On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Strawberry Samples
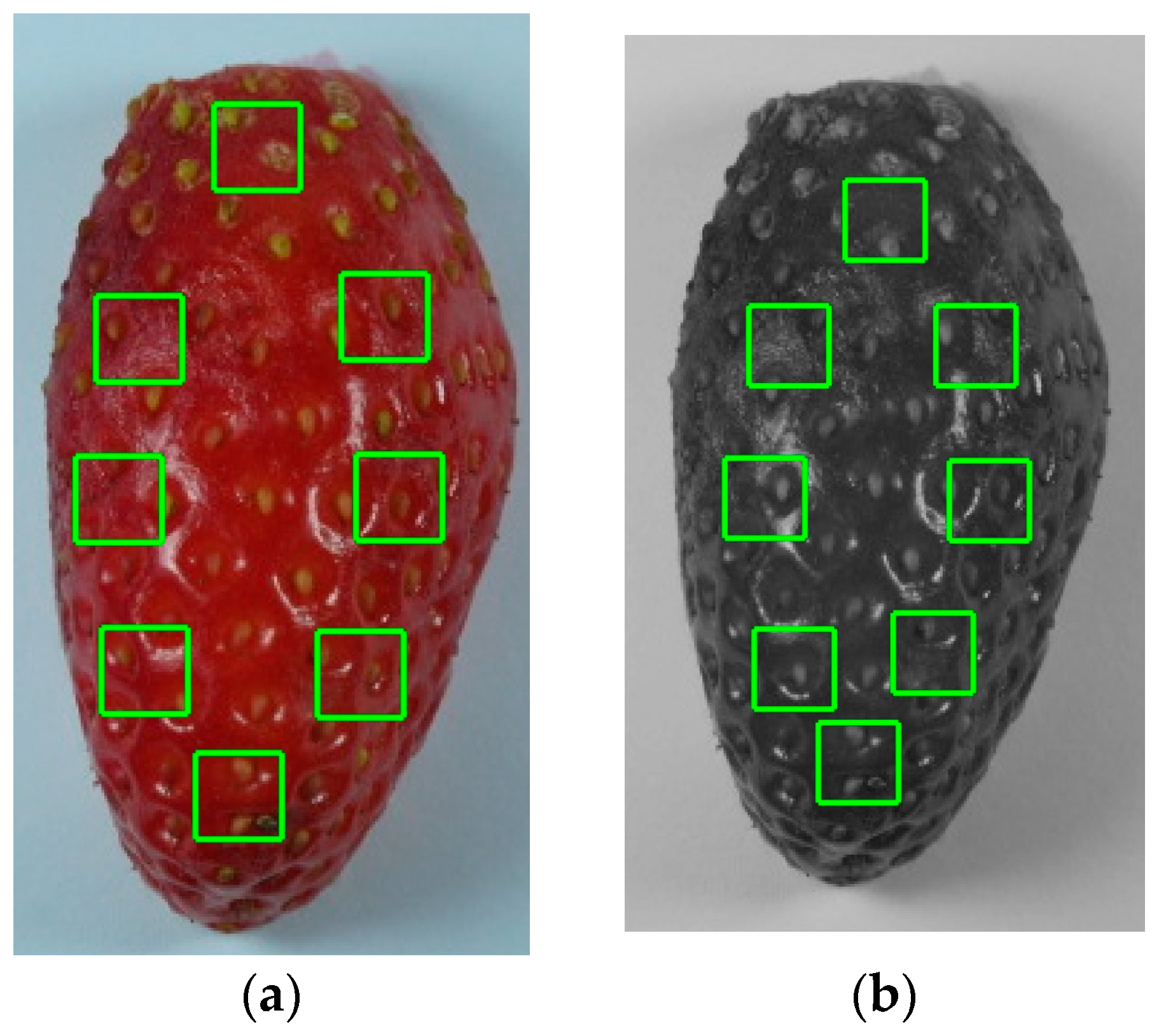
2.2. Image Analysis
2.3. Physicochemical Measurements during Strawberries’ Shelf-Life
2.4. Phenolic Compound Extraction and Analysis Using Spectrophotometric Methods
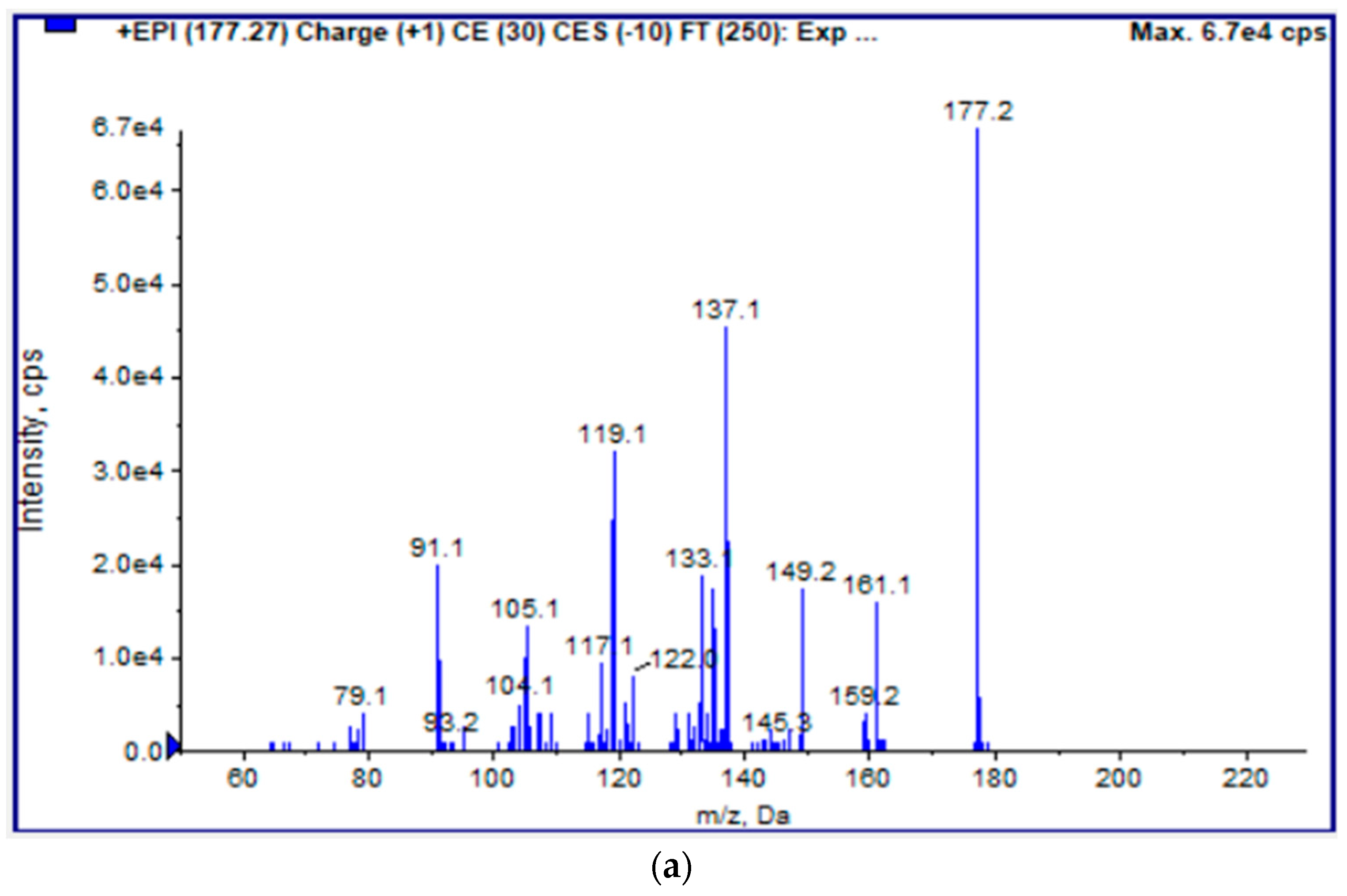
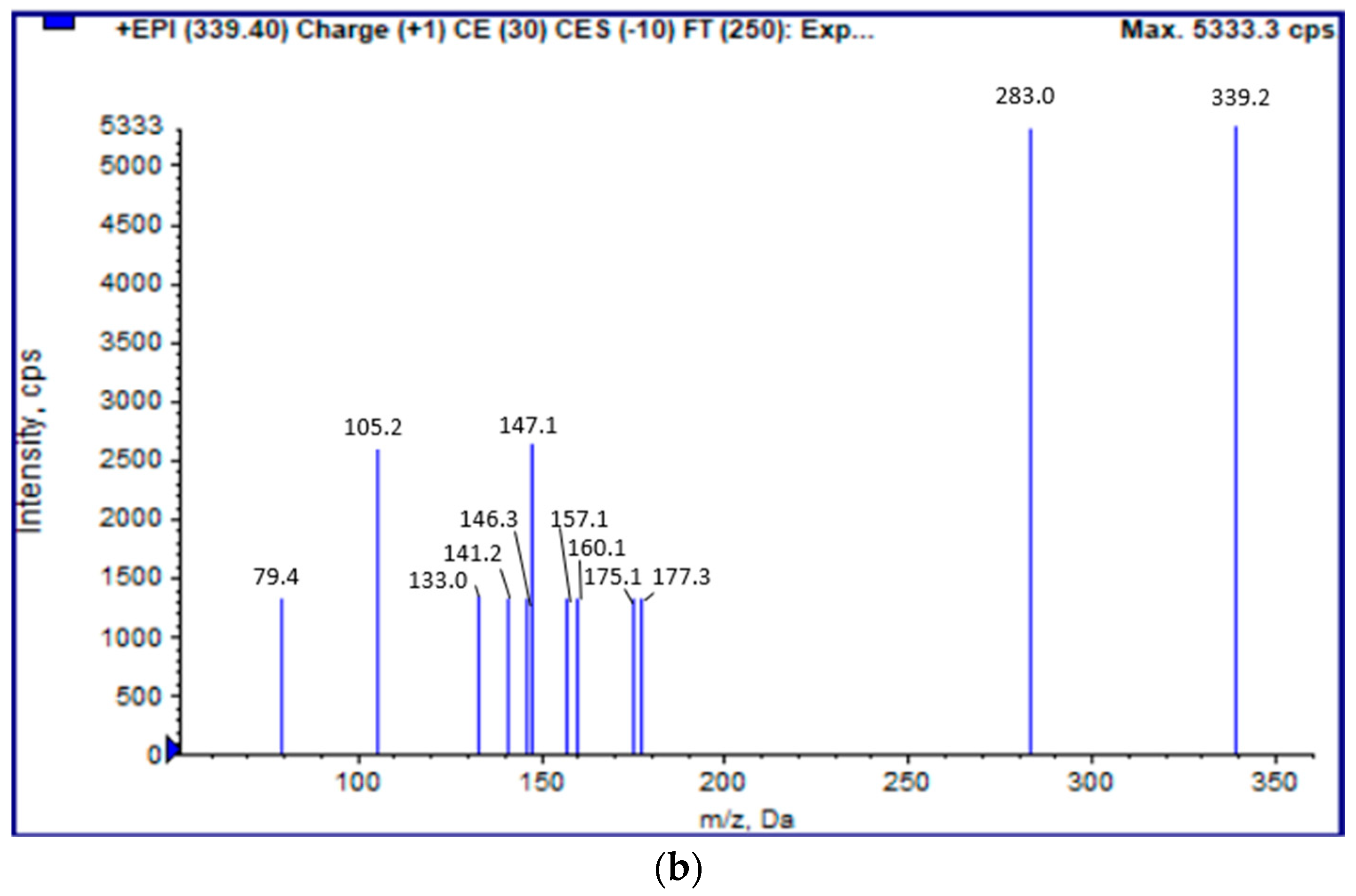
2.5. LC-ESI(+)-MS/MS Analysis
2.6. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (ATR-FTIR)
2.7. Discriminant Analysis
2.8. Statistical Analysis
3. Results and Discussion
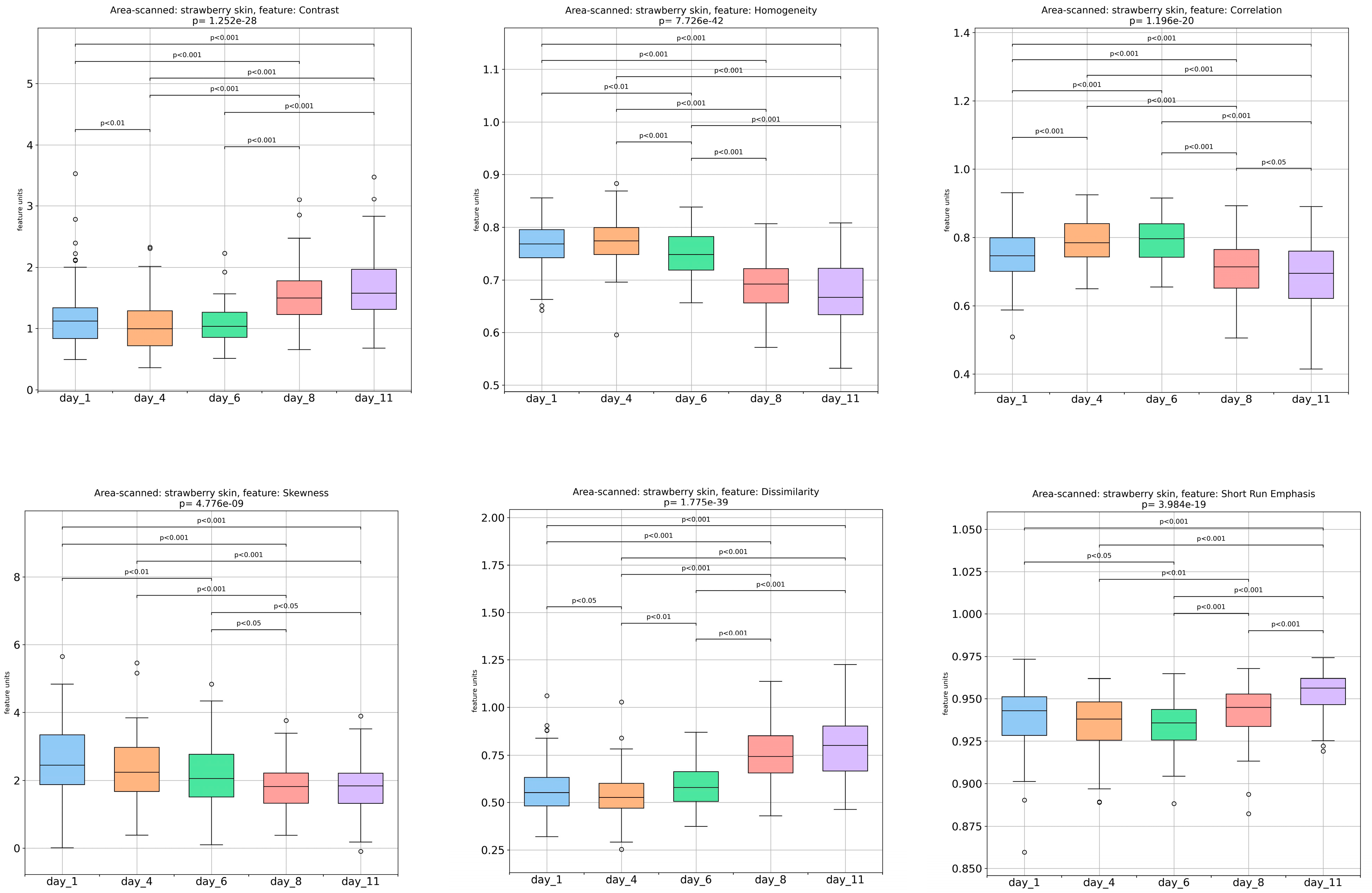
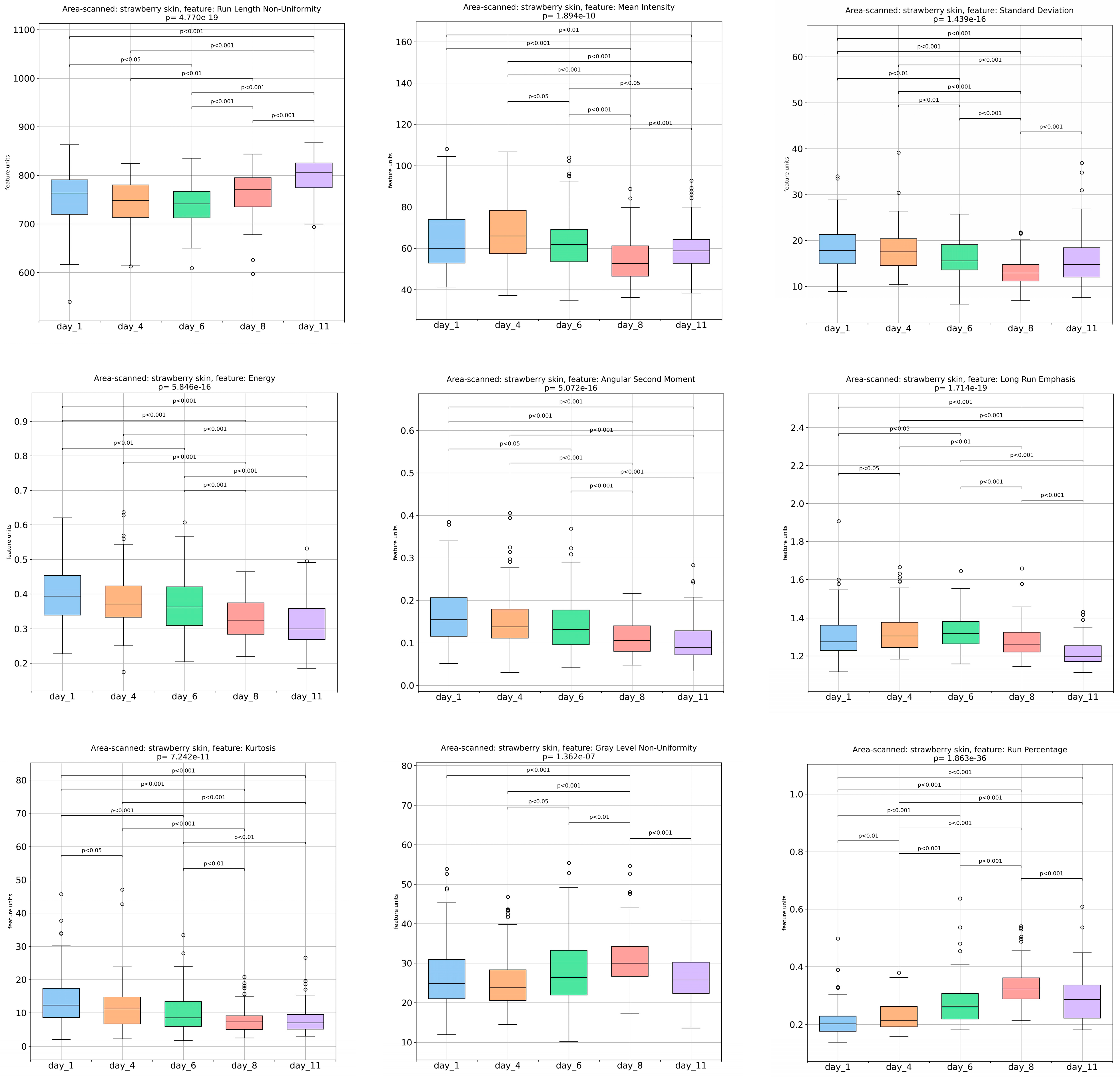
3.1. Textural-Image Analysis of Strawberries’ Outer Surface during Storage
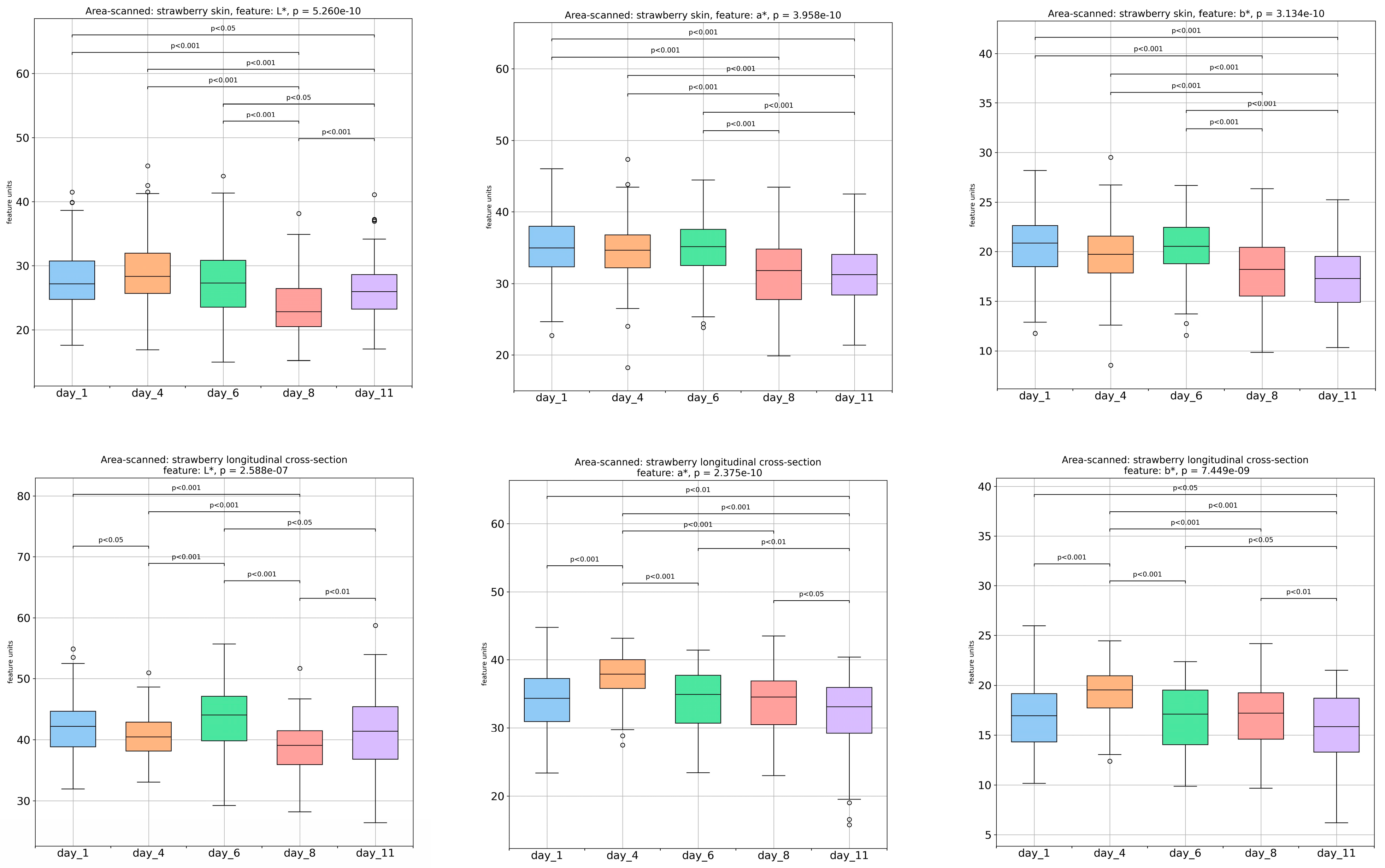
3.2. Image Analysis-Extracted Color Parameters of Strawberries’ Outer and Inner Surface
3.3. Storage Effect on the Physicochemical Features of Strawberries
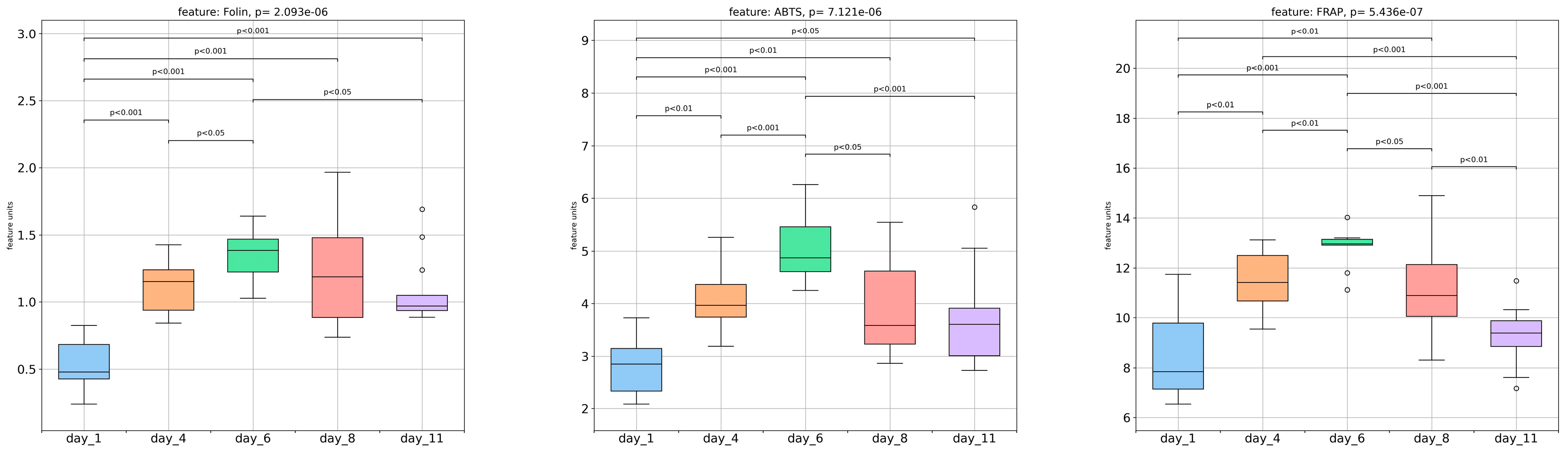
3.4. Spectrophotometric Assays
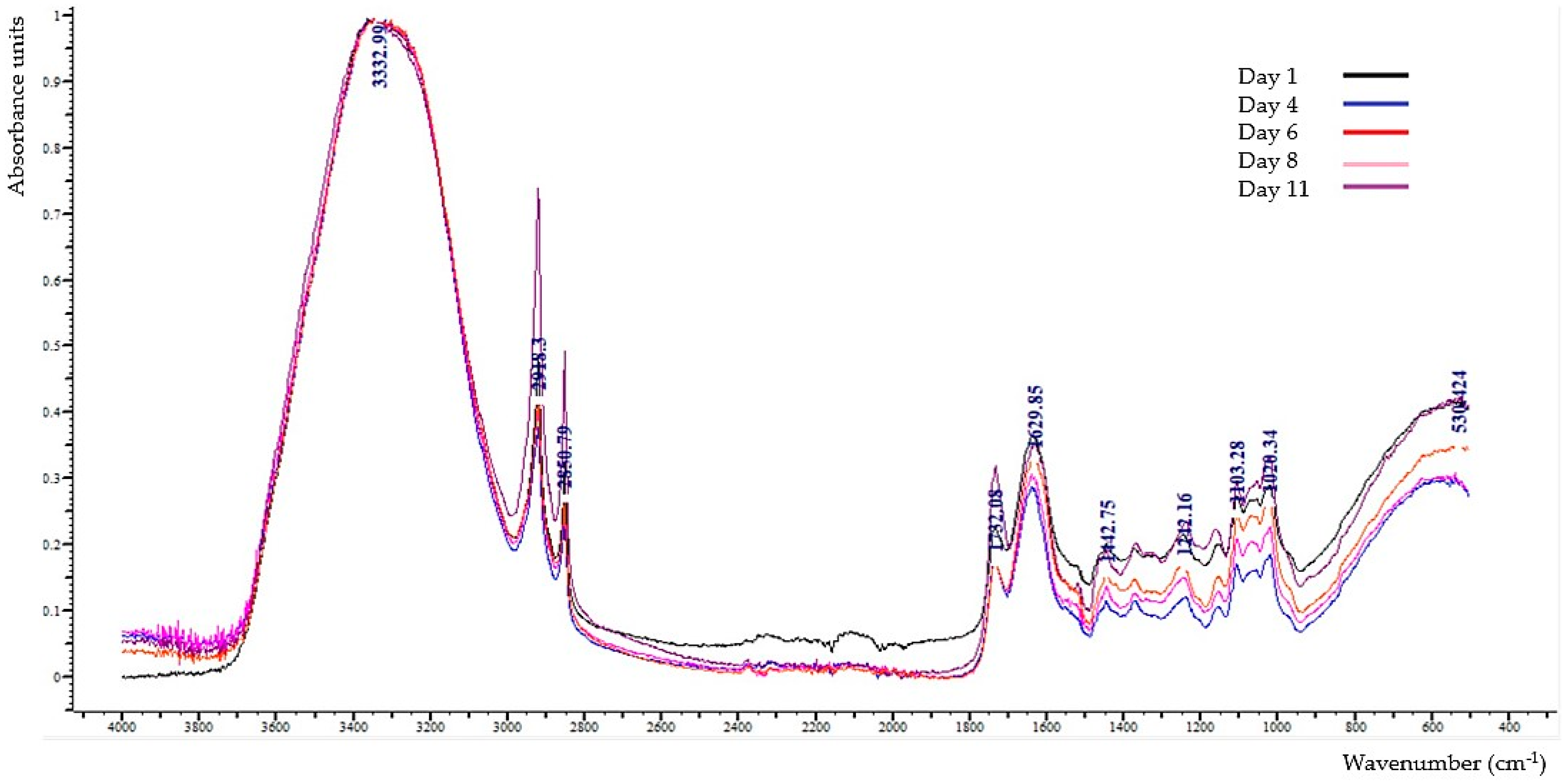
3.5. Interpretation of ATR-FTIR (Attenuated Total Reflection-Fourier Transform Infrared) Spectra
3.6. Statistical Analysis
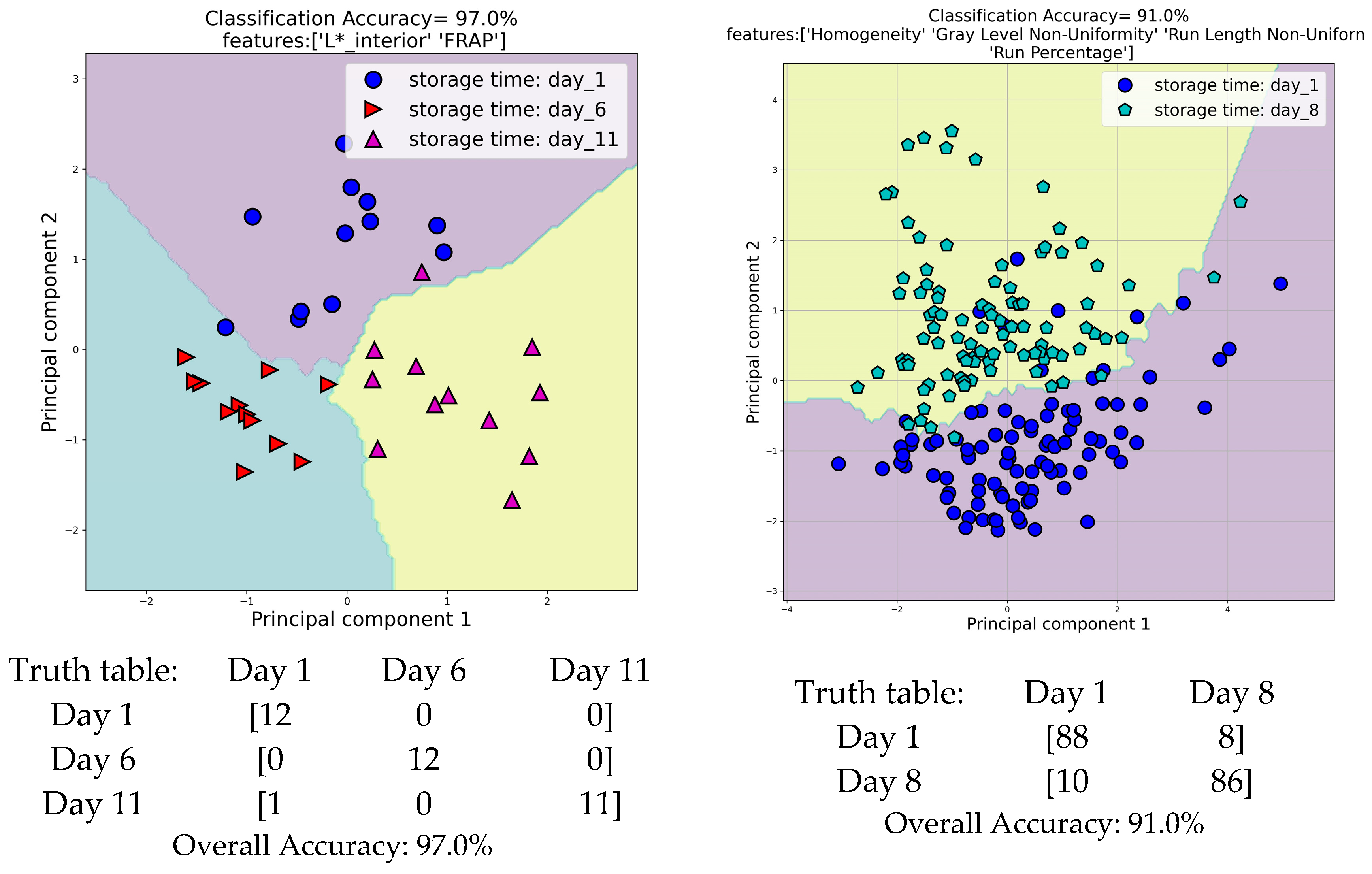
3.6.1. Discriminant Analysis
3.6.2. Regression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A Review of Strawberry Photobiology and Fruit Flavonoids in Controlled Environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Ejaz, S.; Naveed Ur Rehman, R.; Khan, M.; Qadri, R. Postharvest Quality Management of Strawberries. In Strawberry-Pre-and Post-Harvest Management Techniques for Higher Fruit Quality; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83962-253-3. [Google Scholar]
- Drobek, M.; Frąc, M.; Zdunek, A.; Cybulska, J. The Effect of Cultivation Method of Strawberry (Fragaria × ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage. Molecules 2020, 25, 4325. [Google Scholar] [CrossRef]
