Analysis of Polycyclic Aromatic Hydrocarbons via GC-MS/MS and Heterocyclic Amines via UPLC-MS/MS in Crispy Pork Spareribs for Studying Their Formation during Frying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
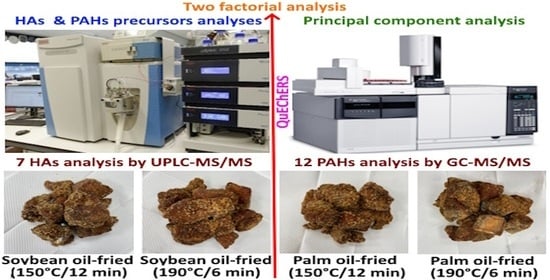
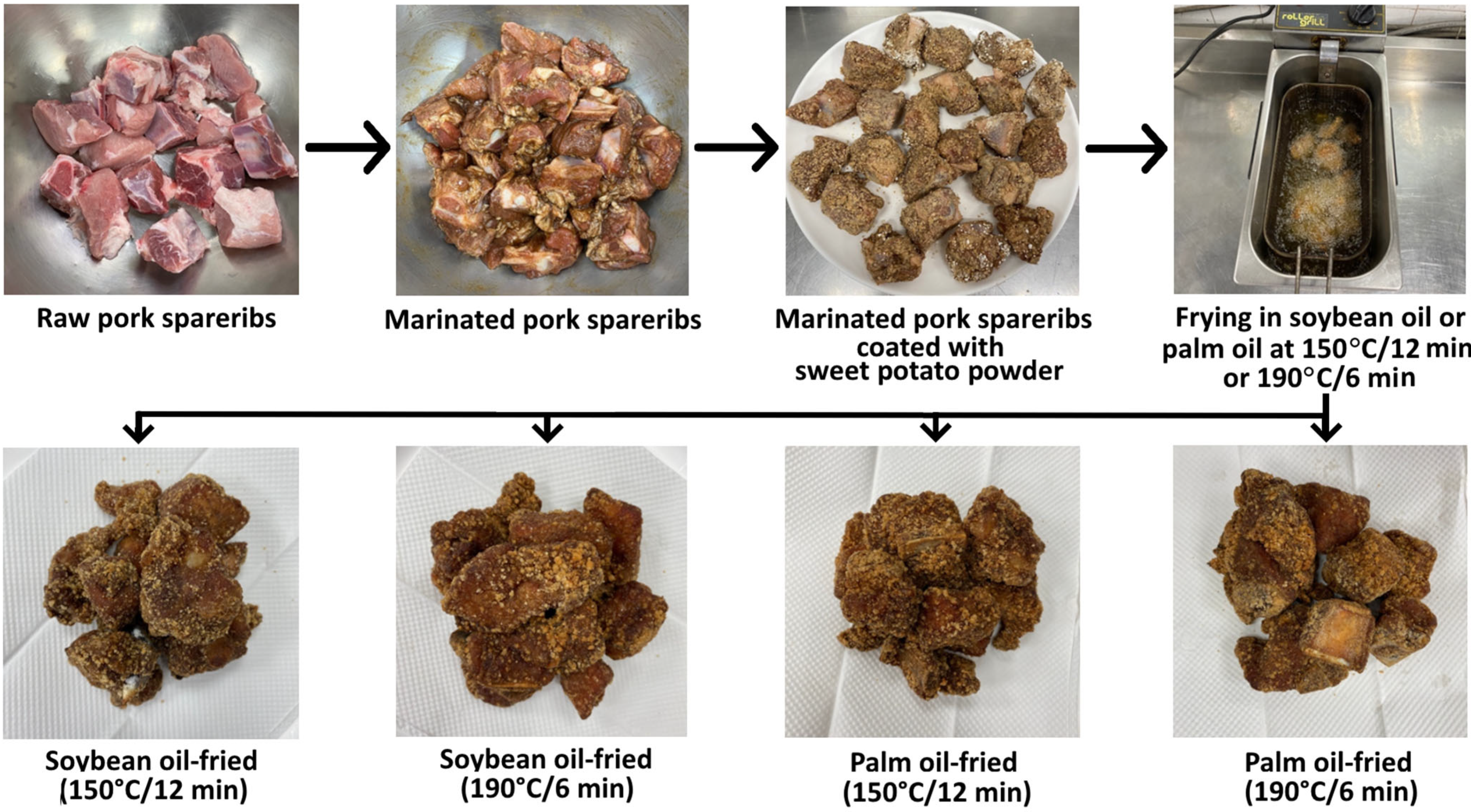
2.2. Processing of Crispy Pork Spareribs
2.3. Simultaneous Extraction and Purification of HAs and PAHs in Raw Pork Spareribs, Marinated Pork Spareribs, and Crispy Pork Spareribs
2.4. Analysis of HAs in Raw Pork Spareribs, Marinated Pork Spareribs, and Crispy Pork Spareribs via UPLC-MS/MS
