Antioxidant and Nitric Oxide Inhibitory Activity of the Six Most Popular Instant Thai Curries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Fresh Curry Paste
2.3. Total Phenolic Content and Antioxidant Activity
2.3.1. Sample Preparation and Extraction
2.3.2. Total Phenolic Content (TPC) Determination
2.3.3. Total Flavonoid Content (TFC) Determination
2.3.4. DPPH Radical Scavenging Activity
2.3.5. ABTS Radical Scavenging Activity
2.3.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.7. Oxygen Radical Absorbance Capacity (ORAC) Determination
2.4. Phenolic and Flavonoid Profiling Determination by LC-MS
2.5. Evaluation of NO Inhibitory Property in Animal Cell Culture
2.5.1. Cytotoxicity of the Macrophage Cell Line RAW 264.7 by MTT Assay
2.5.2. Determination of Extract Anti-Inflammatory Properties by Inhibiting NO Production Using Griess Reagent
- O.D.Bc means absorbance of the blank (RPMI),
- O.D.S means absorbance of the sample solution (Sample + LPS), and
- O.D.Bs means absorbance of the sample blank (Sample + RPMI).
2.6. Statistical Analysis
3. Results and Discussion
3.1. TPC, TFC, and Antioxidant Activity
3.1.1. Total Phenolic Content (TPC)
3.1.2. Total Flavonoid Content (TFC)
3.2. Phytochemicals Profiling by LC-MS
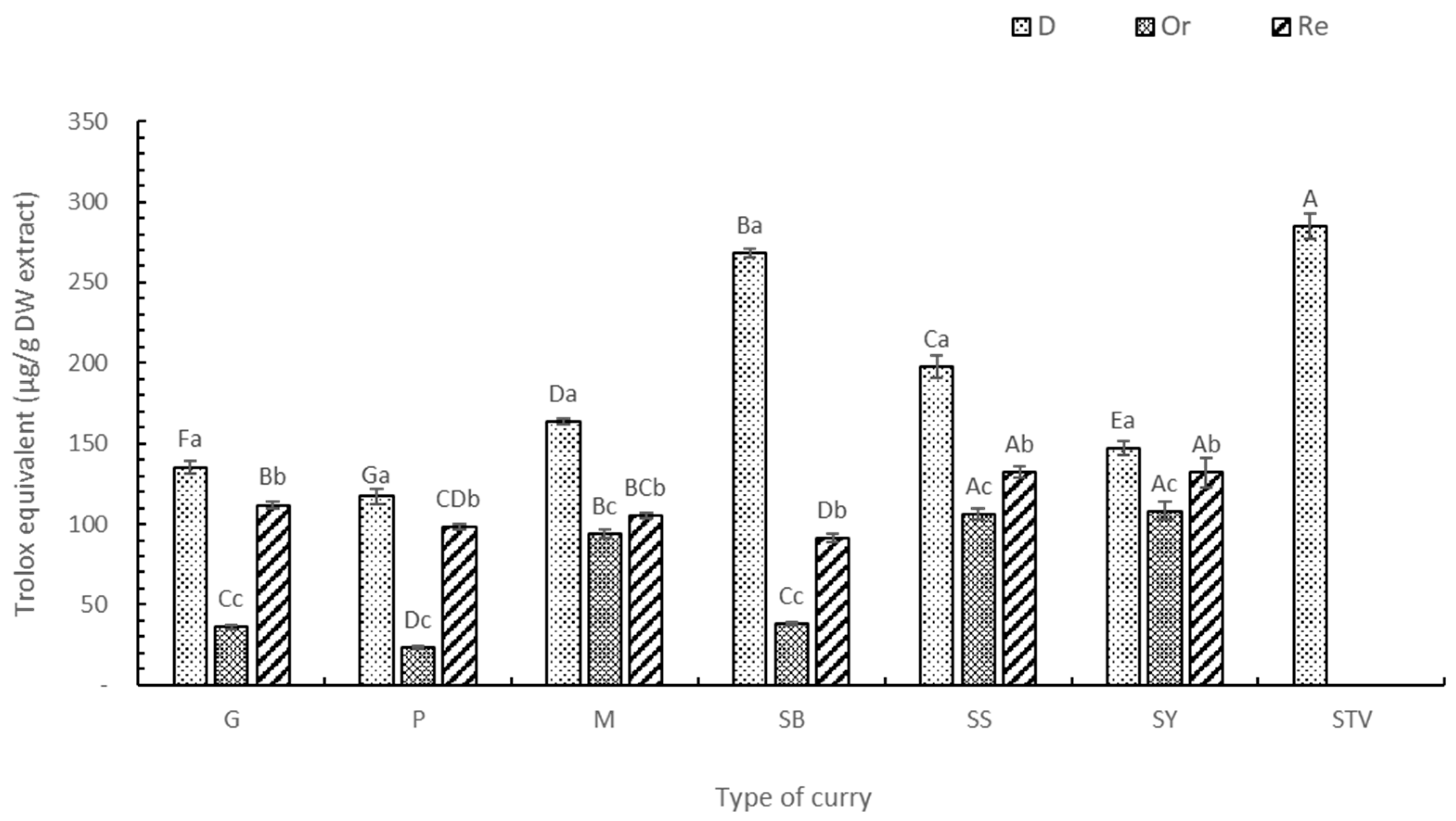
3.3. Antioxidant Activity (DPPH, ABTS, FRAP, and ORAC Assays)
3.4. Cytotoxicity and NO Inhibitory Activity
3.4.1. Cytotoxicity Test Using the Macrophage Cell Line RAW 264.7 Model
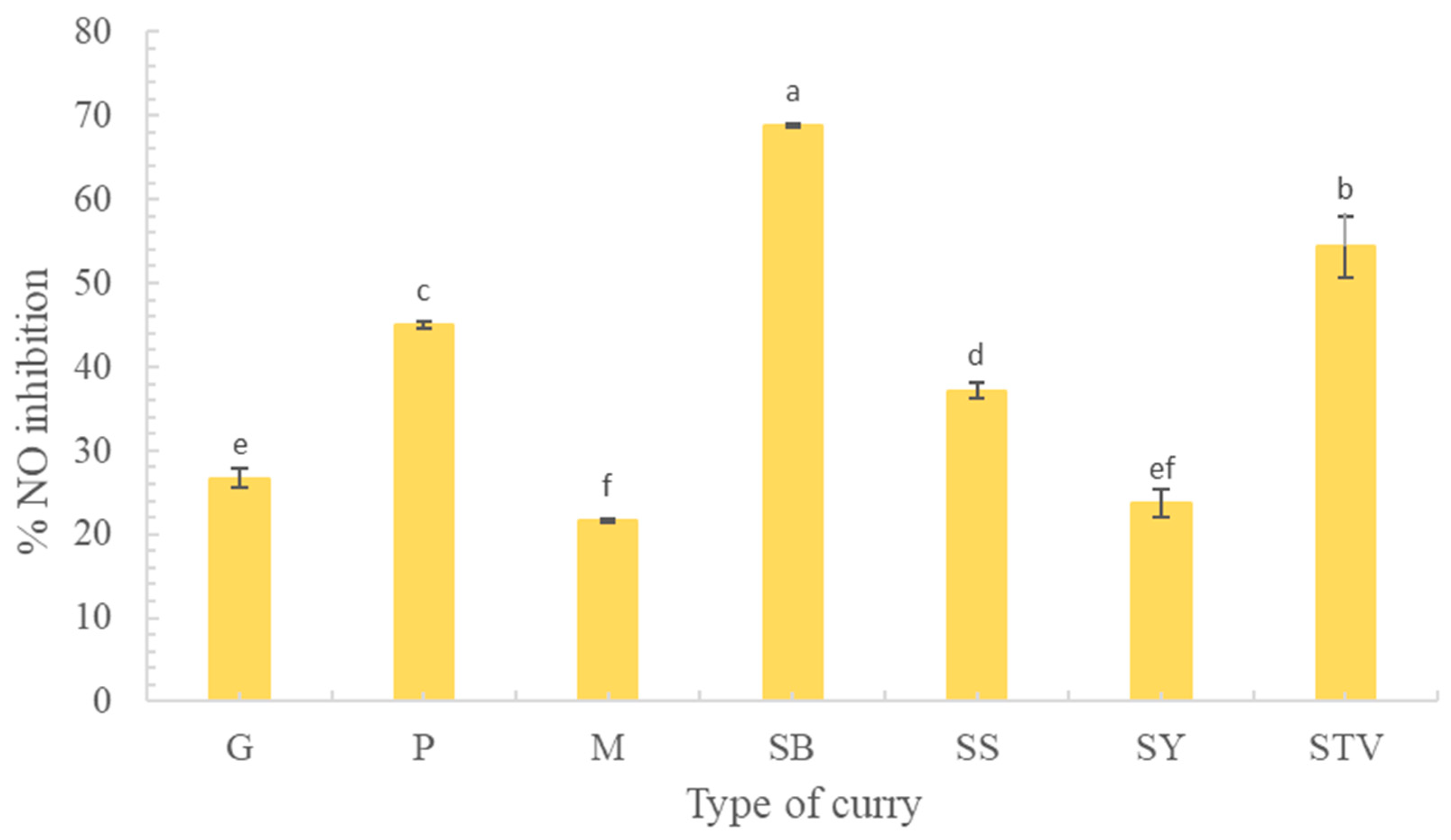
3.4.2. Inhibition of NO Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagchi, K.; Puri, S. Free radicals and antioxidants in health and disease. East. Mediterr. Health J. 1998, 4, 350–360. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants play the role in cellular response against oxidative/nitrosative stress: Current state. J. Nutr. 2015, 15, 71. [Google Scholar] [CrossRef]
- Esfahani, M.F.; Shahpiri, A.; Nasab, A.K. A highly efficient, thermo stable and broad pH adaptable copper-zinc super oxide dismutase (AmSOD1) mediates hydrogen peroxide tolerance in Avicennia marina. Phytochem 2021, 187, 1122766. [Google Scholar] [CrossRef]
- Bahja, J.; Stewart, N.A.; Dymond, M.K. Oxidative stress is inhibited by plant-based supplements: A quantitative lipidomic analysis of antioxidant activity and lipid compositional change. Adv. Redox. Res. 2022, 6, 100054. [Google Scholar] [CrossRef]