- Khammayom, N.; Maruyama, N.; Chaichana, C. The Effect of Climatic Parameters on Strawberry Production in a Small Walk-In Greenhouse. AgriEngineering 2022, 4, 104–121. [Google Scholar] [CrossRef]
- Sreedevi, T.R.; Santosh Kumar, M.B. Digital Twin in Smart Farming: A Categorical Literature Review and Exploring Possibilities in Hydroponics. In Proceedings of the 2020 Advanced Computing and Communication Technologies for High Performance Applications (ACCTHPA), Cochin, India, 2–4 July 2020; pp. 120–124. [Google Scholar]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current State and Future Perspectives of Commercial Strawberry Production: A Review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of Quality and Shelf-Life of Strawberries with Edible Coatings Enriched with Chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Kelly, K.; Madden, R.; Emond, J.P.; Do Nascimento Nunes, M.C. A Novel Approach to Determine the Impact Level of Each Step along the Supply Chain on Strawberry Quality. Postharvest Biol. Technol. 2019, 147, 78–88. [Google Scholar] [CrossRef]
- Bhat, R.; Stamminger, R. Preserving Strawberry Quality by Employing Novel Food Preservation and Processing Techniques—Recent Updates and Future Scope—An Overview: Preservation and Processing Techniques for Strawberries. J. Food Process Eng. 2015, 38, 536–554. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Genovese, M.I.; Oliveira Do Nascimento, J.R.; Aymoto Hassimotto, N.M.; José Dos Santos, R.; Lajolo, F.M. Effects of Temperature on the Chemical Composition and Antioxidant Activity of Three Strawberry Cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Falah, M.A.F.; Husna, H.I.; Andam Dewi, A.R.P.; Jumeri. Quality evaluation of fresh strawberry (Fragaria sp. cv. Earlybrite) during storage in a tropical environment. AIP Conf. Proc. 2016, 1755, 130003. [Google Scholar]