2.5. Analysis of PAHs in Raw Pork Spareribs, Marinated Pork Spareribs, and Crispy Pork Spareribs via GC-MS/MS
2.6. Method Validation of PAHs and HAs in Pork Samples
2.7. Determination of PAH Precursors in Pork Samples via GC-MS
2.8. Determination of HA Precursors
2.8.1. Creatine and Creatinine
2.8.2. Amino Acid
2.8.3. Reducing Sugar
2.9. Principal Component Analysis (PCA)
2.10. Statistical Analysis
3. Results and Discussion
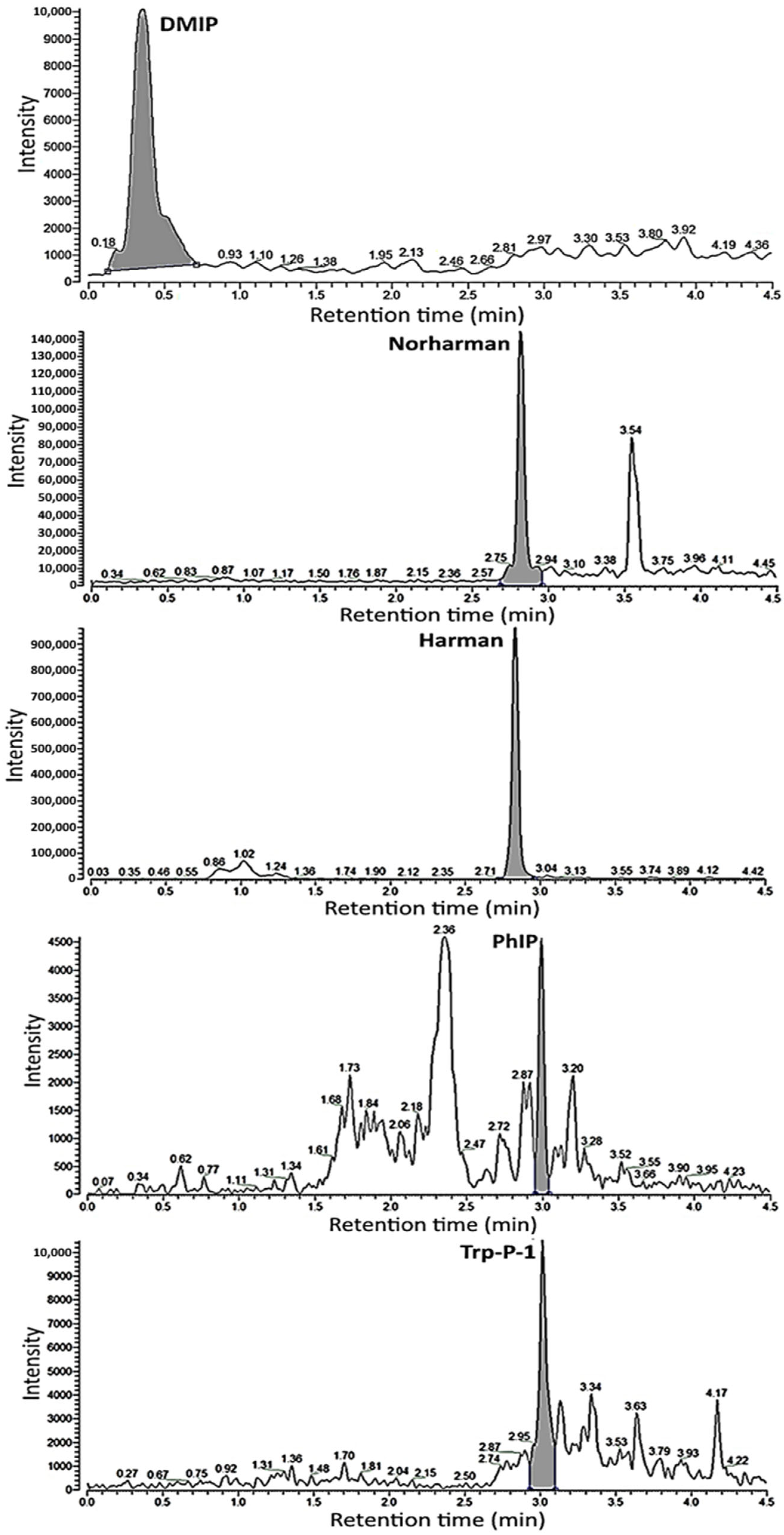
3.1. Separation of HA Standards by UPLC-MS/MS
3.2. Effects of Frying Condition and Oil type on HA Formation in Crispy Pork Spareribs
3.3. Content Changes of HA Precursors in Crispy Pork Spareribs during Processing