- Lounifi, I.; Arc, E.; Molassiotis, A.; Job, D.; Rajjou, L.; Tanou, G. Interplay between protein carbonylation and nitrosylation in plants. J. Proteom. 2013, 13, 568–578. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Suppression of the nuclear factor-kappa B activation pathway by spice-derived phytochemicals: Reasoning for seasoning. Ann. N. Y. Acad. Sci. 2004, 1030, 434–441. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Ghaedi, E.; Sahebkar, A. The effect of Curcumin/Turmeric on blood pressure modulation: A systematic review and meta-analysis. Pharmacol. Res. 2019, 150, 104505. [Google Scholar] [CrossRef]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Saini, S.S.; Varshney, R.; Krishnan, V.; Sircar, D.; Roy, P. Black pepper and piperine induce anticancer effects on leukemia cell line. Toxicol. Res. 2021, 10, 169–182. [Google Scholar] [CrossRef]
- Promjiam, P.; Siripongvutikorn, S.; Wichienchot, S. Functional properties of curry paste in relation to digestibility and fermentation by gut microbiota. Int. J. Food Prop. 2017, 20, 3204–3214. [Google Scholar] [CrossRef]
- Settharaksa, S.; Jongjareonrak, A.; Hmadhlu, P.; Chansuwan, W.; Siripongvutikorn, S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012, 19, 1581–1587. [Google Scholar]
- Amerzadeh, M.; Takian, A.; Pouraram, H.; Sari, A.A.; Ostovar, A. Policy analysis of socio-cultural determinants of salt, sugar and fat consumption in Iran. BMC Nutr. 2022, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Philippaert, K.; Pirone, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef] [PubMed]
- Rujirapong, C.; Siripongvutikorn, S.; Usawakesmanee, W.; Wanikorn, B. Quality changes when replacing NaCl with KCl in shrimp head paste. Food Sci. Technol. 2023, 43, e108121. [Google Scholar] [CrossRef]
- Smith, S.R.; Klotman, P.E.; Svetkey, L.P. Potassium chloride lowers blood pressure and causes natriuresis in older patients with hypertension. J. Am. Soc. Nephrol. 1992, 8, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhu, Y.; Cheng, H.; Yan, H.; Zhao, L.; Tang, J.; Ma, X.; Mao, P. Nitric oxide regulates seedling growth and mitochondrial responses in aged oat seeds. Int. J. Mol. Sci. 2018, 19, 1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Mao, L.; Ying, T. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant efects of vitamins in type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018, 10, 18. [Google Scholar] [CrossRef]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef]
- Srisook, K.; Jinda, S.; Srisook, E. Anti-inflammatory and antioxidant effects of Pluchea Indica leaf extract in TNF-α-Induced human endothelial cells. Wilailak J. Sci. Tech. 2021, 18, 10271. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ha, P.T.T.; Tran, N.T.B.; Tram, N.T.N.; Kha, V.H. Total phenolic, total flavonoid contents and antioxidant potential of Common Bean (Phaseolus vulgaris L.) in Vietnam. AIMS Agric. Food 2020, 5, 635–648. [Google Scholar]