- Joshi, P.; Pahariya, P.; Al-Ani, M.F.; Choudhary, R. Monitoring and Prediction of Sensory Shelf-life in Strawberry with Ultraviolet-visible-near-infrared (UV-VIS-NIR) Spectroscopy. Appl. Food Res. 2022, 2, 100123. [Google Scholar] [CrossRef]
- Jouki, M.; Dadashpour, A. Comparison of Physiochemical Changes in Two Popular Strawberry Cultivars Grown in Iran (Cvs. Kurdistan & Selva) during Storage Time at 4 °C. Genetika 2012, 44, 679–688. [Google Scholar] [CrossRef]
- Koyuncu, M.A. Quality Changes of three Strawberry Cultivars during the Cold Storage. Europ. J. Hort. Sci. 2004, 69, 193–200. [Google Scholar]
- Lee, C.; Lee, J.; Lee, J. Relationship of Fruit Color and Anthocyanin Content with Related Gene Expression Differ in Strawberry Cultivars during Shelf Life. Sci. Hortic. 2022, 301, 111109. [Google Scholar] [CrossRef]
- Pelayo, C.; Ebeler, S.E.; Kader, A.A. Postharvest Life and Flavor Quality of Three Strawberry Cultivars Kept at 5 °C in Air or Air + 20 kPa CO2. Postharvest Biol. Technol. 2003, 27, 171–183. [Google Scholar] [CrossRef]
- Rahman, M.M.; Moniruzzaman, M.; Ahmad, M.R.; Sarker, B.C.; Khurshid Alam, M. Maturity Stages Affect the Postharvest Quality and Shelf-Life of Fruits of Strawberry Genotypes Growing in Subtropical Regions. J. Saudi Soc. Agric. Sci. 2016, 15, 28–37. [Google Scholar] [CrossRef]
- Rutkowski, K.P.; Kruczynska, D.E.; Zurawicz, E. Quality and shelf life of strawberry cultivars in Poland. Acta Hortic. 2006, 708, 329–332. [Google Scholar] [CrossRef]
