Surface Modification of Okara Cellulose Crystals with Phenolic Acids to Prepare Multifunction Emulsifier with Antioxidant Capacity and Lipolysis Retardation Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Okara Cellulose Crystal with Phenolic Compounds
Characterization of the Modified OC
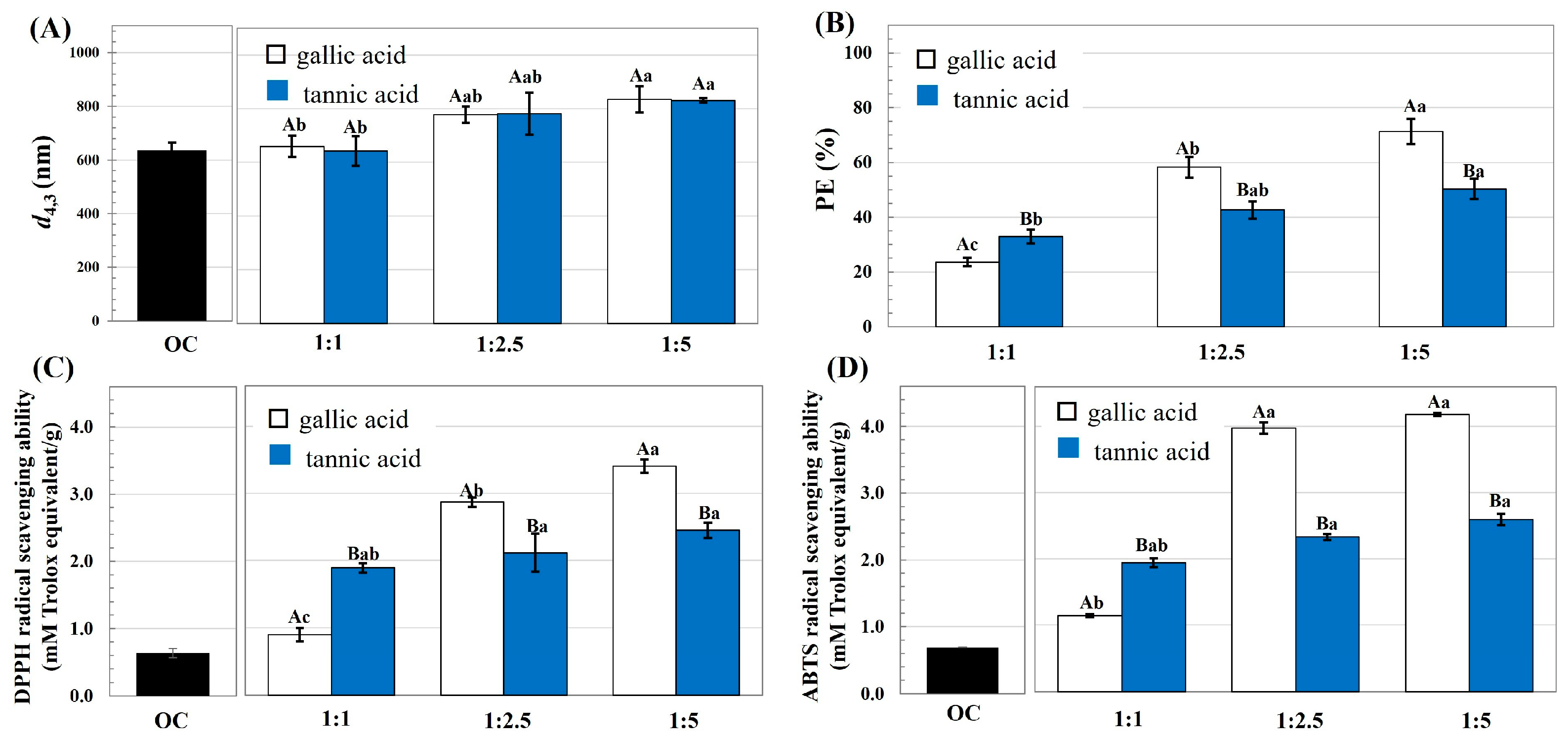
- Particle size: The modified OC samples were dispersed in phosphate buffer (10 mM, pH 7.0) at a concentration of 0.1%. The hydrodynamic size of the particles was determined using a static light particle size analyzer (Zetasizer model nano series; Malvern; Worcestershire, UK) at 25 °C. The volume-weighted diameter (d4,3 = ∑ nidi4/nidi3) was reported, where ni is the number of particles with diameter di.