- Ding, D.; Du, B.; Zhang, C.; Zaman, F.; Huang, Y. Isolation and identification of an antioxidant collagen peptide from skipjack tuna (Katsuwonus pelamis) bone. RSC Adv. 2019, 9, 27032–27041. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) usung a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. ACS Food Sci. Technol. 2002, 50, 4437–4444. [Google Scholar]
- Araujo, N.M.P.; Arruda, H.S.; dos Santos, F.N.; de Morais, D.R.; Pereira, G.A.; Pastore, G.M. LC-MS/MS screening and identification of bioactive compounds in leaves, pulp and seed from Eugenia calycina Cambess. Food Res. Int. 2020, 137, 109556. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ando, S.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003, 11, 1995–2000. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effect of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical Composition and Antioxidant Property of Holy Basil (Ocimum sanctum L.) Leaves, Stems, and Inflorescence and Their in Vitro Callus Cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, N.; Gupta, M.; Darshana, M.; Metha, B.K. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Mayo Clinic. Vitamin C. 2020. Available online: https://www.mayoclinic.org/drugs-supplements-vitamin-c/art-20363932 (accessed on 1 March 2023).
- Lim, J.; Nguyen, T.T.H.; Pal, K.; Kang, C.G.; Park, C.; Kim, S.W.; Kim, D. Phytochemical properties and functional characteristics of wild turmeric (Curcuma aromatica) fermented with Rhizopus oligosporus. Food Chem. X 2022, 13, 100198. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, A.; Roberts, M.F. Phytochemical investigation of the flavonoids of stevia (compositae). J. Pharm. Pharmacol. 1982, 34 (Suppl. S12), 108p. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses pro inflammatory cytokines production through MAP kinases and NF-kB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Wangner, K.-H.; Bulmer, A.C. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology 2010, 278, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Bidian, C.; Mitrea, D.-R.; Vasile, O.G.; Filip, A.; Cătoi, A.F.; Moldovan, R.; Decea, N.; Albu, A. Quercetin and curcumin effects in experimental pleural inflammation. Med. Pharm. Rep. 2020, 93, 260–266. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. FCT 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Elansary, H.O.; Agnieszka, S.