- Shin, Y.; Ryu, J.-A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest Maturity, Storage Temperature and Relative Humidity Affect Fruit Quality, Antioxidant Contents and Activity, and Inhibition of Cell Proliferation of Strawberry Fruit. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, B.G.; Li, W.S.; Yang, Y.; Shi, L.; Yang, J.J. Physical and chemical characteristics of three strawberry cultivars during cold storage. Acta Hortic. 2014, 1049, 939–946. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.; Liu, F.; Kong, W.; He, Y.; Lou, B. Hyperspectral Imaging Analysis for Ripeness Evaluation of Strawberry with Support Vector Machine. J. Food Eng. 2016, 179, 11–18. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Tsiaka, T.; Aouant, K.; Mouka, E.; Ladika, G.; Kritsi, E.; Konteles, S.J.; Ioannou, A.-G.; Zoumpoulakis, P.; Strati, I.F.; et al. Quality Assessment of Banana Ripening Stages by Combining Analytical Methods and Image Analysis. Appl. Sci. 2023, 13, 3533. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Stavropoulou, N.; Tsironi, T.; Lougovois, V.; Kyrana, V.; Konteles, S.J.; Sinanoglou, V.J. Application of Hurdle Technology for the Shelf Life Extension of European Eel (Anguilla anguilla) Fillets. Aquac. Fish. 2023, 8, 393–402. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis Laboratory Manual; Food Science Text Series; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-44125-2. [Google Scholar]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal Distillates: A New Era of Grape Marc Distillates with Enriched Antioxidant Profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical—Antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.; Proestos, C. Comparison of the Antioxidant and Antiradical Activity of Pomegranate (Punica granatum L.) by Ultrasound-Assisted and Classical Extraction. Anal. Lett. 2016, 49, 969–978. [Google Scholar] [CrossRef]