3.4. Separation of PAH Standards via GC-MS/MS
3.5. Effects of Frying Condition and Oil Type on PAH Contents in Crispy Pork Spareribs
3.6. Content Changes of PAH Precursors in Crispy Pork Spareribs during frying
3.7. Factorial Design Analysis
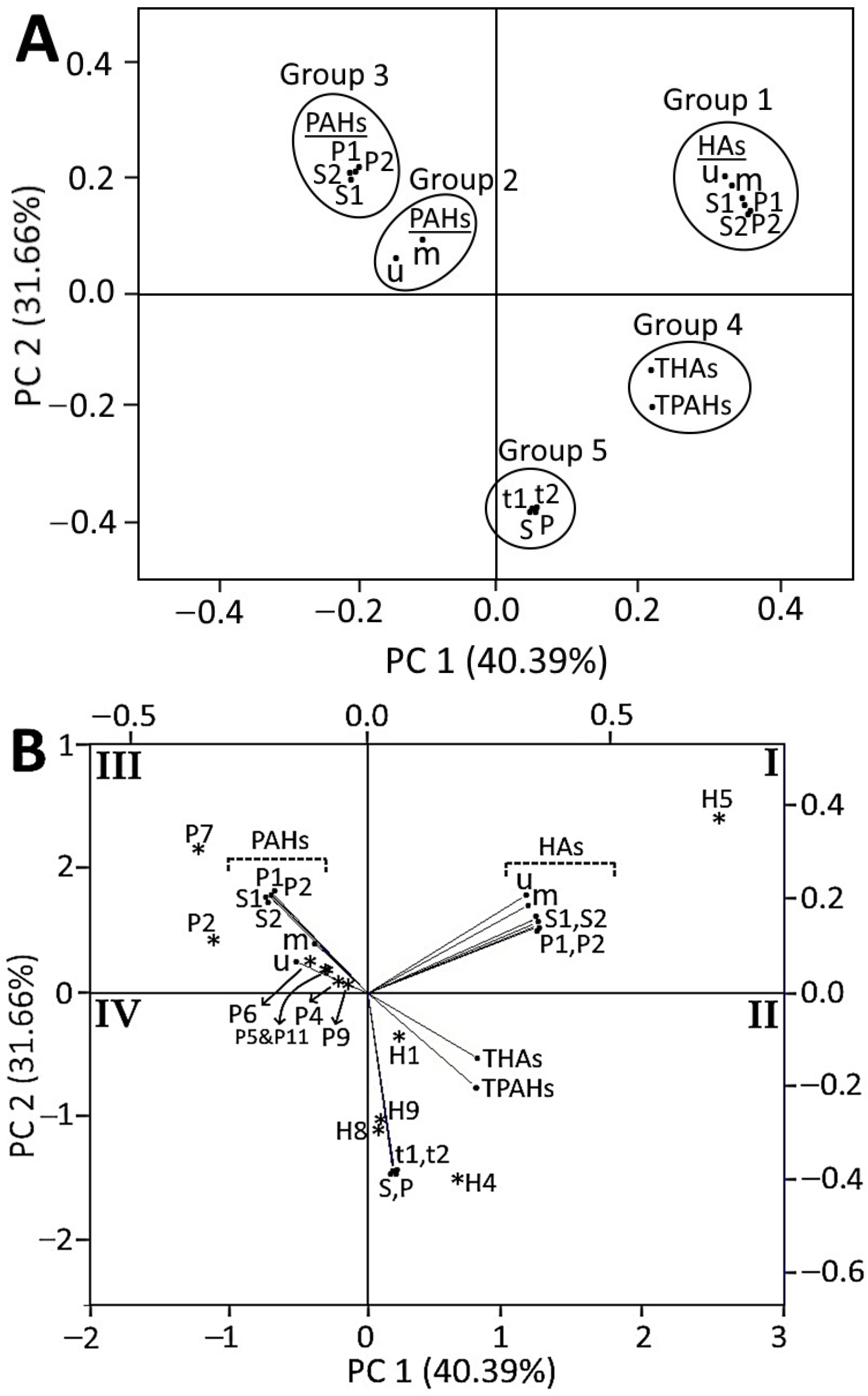
3.8. Correlation between HA and PAH Formation in Crispy Pork Spareribs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Red meat and processed meat. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 114, pp. 1–506. Available online: https://www.ncbi.nlm.nih.gov/books/n/iarcmono114/pdf/ (accessed on 15 November 2023).