- Structural characteristics: The structural characteristics of the OC as affected by the modification were determined using Fourier transform infrared spectrometry (FTIR; Tensor 27 spectrometer; Bruker; Ettlingen, Germany) with 16 scans, at 4 cm−1 resolution over the wavelength range 4000–500 cm−1. Moreover, the chemical characteristics of the modified OC were elucidated using 13C solid-state nuclear magnetic resonance (13C NMR; AVANCE II HD/Ascend 400WB; Bruker; Ettlingen, Germany) at 214 MHz with 4000 scans and a spinning rate of 15 KHz.
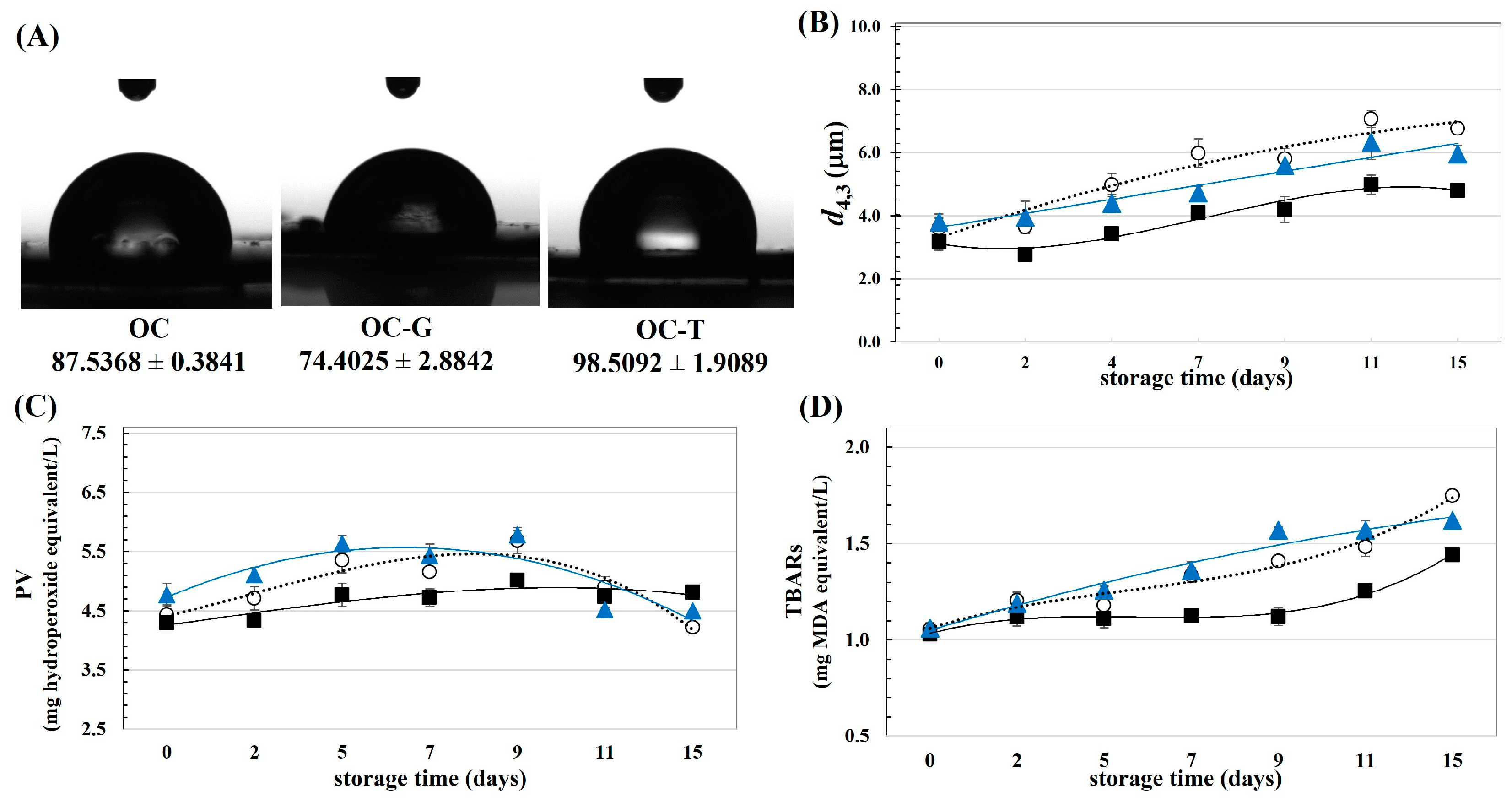
- Contact angle: Pellets of cellulosic materials with a planar surface were dropped with 5 μL of distilled water. Then, the contact angles were determined using a contact angle analyzer (OCA-15EC; Dataphysics GmbH; Filderstadt, Germany). The shape and edge characteristic of the water drop on the OC pellet was recorded by a high-speed video camera accompanied by the software of the apparatus. The contact angles formed by both ends of the water drop were then calculated.