- Tsiaka, T.; Kritsi, E.; Lantzouraki, D.Z.; Christodoulou, P.; Tsigrimani, D.; Strati, I.F.; Sinanoglou, V.J.; Zoumpoulakis, P. Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis. Appl. Sci. 2022, 12, 8361. [Google Scholar] [CrossRef]
- Ioannou, A.G.; Kritsi, E.; Sinanoglou, V.J.; Cavouras, D.; Tsiaka, T.; Houhoula, D.; Zoumpoulakis, P.; Strati, I.F. Highlighting the Potential of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy to Characterize Honey Samples with Principal Component Analysis (PCA). Anal. Lett. 2023, 56, 789–806. [Google Scholar] [CrossRef]
- Takemoto, R.; Koyama, K.; Watanabe, T.; Koseki, S.; Nakamura, N. Mathematical Model for Analyzing the Effect of Storage Conditions on the Visually Perceived Freshness of Strawberries via Surface Luminance Distribution. Food Packag. Shelf Life 2022, 34, 100965. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.T.; Cho, J.; McGhie, T.K.; Larsen, D.S.; Schaffer, R.J.; Espley, R.V.; Perry, N.B. Discovery of a stable vitamin C glycoside in crab apples (Malus sylvestris). Phytochemistry 2020, 173, 112297. [Google Scholar] [CrossRef] [PubMed]
- Nyorere, O. Instrumental Texture Profile Analysis (TPA) of Cucumber Fruit as Influenced by Its Part and Maturity Stage. Am. J. Eng. Technol. Manag. 2018, 3, 54. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR Spectroscopy to the Quantification of Sugar in Honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of Sugars and Phenolic Compounds in Honey Powders with the Use of GC–MS, FTIR Spectroscopy, and X-Ray Diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef] [PubMed]