- Lai, Y.W.; Lee, Y.T.; Cao, H.; Zhang, H.L.; Chen, B.H. Extraction of heterocyclic amines and polycyclic aromatic hydrocarbons from pork jerky and the effect of flavoring on formation and inhibition. Food Chem. 2023, 402, 134291. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 92, pp. 1–853. Available online: https://www.ncbi.nlm.nih.gov/books/n/iarcmono92/pdf/ (accessed on 17 October 2023).
- IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993; Volume 56, pp. 1–609. Available online: https://www.ncbi.nlm.nih.gov/books/n/iarcmono056/pdf/ (accessed on 15 November 2023).
- Pouzou, J.G.; Costard, S.; Zagmutt, F.J. Probabilistic estimates of heterocyclic amines and polycyclic aromatic hydrocarbons concentrations in meats and breads applicable to exposure assessments. Food Chem. Toxicol. 2018, 114, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Neves, T.M.; da Cunha, D.T.; de Rosso, V.V.; Domene, S.M.Á. Effects of seasoning on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in meats: A meta-analysis. Compr. Rev. Food Sci. Food Saf. 2021, 20, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1598–1626. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef]
- Lai, Y.W.; Lee, Y.T.; Inbaraj, B.S.; Chen, B.H. Formation and inhibition of heterocyclic amines and polycyclic aromatic hydrocarbons in ground pork during marinating. Foods 2022, 11, 3080. [Google Scholar] [CrossRef]
- Lai, Y.W.; Inbaraj, B.S.; Chen, B.H. Effects of oil and processing condition on formation of heterocyclic amines and polycyclic aromatic hydrocarbons in pork fiber. Foods 2023, 12, 3504. [Google Scholar] [CrossRef]
- Gibis, M.; Loeffler, M. Effect of creatine and glucose on formation of heterocyclic amines in grilled chicken breasts. Foods 2019, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- TFDA. Method of Test for Free Amino Acids, Glucosamine and Taurine in Foods, TFDAA0060.00; Taiwan Food and Drug Administration: Taipei, Taiwan, 2017. [Google Scholar]
- Chen, Y.; Chien, J.; Inbaraj, B.S.; Chen, B.H. Formation and inhibition of cholesterol oxidation products during marinating of pig feet. J. Agric. Food Chem. 2012, 60, 173–179. [Google Scholar] [CrossRef]
- SAS. SAS® 9.4 Output Delivery System: User’s Guide, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Pfau, W.; Skog, K. Exposure to β-carbolines norharman and harman. J. Chromatogr. B 2004, 802, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, Z.; Guo, H.; Na, N.; Chen, L.; Zhang, D. Potential sources of β-carbolines harman and norharman in braised sauce meat. Sci. Agric. Sinica 2013, 46, 3003–3009. [Google Scholar] [CrossRef]
