Control | 81.25 ± 1.14 e | −4.23 ± 0.01 a | 13.14 ± 0.09 g | 76.72 ± 1.23 f | |
| RN0.5 | 56.38 ± 0.89 c | 5.65 ± 0.23 d | 2.45 ± 0.01 d | 28.82 ± 0.56 b | 55.95 ± 0.85 d |
| RN1.0 | 51.44 ± 0.57 b | 6.42 ± 0.21 de | 0.35 ± 0.01 c | 34.14 ± 0.18 c | 51.02 ± 0.78 c |
| RN1.5 | 40.28 ± 0.63 a | 7.47 ± 0.35 e | −1.21 ± 0.02 b | 44.96 ± 0.28 e | 39.80 ± 0.52 a |
| FN0.5 | 67.65 ± 1.05 d | 2.14 ± 0.02 b | 5.43 ± 0.11 f | 16.88 ± 0.15 a | 67.13 ± 1.05 e |
| FN1.0 | 49.58 ± 0.65 b | 4.63 ± 0.04 c | 3.56 ± 0.08 e | 34.25 ± 0.42 c | 49.24 ± 0.79 c |
| FN1.5 | 47.15 ± 0.85 b | 4.78 ± 0.03 c | −2.37 ± 0.03 a | 38.53 ± 0.24 d | 46.88 ± 0.63 b |
| Sample | Storage Time, Days | ||||
|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | |
| Control | 23.11 ± 0.14 b | 24.32 ± 0.10 bc | 25.34 ± 0.14 b | 26.41 ± 0.10 b | 28.06 ± 0.14 c |
| FN0.5 | 24.15 ± 0.09 c | 26.52 ± 0.15 c | 26.95 ± 0.10 c | 27.24 ± 0.08 c | 29.34 ± 0.14 d |
| FN1.0 | 22.25 ± 0.13 ab | 23.28 ± 0.08 b | 23.68 ± 0.08 a | 24.52 ± 0.08 ab | 26.04 ± 0.10 b |
| FN1.5 | 22.03 ± 0.06 ab | 22.75 ± 0.12 a | 23.22 ± 0.12 a | 23.84 ± 0.10 a | 24.48 ± 0.09 a |
| RN0.5 | 24.23 ± 0.12 c | 25.71 ± 0.08 c | 26.06 ± 0.10 b | 27.05 ± 0.11 c | 28.92 ± 0.12 c |
| RN1.0 | 21.94 ± 0.07 a | 22.85 ± 0.12 a | 23.32 ± 0.16 a | 24.56 ± 0.14 ab | 26.22 ± 0.08 b |
| RN1.5 | 21.48 ± 0.12 a | 22.07 ± 0.12 a | 22.78 ± 0.12 a | 24.24 ± 0.12 ab | 25.04 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covaliov, E.; Capcanari, T.; Reșitca, V.; Chirsanova, A.; Boiștean, A.; Sturza, R.; Patras, A.; Pocol, C.B.; Ruseva, O.; Chioru, A. Exploring the Biological Value of Red Grape Skin: Its Incorporation and Impact on Yogurt Quality. Foods 2024, 13, 3254. https://doi.org/10.3390/foods13203254
Covaliov E, Capcanari T, Reșitca V, Chirsanova A, Boiștean A, Sturza R, Patras A, Pocol CB, Ruseva O, Chioru A. Exploring the Biological Value of Red Grape Skin: Its Incorporation and Impact on Yogurt Quality. Foods. 2024; 13(20):3254. https://doi.org/10.3390/foods13203254
Chicago/Turabian StyleCovaliov, Eugenia, Tatiana Capcanari, Vladislav Reșitca, Aurica Chirsanova, Alina Boiștean, Rodica Sturza, Antoanela Patras, Cristina Bianca Pocol, Olga Ruseva, and Ana Chioru. 2024. "Exploring the Biological Value of Red Grape Skin: Its Incorporation and Impact on Yogurt Quality" Foods 13, no. 20: 3254. https://doi.org/10.3390/foods13203254
APA StyleCovaliov, E., Capcanari, T., Reșitca, V., Chirsanova, A., Boiștean, A., Sturza, R., Patras, A., Pocol, C. B., Ruseva, O., & Chioru, A. (2024). Exploring the Biological Value of Red Grape Skin: Its Incorporation and Impact on Yogurt Quality. Foods, 13(20), 3254. https://doi.org/10.3390/foods13203254