- Minutti-López Sierra, P.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Meza-Márquez, O.G. Chemical Composition and Antioxidant Capacity in Strawberry Cultivars (Fragaria × ananassa Duch.) by FT-MIR Spectroscopy and Chemometrics. CyTA J. Food 2019, 17, 724–732. [Google Scholar] [CrossRef]
- Brangule, A.; Šukele, R.; Bandere, D. Herbal Medicine Characterization Perspectives Using Advanced FTIR Sample Techniques—Diffuse Reflectance (DRIFT) and Photoacoustic Spectroscopy (PAS). Front. Plant Sci. 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Bello-Pérez, L.A.; Ottenhof, M.-A.; Agama-Acevedo, E.; Farhat, I.A. Effect of Storage Time on the Retrogradation of Banana Starch Extrudate. J. Agric. Food Chem. 2005, 53, 1081–1086. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared Spectroscopy of Carbohydrates: A Review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Shimomura, K.; Horie, H.; Sugiyama, M.; Kawazu, Y.; Yoshioka, Y. Quantitative Evaluation of Cucumber Fruit Texture and Shape Traits Reveals Extensive Diversity and Differentiation. Sci. Hortic. 2016, 199, 133–141. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.D.; Passos, T.M.; Quilty, B.; Thiré, R.M.D.S.M.; McGuinness, G.B. FTIR Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing. Matér. Rio Jan. 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Cassani, L.; Santos, M.; Gerbino, E.; Del Rosario Moreira, M.; Gómez-Zavaglia, A. A Combined Approach of Infrared Spectroscopy and Multivariate Analysis for the Simultaneous Determination of Sugars and Fructans in Strawberry Juices during Storage. J. Food Sci. 2018, 83, 631–638. [Google Scholar] [CrossRef]