- Fan, H.; Hu, H.; Li, C.; Xie, J.; Chen, J.; Zeng, M.; Shen, M.; Xie, M. Effects of cooking factors on the formation of heterocyclic aromatic amines in fried beef patties. J. Food Process. Preserv. 2022, 46, e16288. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Huang, Z.; Kong, B.; Liu, Q.; Wang, H.; Ming, X.; Xia, X. Dynamic changes in the qualities and heterocyclic aromatic amines of roasted pork induced by frying temperature and time. Meat Sci. 2021, 176, 108457. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Z.; Chen, B.H.; Hui, T.; Zhang, D. Frying oils with lower levels of saturated fatty acids induce less heterocyclic amine formation in meat floss (boiled, shredded and fried pork). Int. J. Food Sci. Technol. 2020, 55, 823–832. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α, β unsaturated aldehydes. Food Chem. 2011, 131, 915–926. [Google Scholar] [CrossRef]
- Tai, C.Y.; Lee, K.H.; Chen, B.H. Effects of various additives on the formation of heterocyclic amines in fried fish fibre. Food Chem. 2001, 75, 309–316. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, F. The effects of different frying oils on the formation of heterocyclic aromatic amines in meatballs and the changes in fatty acid compositions of meatballs and frying oils. J. Sci. Food Agric. 2019, 99, 1509–1518. [Google Scholar] [CrossRef]
- Turesky, R.J. Heterocyclic aromatic amines: Potential human carcinogens. Adv. Mol. Toxicol. 2010, 4, 37–83. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgom, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Quelhas, I.; Petisca, C.; Viegas, O.; Melo, A.; Pinho, O.; Ferreira, I. Effect of green tea marinades on the formation of heterocyclic amines and sensory quality of pan-fried beef. Food Chem. 2010, 122, 98–104. [Google Scholar] [CrossRef]
- Chen, B.H.; Inbaraj, B.S.; Hsu, K.C. Recent advances in the analysis of polycyclic aromatic hydrocarbons in food and water. J. Food Drug Anal. 2022, 30, 494–522. [Google Scholar] [CrossRef] [PubMed]
- Kholghy, M.R. The Evolution of Soot Morphology in Laminar Co-Flow Diffusion Flames of the Surrogates for Jet A-1 and a Synthetic Kerosene. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2012. [Google Scholar]
- Hu, M.; Zhu, M.; Xin, L.; Zhang, G.; Wu, S.; Hu, X.; Gong, D. Change of benzo (a) pyrene during frying and its groove binding to calf thymus DNA. Food Chem. 2021, 350, 129276. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, J.; Yao, Z. Changes in PAHs levels in edible oils during deep-frying process. Food Cont. 2016, 66, 233–240. [Google Scholar] [CrossRef]
- An, K.J.; Liu, Y.L.; Liu, H.L. Relationship between total polar components and polycyclic aromatic hydrocarbons in fried edible oil. Food Addit. Contam. Part A 2017, 34, 1596–1605. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Zhang, J.; Liang, L.; Wen, C.; Li, Y.; Shen, M.; Wu, Y.; He, X.; Liu, G. Formation, migration, derivation, and generation mechanism of polycyclic aromatic hydrocarbons during frying. Food Chem. 2023, 425, 136485. [Google Scholar] [CrossRef]