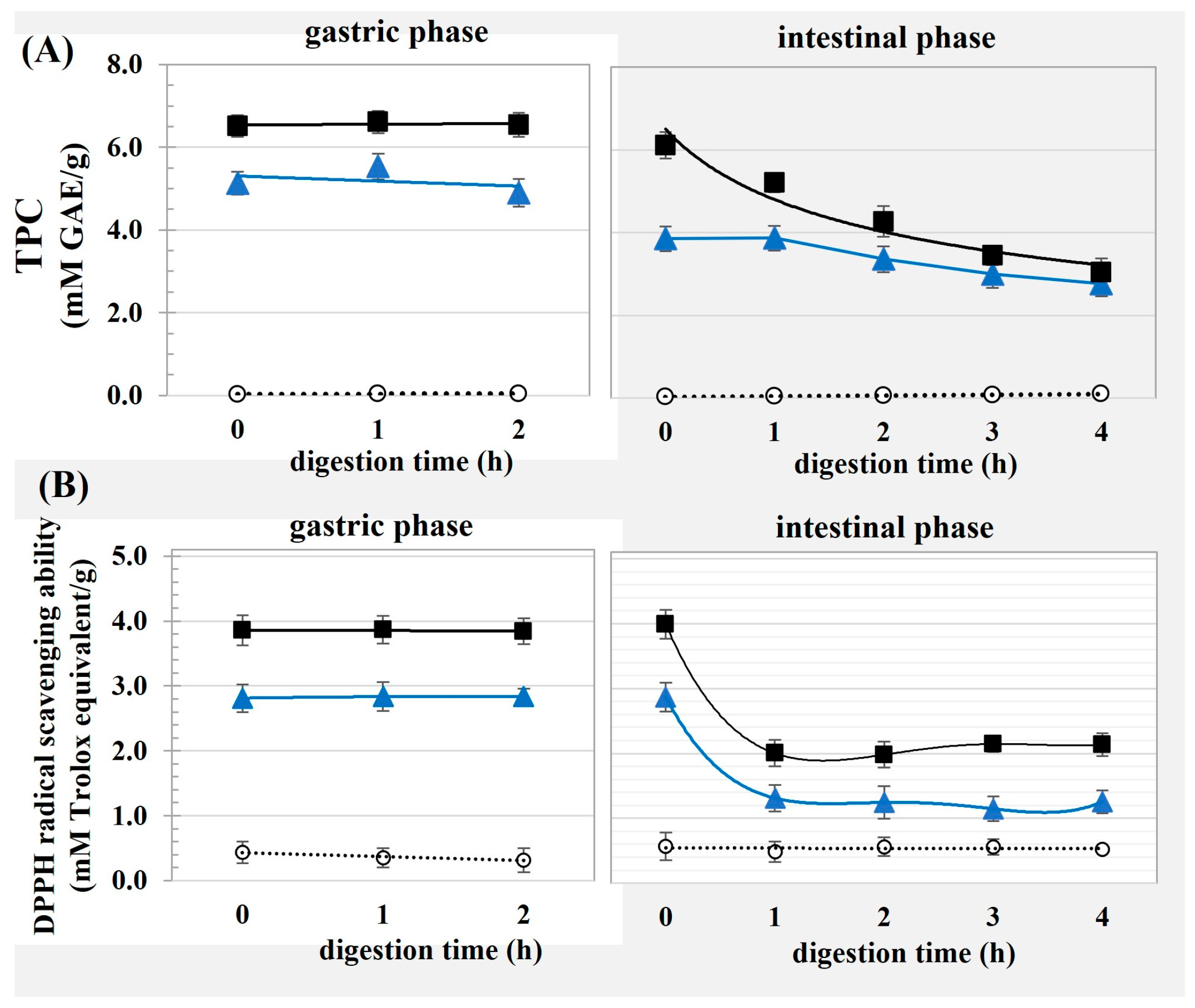
- Phenolic loading efficiency (PE): The cellulose samples were dissolved in ethanol to determine the total phenolic content (TPC) using the Folin–Ciocalteu assay as per the method of Javanmardi et al. [10]. TPC was quantified using gallic acid as a standard, with the PE calculated using Equation (1).where TPCcomplex and TPCOC represent the TPC values of the modified OC and unmodified OC, respectively, and WOC is the weight of the OC.