- Grassi, S.; Amigo, J.M.; Lyndgaard, C.B.; Foschino, R.; Casiraghi, E. Assessment of the Sugars and Ethanol Development in Beer Fermentation with FT-IR and Multivariate Curve Resolution Models. Food Res. Int. 2014, 62, 602–608. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; Calvo-Gómez, O.; Jofre-Garfias, A.E.; Dávalos-González, P.A.; Desjardins, Y.; López, M.G. Fructooligosaccharides as Molecular Markers of Geographic Origin, Growing Region, Genetic Background and Prebiotic Potential in Strawberries: A TLC, HPAEC-PAD and FTIR Study. Food Chem. Adv. 2022, 1, 100064. [Google Scholar] [CrossRef]


| Days | h (Internal Surface) | h (External Surface) |
|---|---|---|
| 1 | 46.62 ± 2.04 | 24.86 ± 3. 40 |
| 4 | 44.45 ± 1.68 | 26.60 ± 4.46 |
| 6 | 45.70 ± 1.61 | 25.74 ± 4.19 |
| 8 | 45.36 ± 0.70 | 26.81 ± 4.71 |
| 11 | 44.26 ± 6.74 | 25.26 ± 2.38 |
| Days | Firmness | Adhesiveness | Cohesiveness |
|---|---|---|---|
| 1 | 4.70 ± 1.66 ab 1 | −0.024 ± 0.018 a | 0.26 ± 0.03 a |
| 4 | 6.40 ± 1.91 a | −0.023 ± 0.013 a | 0.30 ± 0.04 ab |
| 6 | 4.24 ± 1.17 b | −0.039 ± 0.013 ab | 0.25 ± 0.04 a |
| 8 | 4.88 ± 1.20 ab | −0.031 ± 0.015 ab | 0.27 ± 0.03 a |
| 11 | 5.07 ± 1.44 ab | −0.044 ± 0.019 b | 0.34 ± 0.10 b |
| Regions (cm−1) | Day 1 | Day 4 | Day 6 | Day 8 | Day 11 |
|---|---|---|---|---|---|
| 3645–3600 | - | 0.020 ± 0.003 b 1 | 0.011 ± 0.003 c | 0.023 ± 0.009 b | 0.011 ± 0.003 c |
| 3380 | 0.006 ± 0.001 ac | 0.004 ± 0.002 b | 0.007 ± 0.001 c | 0.006 ± 0.001 ac | 0.005 ± 0.001 ab |
| 2918–2920 | 0.317 ± 0.040 ab | 0.251 ± 0.070 a | 0.370 ± 0.063 b | 0.292 ± 0.072 a | 0.279 ± 0.062 a |
| 2850–2855 | 0.181 ± 0.030 ab | 0.134 ± 0.048 a | 0.210 ± 0.053 b | 0.174 ± 0.040 ab | 0.202 ± 0.038 b |
| 1730–1742 | 0.111 ± 0.014 a | 0.100 ± 0.021 a | 0.109 ± 0.030 a | 0.104 ± 0.017 a | 0.093 ± 0.016 a |
| 1632–1647 | 0.171 ± 0.012 a | 0.184 ± 0.012 ab | 0.188 ± 0.010 ab | 0.197 ± 0.023 b | 0.190 ± 0.013 b |
| 1510–1520 | 0.010 ± 0.005 ac | 0.007 ± 0.003 c | 0.017 ± 0.006 b | 0.012 ± 0.004 a | 0.011 ± 0.003 a |