- Siddique, R.; Zahoor, A.F.; Ahmad, H.; Zahid, F.M.; Abid, M.; Siddeeg, A. Probing the impact of conventional oil frying on the formation of polycyclic aromatic hydrocarbons in rabbit meat. Food Sci. Nutr. 2021, 9, 1698–1703. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, L.; Zhong, H.; Gao, T.; Jiao, Y.; Liu, Y. The effects of various Chinese processing methods on the nutritional and safety properties of four kinds of meats. Innov. Food Sci. Emerg. Technol. 2021, 70, 102674. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, J.W.; Jung, M.; Lee, K.W.; Hung, M.S. Effects of thawing and frying methods on the formation of acrylamide and polycyclic aromatic hydrocarbons in chicken meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Zahoor, A.F.; Ahmad, H.; Zahid, F.M.; Karrar, E. Impact of different cooking methods on polycyclic aromatic hydrocarbons in rabbit meat. Food Sci. Nutr. 2021, 9, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.A.; Wu, P.F.; Wang, L.F.; Lee, H.; Lee, C.H.; Ko, Y.C. Mutagenicity and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1997, 381, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Chen, Y.C. Formation of polycyclic aromatic hydrocarbons in the smoke from heated model lipids and food lipids. J. Agric. Food Chem. 2001, 49, 5238–5243. [Google Scholar] [CrossRef]
- Gong, G.; Zhao, X.; Wu, S. Effect of natural antioxidants on inhibition of parent and oxygenated polycyclic aromatic hydrocarbons in Chinese fried bread youtiao. Food Cont. 2018, 87, 117–125. [Google Scholar] [CrossRef]
- EU Commission. Commission regulation 835/2011 of 19 August 2011 amending Regulation (EC) EC no 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 214–218. [Google Scholar]
- TFDA. Sanitation Standard for Contaminants and Toxins in Food; Taiwan Food and Drug Administration: Taipei, Taiwan, 2018. [Google Scholar]





| HA | Raw Pork Spareribs | Marinated Pork Spareribs | Crispy Pork Spareribs | |||
|---|---|---|---|---|---|---|
| Soybean Oil | Palm Oil | |||||
| 150 °C/12 min | 190 °C/6 min | 150 °C/12 min | 190 °C/6 min | |||
| DMIP | nd 3 | nd | 1.44 ± 0.06 d | 5.26 ± 0.46 b | 2.67 ± 0.42 a | 6.63 ± 0.45 c |
| Norharman | trace 4 | 0.62 ± 0.32 c | 3.41 ± 0.37 b | 4.54 ± 0.31 a | 4.42 ± 0.34 a | 4.55 ± 0.16 a |
| Harman | 0.16 ± 0.02 d | 6.68 ± 1.92 c | 14.46 ± 0.74 b | 17.64 ± 0.62 a | 16.87 ± 1.04 a | 17.91 ± 1.45 a |
| Trp-P-2 | nd | nd | trace | trace | trace | trace |
| PhIP | nd | nd | 0.91 ± 0.09 b | 1.74 ± 0.03 a | 1.51 ± 0.03 a | 1.53 ± 0.25 a |
| Trp-P-1 | nd | nd | 1.55 ± 0.04 c | 1.89 ± 0.06 a | 1.72 ± 0.06 a | 1.98 ± 0.05 b |
| MeAαC | nd | nd | trace | trace | trace | trace |
| Total | 0.16 ± 0.02 d | 7.30 ± 1.60 c | 20.34 ± 0.96 b | 25.81 ± 0.86 a | 24.52 ± 0.76 a | 25.97 ± 1.65 a |
| AA | Raw Pork Spareribs | Marinated Pork Spareribs | Crispy Pork Spareribs | |||
|---|---|---|---|---|---|---|
| Soybean Oil | Palm Oil | |||||
| 150 °C/12 min | 190 °C/6 min | 150 °C/12 min | 190 °C/6 min | |||