- Antioxidant activity: The DPPH and ABTS radical inhibition capacities were determined as per the method of Thaipong et al. [11], and reported as milli moles Trolox equivalent per gram of sample.
2.3. Effect of Modified Okara Cellulose Crystal on Emulsion Stability
- Colloidal stability: Dispersibility of the emulsions was evaluated by measuring the d4,3 of the oil droplets.
- Oxidative stability: The oxidative degree of the emulsions was quantified by measuring the peroxide value (PV) and the content of thiobarbituric acid reactive substances (TBARs) as per the methods of Prabsangob and Benjakul [12]. PV and TBARs were reported as milligrams hydroperoxide equivalent per liter and milligrams malondialdehyde (MDA) equivalent per liter, respectively.
2.4. In Vitro Digestion of the Emulsion
2.5. Statistical Analysis
3. Results
3.1. Characteristics of OC Modified by Phenolic Compounds
3.2. Effect of Modified OC on the Physical and Chemical Stability of Emulsion
3.3. In Vitro Digestion of Emulsions Stabilized Using Modified OC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Meng, R.; Li, X.L.; Liu, W.J.; Cheng, J.S.; Wang, W. Preparation of Pickering emulsion gels based on k-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibiltiy of curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-cellulose on starch and milk digestion and mineral adsorption. Int. J. Biol. Macromol. 2019, 137, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Prabsangob, N. Preparation and characterization of okara cellulose crystals as the emulsifier in a Pickering emulsion. J. Food Meas. Charact. 2022, 16, 4433–4443. [Google Scholar] [CrossRef]
- Ruiz-Henestrosa, V.M.; Bellesi, F.A.; Camino, N.A.; Pilosof, A.M.R. The impact of HPMC structure in the modulation of in vitro lipolysis: The role of bile salts. Food Hydrocoll. 2017, 62, 251–261. [Google Scholar] [CrossRef]
- Zeng, X.; Sheng, Z.; Li, X.; Fan, X.; Jiang, W. In vitro studies on the interactions of blood lipid level-related biological molecules with gallic acid and tannic acid. J. Sci. Food Agric. 2019, 99, 6882–6892. [Google Scholar] [CrossRef]
- Alzate-Arbeláez, F.A.; Dorta, E.; López-Alarcón, C.; Cortés, F.B.; Rojano, B.A. Immobilization of Andean berry (Vaccinium meridionale) polyphenols on nanocellulose isolated from banana residues: A natural food additive with antioxidant properties. Food Chem. 2019, 294, 503–517. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Xu, X.; Chen, Y.; Li, K.; Fan, X.; Pang, J.; Teng, H. Self-nano-emulsifying formulation of Sonchus oleraceus Linn for improved stability: Implications for phenolic degradation under in vitro gastrointestinal digestion. J. Funct. Foods 2019, 53, 28–35. [Google Scholar] [CrossRef]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-pot water based hydrophobic surface modification of cellulose nanocrystals using plant polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Akl, E.M.; Dacrory, S.; Abdel-Aziz, M.; Kamel, S.; Fahim, A.M. Preparation and characterization of novel antibacterial blended films based on modified carboxymethylcellulose/phenolic compounds. Polym. Bull. 2020, 78, 1061–1085. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Prabsangob, N.; Benjakul, S. Effect of tea catechin derivatives on stability of soybean oil/tea seed oil blend and oxidative stability of fried fish crackers during storage. Food Sci. Biotechnol. 2019, 28, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Le, H.D.; Loveday, S.M.; Singh, H.; Sarkar, A. Gastrointestinal digestion of Pickering emulsions stabilized by hydrophobically modified cellulose nanocrystals: Release of short-chain fatty acids. Food Chem. 2020, 320, 126650. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lopez-Sanchez, P.; Martinez-Sanz, M.; Gilbert, E.P.; Gidley, M.J. Adsorption isotherm studies on the interaction between polyphenols and apple cell walls: Effects of variety, heating and drying. Food Chem. 2019, 282, 58–66. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouchet, B.; Renard, C. Non-covalent interaction between procyanidins and apple cell wall material: Part III: Study on model polysaccharides. Biochem. Biophys. Acta 2005, 1725, 10–18. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Dongjie, W.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Hager, A.S.; Vallons, K.J.R.; Arendt, E.K. Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J. Agric. Food Chem. 2012, 60, 6157–6163. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. Dual-grafting of microcrystalline cellulose by tea polyphenols and cationic ε-polysine to tailor a structured antimicrobial soy-based emulsions for 3D printing. ACS Appl. Mater. Interfaces 2022, 14, 21392–21405. [Google Scholar] [CrossRef]