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; El-Shafei, A.A. Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities. Plants 2020, 9, 1086. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, D.S. Anti-oxidant, Anti-inflammatory, and Anti-pancreatic Cancer Activities of Cynaroside and n-butanol Fraction of Orostachys japonicus. J. Food Nutr. Res. 2023, 11, 404–418. [Google Scholar]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Wiesz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-L.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sharma, S.; Mittal, A.; Gupta, S.; Dua, A. Phytochemical and antioxidant profiling of Ocimum sanctum. J. Food Sci. Technol. 2020, 57, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the Trolox equivalent antioxidant capacity assay with ABTS(+•). J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods. 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Rojas, P.; Stucken, K.; Delpote, C.; Valenzyela-Barra, G.; Jagus, R.J.; Agüero, M.V.; Psaten, A. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J. Appl. Res. Med. Aromat. Plants 2018, 11, 37–46. [Google Scholar] [CrossRef]
- Oliveira, H.; Wulff, A.; Saviani, E.; Salgado, I. Involvement of external NAD(P)H dehydrogenases in nitric oxide degradation by plant mitochondria. CBPA 2008, 150, S187. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jing, G.; Zhu, S. Nitric oxide (NO) involved in antioxidant enzyme gene regulation to delay mitochondrial damage in peach fruit. Postharvest Biol. Technol. 2022, 192, 111993. [Google Scholar] [CrossRef]
- Tuntipopipat, S.; Muangnoi, C.; Chingsuwanrote, P.; Parengam, M.; Chantravisut, P.; Charoenkiatkul, S.; Svasti, S. Anti-Inflammatory activities of red curry paste extract on lipopolysaccharide-activated murine macrophage cell line. Nutrition 2011, 27, 479–487. [Google Scholar] [CrossRef]







| Ingredients | Amount (%) | |||||
|---|---|---|---|---|---|---|
| G | P | M | SB | SS | SY | |
| Fresh lemongrass (Cymbopogon citrates L.) | 30 | 10 | 5 | - | - | 40 |
| Fresh green chili (Capsicum annuum L.) | 35 | - | - | 5.5 | - | - |
| Fresh galangal (Alpinia galanga L.) | 5 | 10 | 5 | - | - | - |
| Fresh shallot bulb (Allium ascalonicum L.) | 15 | 30 | 35 | - | 5 | 5 |
| Fresh garlic bulb (Allium sativum L.) | 10 | 25 | 15 | 45 | 10 | 10 |
| Dried chili pepper (Capsicum annuum L.) | - | 20 | 15 | - | - | - |
| Dried black pepper (Piper nigrum L.) | - | - | 1 | 7 | - | 5 |
| Fresh ginger (Zingiber officinale Roscoe.) | - | - | 4 | - | - | - |