| Aspartic acid | 0.56 ± 0.04 | 0.70 ± 0.04 | 0.61 ± 0.05 | 0.65 ± 0.02 | 0.69 ± 0.02 | 0.56 ± 0.01 |
| Glutamic acid | 0.91 ± 0.05 | 1.06 ± 0.04 | 1.09 ± 0.02 | 1.18 ± 0.02 | 1.00 ± 0.02 | 0.87 ± 0.02 |
| Serine | 0.30 ± 0.01 | 0.42 ± 0.03 | 0.45 ± 0.04 | 0.32 ± 0.01 | 0.37 ± 0.01 | 0.35 ± 0.02 |
| Histidine | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.16 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| Glycine | 0.82 ± 0.02 | 1.34 ± 0.12 | 1.06 ± 0.02 | 0.85 ± 0.04 | 1.20 ± 0.06 | 1.44 ± 0.03 |
| Threonine | 0.15 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.17 ± 0.02 | 0.20 ± 0.01 | 0.16 ± 0.01 |
| Arginine | 0.50 ± 0.01 | 0.45 ± 0.01 | 0.55 ± 0.01 | 0.61 ± 0.02 | 0.42 ± 0.02 | 0.40 ± 0.01 |
| Alanine | 0.79 ± 0.02 | 1.08 ± 0.03 | 0.91 ± 0.01 | 0.75 ± 0.04 | 0.96 ± 0.02 | 0.97 ± 0.03 |
| Tyrosine | 0.16 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.25 ± 0.01 | 0.18 ± 0.01 | 0.15 ± 0.00 |
| Cystine | 0.06 ± 0.00 | 0.08 ± 0.01 | 0.81 ± 0.02 | 0.04 ± 0.00 | 0.07 ± 0.03 | 0.07 ± 0.03 |
| Valine | 0.07 ± 0.01 | 0.46 ± 0.03 | 0.36 ± 0.02 | 0.41 ± 0.01 | 0.46 ± 0.01 | 0.39 ± 0.01 |
| Methionine | 0.19 ± 0.01 | 0.22 ± 0.01 | 0.39 ± 0.03 | 0.20 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.00 |
| Phenylalanine | 0.20 ± 0.01 | 0.29 ± 0.02 | 0.16 ± 0.01 | 0.35 ± 0.01 | 0.28 ± 0.01 | 0.23 ± 0.00 |
| Isoleucine | 0.24 ± 0.01 | 0.41 ± 0.02 | 0.20 ± 0.01 | 0.40 ± 0.07 | 0.39 ± 0.01 | 0.33 ± 0.01 |
| Leucine | 0.33 ± 0.02 | 1.03 ± 0.03 | 0.68 ± 0.03 | 0.97 ± 0.04 | 0.87 ± 0.02 | 0.83 ± 0.02 |
| Lysine | 0.78 ± 0.00 | 0.91 ± 0.06 | 0.65 ± 0.00 | 1.01 ± 0.10 | 0.88 ± 0.09 | 0.87 ± 0.03 |
| Proline | 0.51 ± 0.05 | 0.97 ± 0.08 | 0.76 ± 0.06 | 0.59 ± 0.02 | 0.90 ± 0.12 | 0.78 ± 0.17 |
| Total AA | 6.61 ± 0.10 d | 9.87 ± 0.17 a | 9.26 ± 0.14 b | 8.78 ± 0.13 c | 9.07 ± 0.07 b | 8.59 ± 0.04 c |
| PAH | Raw Pork Spareribs | Marinated Pork Spareribs | Crispy Pork Spareribs | |||
|---|---|---|---|---|---|---|
| Soybean Oil | Palm Oil | |||||
| 150 °C/12 min | 190 °C/6 min | 150 °C/12 min | 190 °C/6 min | |||
| Acenaphthylene (AcPy) | nd 3 | nd | 0.08 ± 0.00 a | 0.07 ± 0.01 b | trace | trace |
| Acenaphthene (AcP) | nd | nd | trace | trace | nd | nd |
| Pyrene (Pyr) | 3.52 ± 0.25 e | 10.66 ± 0.08 d | 22.36 ± 1.03 a | 20.87 ± 0.24 b | 17.89 ± 0.02 c | 16.70 ± 0.76 c |
| Benzo[c]fluorene (BcF) | nd | nd | 1.56 ± 0.02 b | 1.59 ± 0.01 a | 1.56 ± 0.01 b | 1.55 ± 0.02 b |
| Benzo[a]anthracene (BaA) | nd | 0.87 ± 0.05 d | 3.85 ± 0.01 b | 3.87 ± 0.01 a | 3.84 ± 0.00 bc | 3.84 ± 0.00 c |
| Benzo[b]fluoranthene (BbF) | nd | 1.70 ± 0.26 c | 5.27 ± 0.03 a | 4.78 ± 0.00 b | 4.77 ± 0.01 b | 4.77 ± 0.00 b |
| Benzo[j]fluoranthene (BjF) | nd | trace | 10.15 ± 0.00 a | 10.15 ± 0.00 a | 9.65 ± 0.00 b | 9.71 ± 0.10 b |
| Cyclopenta[cd]pyrene (CcdP) | nd | 1.37 ± 0.04 d | 32.78 ± 0.70 a | 27.92 ± 2.48 b | 24.33 ± 0.84 c | 22.67 ± 0.09 c |