| 1420–1457 | 0.020 ± 0.006 a | 0.019 ± 0.006 a | 0.012 ± 0.003 b | 0.017 ± 0.005 ab | 0.019 ± 0.004 a |
| 1351–1378 | 0.017 ± 0.004 a | 0.006 ± 0.004 b | 0.018 ± 0.004 a | 0.015 ± 0.005 a | 0.021 ± 0.003 a |
| 1245–1230 | 0.046 ± 0.006 a | 0.035 ± 0.007 b | 0.047 ± 0.012 a | 0.043 ± 0.014 ab | 0.054 ± 0.009 a |
| 1149–1155 | 0.023 ± 0.009 a | 0.021 ± 0.008 a | 0.026 ± 0.004 a | 0.020 ± 0.010 a | 0.022 ± 0.011 a |
| 1105 | 0.058 ± 0.011 a | 0.052 ± 0.012 a | 0.054 ± 0.007 a | 0.053 ± 0.009 a | 0.056 ± 0.010 a |
| 1050–1055 | 0.011 ± 0.003 a | 0.006 ± 0.003 b | 0.011 ± 0.004 a | 0.008 ± 0.001 ab | 0.015 ± 0.002 c |
| 1022–1028 | 0.068 ± 0.013 ab | 0.054 ± 0.013 a | 0.060 ± 0.015 a | 0.064 ± 0.018 a | 0.081 ± 0.008 b |
| 750–720 | 0.004 ± 0.002 a | 0.003 ± 0.001 ab | 0.002 ± 0.001 b | 0.003 ± 0.001 ab | 0.002 ± 0.001 b |
| 680–610 | 0.001 ± 0.001 a | 0.002 ± 0.002 a | 0.001 ± 0.003 a | 0.001 ± 0.001 a | - |
| 523 | 0.009 ± 0.002 a | 0.010 ± 0.003 a | 0.010 ± 0.003 a | 0.009 ± 0.002 a | 0.008 ± 0.002 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods 2024, 13, 234. https://doi.org/10.3390/foods13020234
Ladika G, Strati IF, Tsiaka T, Cavouras D, Sinanoglou VJ. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods. 2024; 13(2):234. https://doi.org/10.3390/foods13020234
Chicago/Turabian StyleLadika, Georgia, Irini F. Strati, Thalia Tsiaka, Dionisis Cavouras, and Vassilia J. Sinanoglou. 2024. "On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics" Foods 13, no. 2: 234. https://doi.org/10.3390/foods13020234
APA StyleLadika, G., Strati, I. F., Tsiaka, T., Cavouras, D., & Sinanoglou, V. J. (2024). On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods, 13(2), 234. https://doi.org/10.3390/foods13020234