- Yadav, S.; Methrotra, G.K.; Dutta, P.L. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Mortada, W.I.; Kenawy, I.M.M.; El-Reash, Y.G.A.; Mousa, A.A. Microwave assisted modification of cellulose by gallic acid and its application for removal of aluminium from real samples. Int. J. Biol. Macromol. 2017, 101, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Amezcua, R.M.; Villanueva-Silva, R.J.; Muñoz-García, R.O.; Macias-Balleza, E.R.; Sydenstricker Flores-Sahagun, M.T.; Lomelí-Ramírez, M.G.; Torres-Rendón, J.G.; Garcia-Enriquez, S. Preparation of Agave tequilana Weber nanocrystalline cellulose and its use as reinforcement for acrylic hydrogels. BioResources 2021, 16, 2731–2746. [Google Scholar] [CrossRef]
- Sarria-Villa, R.A.; Gallo-Corredor, J.A.; Páez, M.I. Isolation of catechin and gallic acid from Colombian bark of Pinus patula. Chem. Sci. J. 2017, 8, 1000174. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hadadw, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Kibbelaar, H.V.M.; Dekker, R.I.; Morcy, A.; Kegal, W.K.; Velikov, K.P.; Bonn, D. Ethyl cellulose nanoparticles as stabilizers for Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128512. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent studies of Pickering emulsions; particles make the difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial concentrations of hydroxytyrosol and its lipophilic esters in intact olive oil-in-water emulsions: Effects of antioxidant hydrophobicity, surfactant concentration, and the oil-to-water ratio on the oxidative stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef]
- Kumagai, A.; Endo, T. Tannic acid-immobilized cellulose nanofiber prepared by esterification using polycarboxylic acid. Cellul. Chem. Technol. 2020, 54, 415–419. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, N.; Tang, C.H. Influence of nanocomplexation with curcumin on emulsifying properties and emulsion oxidative stability of soy protein isolate at pH 3.0 and 7.0. Food Hydrocoll. 2016, 61, 102–112. [Google Scholar] [CrossRef]
- López-Martinez, A.; Rocha-Uribe, A. Influences the partitioning of antioxidants in the interface improving oxidative stability in O/W emulsions rich in n-3 fatty acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700277. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based system? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Suleria, H.A.R.; Dunshea, F.R.; Howell, K. Dietary lipids influence bioaccessibility of polyphenols from black carrots and affect microbial diversity under simulated gastrointestinal digestion. Antioxidants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevonuc, A.; Demokritou, P. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2020, 101, 105503. [Google Scholar] [CrossRef]
- Mackie, A.; Gourcy, S.; Rigby, N.; Moffat, J.; Capron, I.; Bajka, B. The fate of cellulose nanocrystal stabilized emulsion after simulated gastrointestinal digestion and exposure to intestinal mucosa. Nanoscale 2019, 11, 2991–2998. [Google Scholar] [CrossRef]
- Bai, L.; Ly, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 2. In vitro lipid digestion. Food Hydrocoll. 2019, 96, 709–716. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabsangob, N.; Hangsalad, S.; Udomrati, S. Surface Modification of Okara Cellulose Crystals with Phenolic Acids to Prepare Multifunction Emulsifier with Antioxidant Capacity and Lipolysis Retardation Effect. Foods 2024, 13, 184. https://doi.org/10.3390/foods13020184
Prabsangob N, Hangsalad S, Udomrati S. Surface Modification of Okara Cellulose Crystals with Phenolic Acids to Prepare Multifunction Emulsifier with Antioxidant Capacity and Lipolysis Retardation Effect. Foods. 2024; 13(2):184. https://doi.org/10.3390/foods13020184
Chicago/Turabian StylePrabsangob, Nopparat, Sasithorn Hangsalad, and Sunsanee Udomrati. 2024. "Surface Modification of Okara Cellulose Crystals with Phenolic Acids to Prepare Multifunction Emulsifier with Antioxidant Capacity and Lipolysis Retardation Effect" Foods 13, no. 2: 184. https://doi.org/10.3390/foods13020184
APA StylePrabsangob, N., Hangsalad, S., & Udomrati, S. (2024). Surface Modification of Okara Cellulose Crystals with Phenolic Acids to Prepare Multifunction Emulsifier with Antioxidant Capacity and Lipolysis Retardation Effect. Foods, 13(2), 184. https://doi.org/10.3390/foods13020184