| Fresh coriander root (Coriandrum sativum L.) | - | - | - | 3 | - | - |
| Fresh red chili (Dendranthema indicum L.) | - | - | - | 5.5 | 30 | 7 |
| Dried chili (Dendranthema indicum L.) | - | - | - | - | 40 | 25 |
| Fresh turmeric (Curcuma longa L.) | - | - | - | - | 15 | 5 |
| Mixed spices | 5 | 5 | 20 | - | - | 3 |
| Dried holy basil leaves (Ocimum sanctum L.) | - | - | - | 34 | - | - |
| Ingredient | Amount (%) | |||||
|---|---|---|---|---|---|---|
| G | P | M | SB | SS | SY | |
| Dried curry powder | 33 | 45 | 33 | 13 | 80 | 64 |
| Mixed spices | 4 | - | - | - | - | 5 |
| Salt | 13 | 15 | 12 | 24 | 17 | 22 |
| Sugar | 50 | 50 | 55 | 59 | 3 | 9 |
| Dried basil leaves | - | - | - | 4 | - | - |
| Ingredient | Amount (%) | |||||
|---|---|---|---|---|---|---|
| G | P | M | SB | SS | SY | |
| Dried curry powder | 46 | 45 | 43 | 19 | 80 | 71 |
| Mixed spices | 6 | - | - | - | - | 5 |
| Salt | 9 | 10 | 8 | 18 | 8.5 | 12 |
| Sugar | 35 | 32 | 36 | 45 | 1.5 | 5 |
| KCl | 0.5 | 10 | 9 | 9 | 8.5 | 6 |
| Stevia powder (STV) | 3 | 3 | 2 | 2 | 1.5 | 1 |
| Dried basil leaves | - | - | - | 7 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siripongvutikorn, S.; Pumethakul, K.; Yupanqui, C.T.; Seechamnanturakit, V.; Detarun, P.; Utaipan, T.; Sirinupong, N.; Chansuwan, W.; Wittaya, T.; Samakradhamrongthai, R.S. Antioxidant and Nitric Oxide Inhibitory Activity of the Six Most Popular Instant Thai Curries. Foods 2024, 13, 178. https://doi.org/10.3390/foods13020178
Siripongvutikorn S, Pumethakul K, Yupanqui CT, Seechamnanturakit V, Detarun P, Utaipan T, Sirinupong N, Chansuwan W, Wittaya T, Samakradhamrongthai RS. Antioxidant and Nitric Oxide Inhibitory Activity of the Six Most Popular Instant Thai Curries. Foods. 2024; 13(2):178. https://doi.org/10.3390/foods13020178
Chicago/Turabian StyleSiripongvutikorn, Sunisa, Kanyamanee Pumethakul, Chutha Takahashi Yupanqui, Vatcharee Seechamnanturakit, Preeyabhorn Detarun, Tanyarath Utaipan, Nualpun Sirinupong, Worrapanit Chansuwan, Thawien Wittaya, and Rajnibhas Sukeaw Samakradhamrongthai. 2024. "Antioxidant and Nitric Oxide Inhibitory Activity of the Six Most Popular Instant Thai Curries" Foods 13, no. 2: 178. https://doi.org/10.3390/foods13020178
APA StyleSiripongvutikorn, S., Pumethakul, K., Yupanqui, C. T., Seechamnanturakit, V., Detarun, P., Utaipan, T., Sirinupong, N., Chansuwan, W., Wittaya, T., & Samakradhamrongthai, R. S. (2024). Antioxidant and Nitric Oxide Inhibitory Activity of the Six Most Popular Instant Thai Curries. Foods, 13(2), 178. https://doi.org/10.3390/foods13020178