| Benzo[ghi]perylene (BghiP) | nd | nd | 1.27 ± 0.00 a | 1.27 ± 0.00 ab | 1.27 ± 0.00 b | 1.27 ± 0.00 ab |
| Dibenzo[a,h]anthracene (DBahA) | nd | nd | 1.86 ± 0.0 a | 1.85 ± 0.00 a | 1.86 ± 0.00 a | 1.86 ± 0.01 a |
| Dienzo[a,e]pyrene (DBaeP) | trace 4 | trace | 0.59 ± 0.06 a | 0.55 ± 0.03 a | trace | trace |
| Dienzo[a,i]pyrene (DBaiP) | trace | trace | 5.32 ± 0.10 a | 5.37 ± 0.03 a | 5.28 ± 0.03 a | 5.32 ± 0.07 a |
| Total | 3.52 ± 0.25 e | 14.61 ± 0.36 d | 85.10 ± 1.58 a | 78.21 ± 2.67 b | 70.44 ± 0.85 c | 67.69 ± 0.83 c |
| PAH Precursors | Raw Pork Spareribs | Marinated Pork Spareribs | Crispy Pork Spareribs | |||
|---|---|---|---|---|---|---|
| Soybean Oil | Palm Oil | |||||
| 150 °C/12 min | 190 °C/6 min | 150 °C/12 min | 190 °C/6 min | |||
| 4,4-dimethyl-2-cyclohexene-1-one | nd 3 | nd | nd | 0.60 ± 0.08 a | 0.38 ± 0.04 b | 0.50 ± 0.05 a |
| 2-cyclohexene-1-one | 1.80 ± 0.10 d | 0.76 ± 0.13 d | 10.58 ± 1.12 a | 5.14 ± 1.35 bc | 7.30 ± 1.41 b | 4.19 ± 0.85 c |
| Benzaldehyde | 5.21 ± 0.84 e | 12.95 ± 0.79 d | 275.09 ± 21.29 a | 115.53 ± 10.51 b | 29.52 ± 2.01 c | 21.41 ± 1.46 c |
| trans,trans-2,4-decadienal | nd | nd | 1.74 ± 0.20 c | 5.76 ± 1.24 a | 4.22 ± 0.49 b | 3.95 ± 0.50 b |
| Total | 7.01 ± 0.51 e | 13.71 ± 1.83 d | 287.42 ± 21.30 a | 127.04 ± 12.60 b | 41.42 ± 1.53 c | 30.05 ± 2.03 c |
| Factor | DF 1 | SS 2 | MS 3 | F-Value | p-Value |
|---|---|---|---|---|---|
| HAs | |||||
| Frying condition | 1 | 35.90 | 35.90 | 28.92 | 0.0007 |
| Oil type | 1 | 14.14 | 14.14 | 11.39 | 0.0097 |
| Frying condition × Oil type | 1 | 12.12 | 12.12 | 9.76 | 0.0141 |
| PAHs | |||||
| Frying condition | 1 | 69.83 | 69.83 | 25.30 | 0.0010 |
| Oil type | 1 | 475.33 | 475.33 | 172.24 | <0.0001 |
| Frying condition × Oil type | 1 | 12.89 | 12.89 | 4.67 | 0.0627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-W.; Inbaraj, B.S.; Chen, B.-H. Analysis of Polycyclic Aromatic Hydrocarbons via GC-MS/MS and Heterocyclic Amines via UPLC-MS/MS in Crispy Pork Spareribs for Studying Their Formation during Frying. Foods 2024, 13, 185. https://doi.org/10.3390/foods13020185
Lai Y-W, Inbaraj BS, Chen B-H. Analysis of Polycyclic Aromatic Hydrocarbons via GC-MS/MS and Heterocyclic Amines via UPLC-MS/MS in Crispy Pork Spareribs for Studying Their Formation during Frying. Foods. 2024; 13(2):185. https://doi.org/10.3390/foods13020185
Chicago/Turabian StyleLai, Yu-Wen, Baskaran Stephen Inbaraj, and Bing-Huei Chen. 2024. "Analysis of Polycyclic Aromatic Hydrocarbons via GC-MS/MS and Heterocyclic Amines via UPLC-MS/MS in Crispy Pork Spareribs for Studying Their Formation during Frying" Foods 13, no. 2: 185. https://doi.org/10.3390/foods13020185
APA StyleLai, Y.-W., Inbaraj, B. S., & Chen, B.-H. (2024). Analysis of Polycyclic Aromatic Hydrocarbons via GC-MS/MS and Heterocyclic Amines via UPLC-MS/MS in Crispy Pork Spareribs for Studying Their Formation during Frying. Foods, 13(2), 185. https://doi.org/10.3390/foods13020185