Effects of Cactus Polysaccharide on Pasting, Rheology, Structural Properties, In Vitro Digestibility, and Freeze–Thaw Stability of Rice Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. CP Preparation
2.3. Preparation of CP-RS Composites
2.4. Pasting Properties
2.5. Rheological Measurements
2.5.1. Dynamic Rheological Properties
2.5.2. Steady Shear Rheology
2.6. Thermal Property Analysis
2.7. Scanning Electron Microscope (SEM)
2.8. X-ray Diffraction (XRD)
2.9. Fourier Transforms Infrared Spectroscopy (FT-IR)
2.10. In Vitro Digestion
2.11. Freeze–Thaw Stability
2.12. Statistical Analysis
3. Results and Discussion
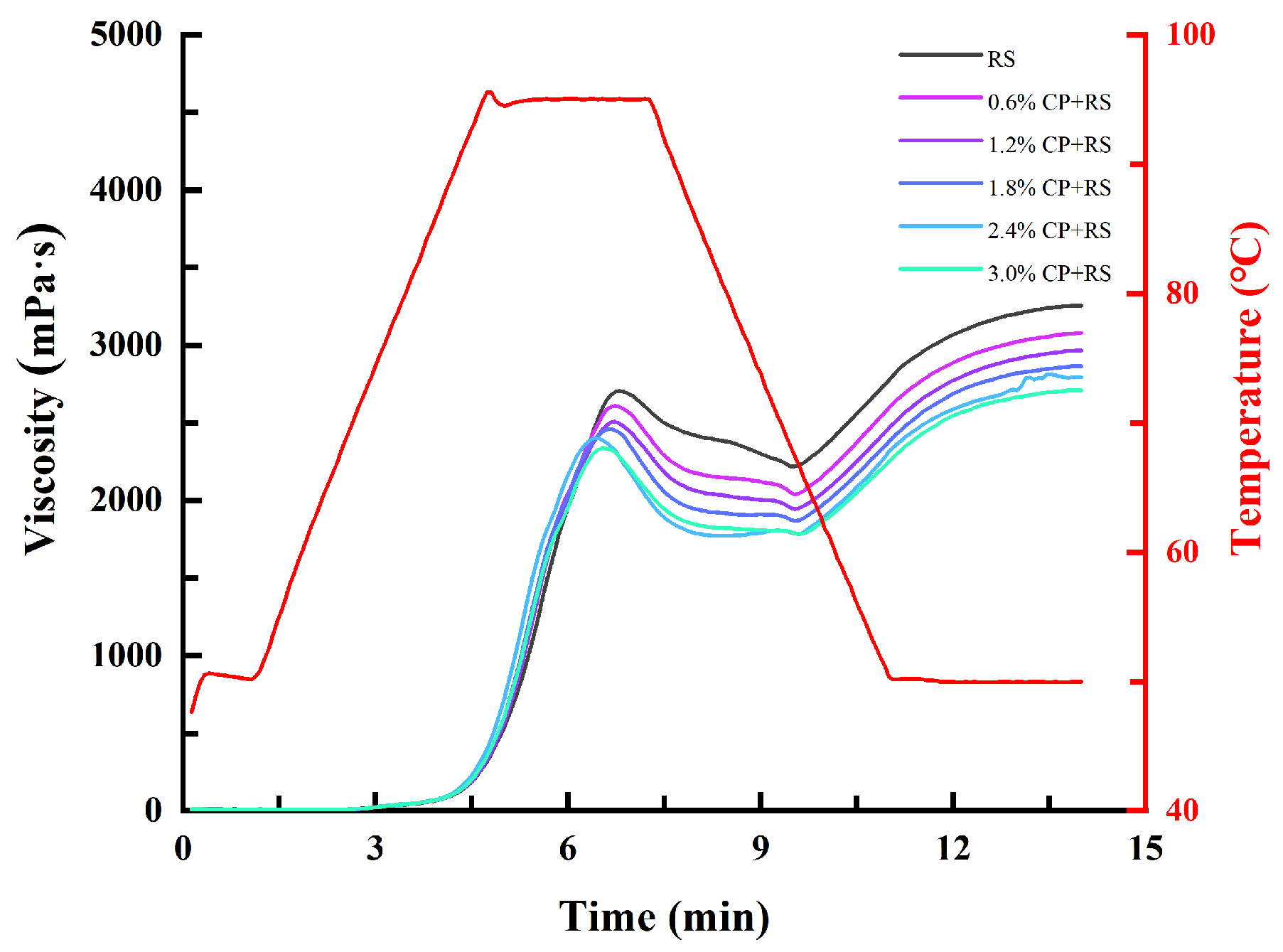
3.1. Pasting Characteristics
3.2. Rheological Properties
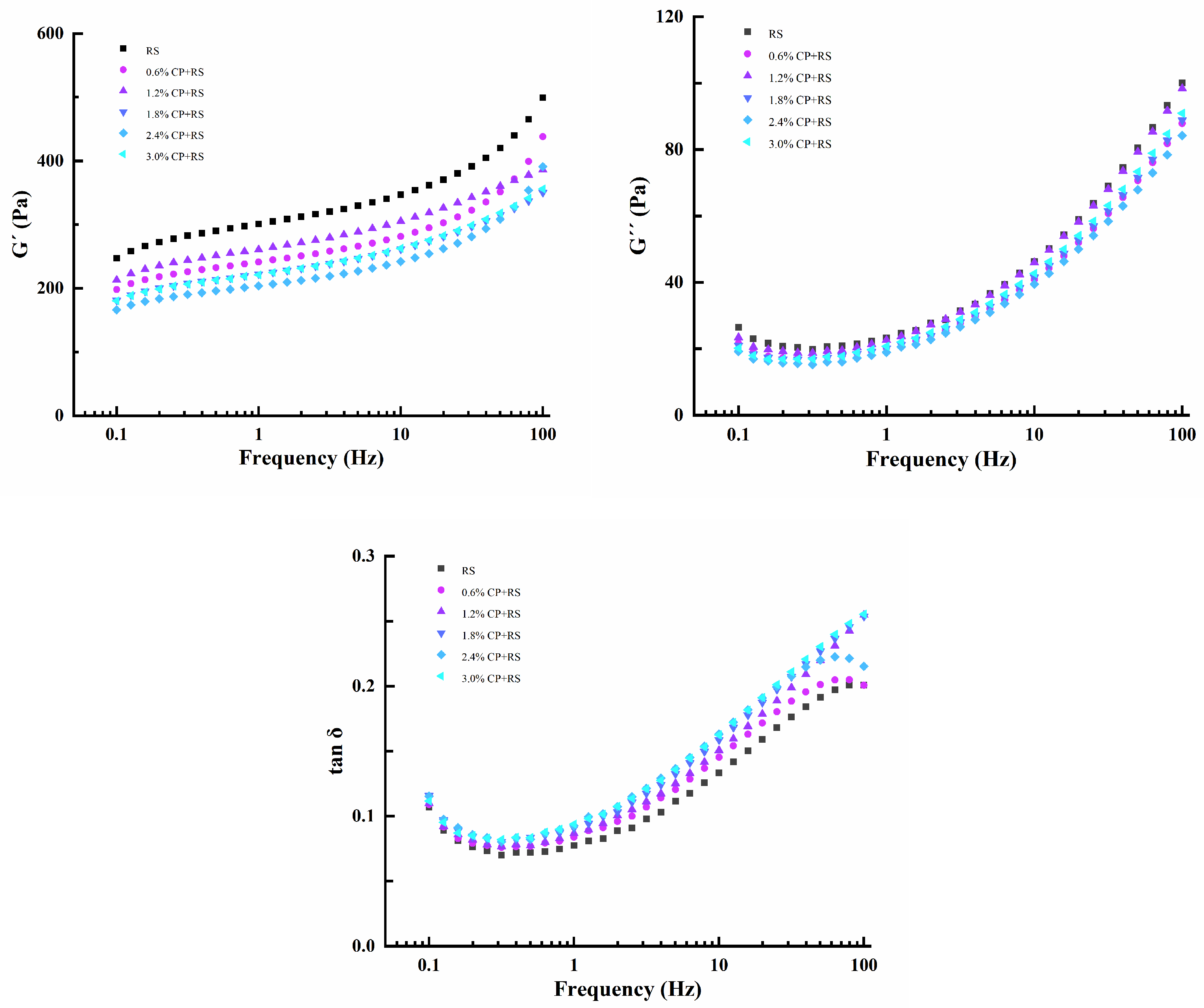
3.2.1. Dynamic Rheological Properties
3.2.2. Static Rheological Measurements
3.3. Thermal Properties
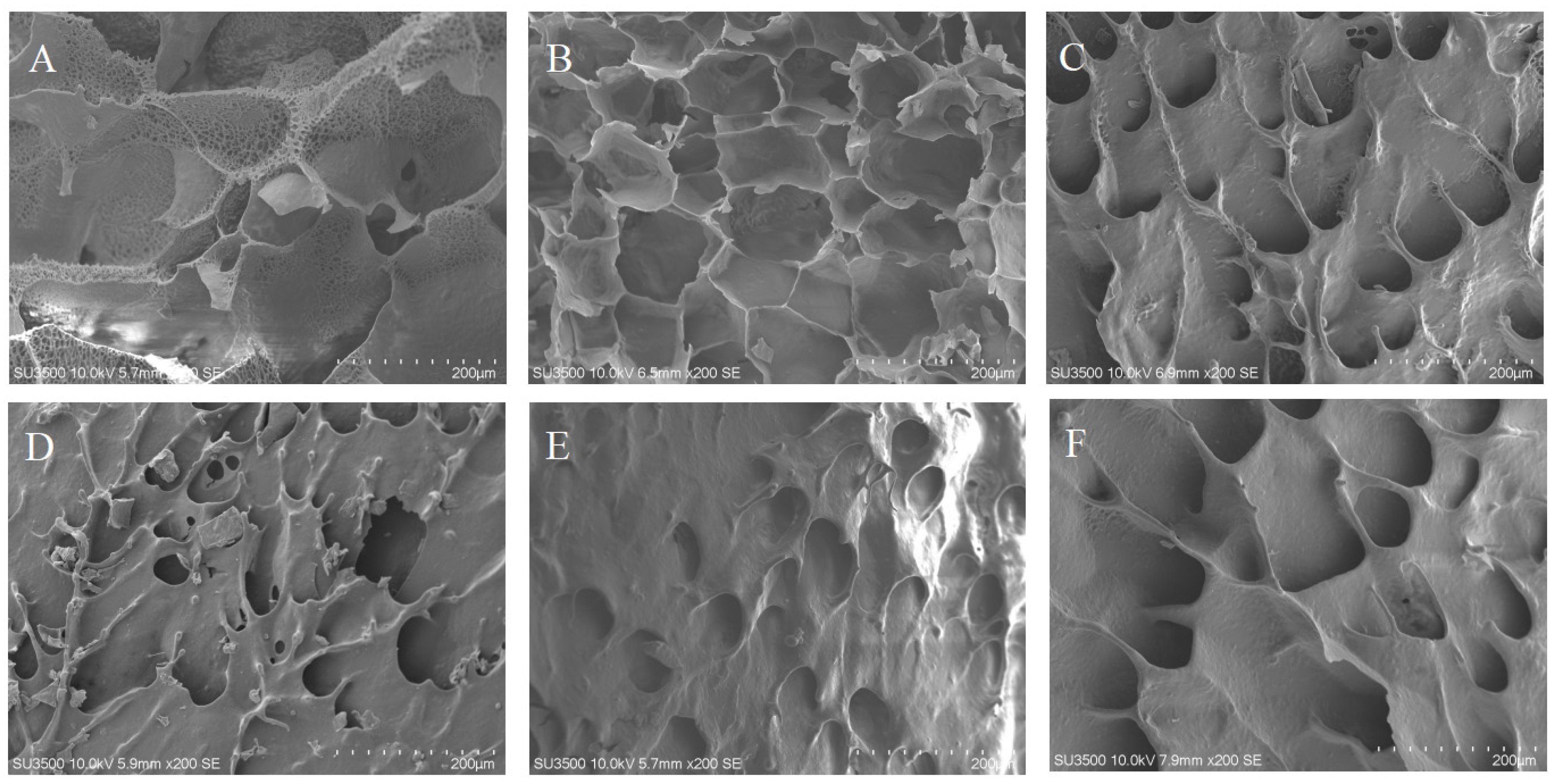
3.4. SEM Analysis
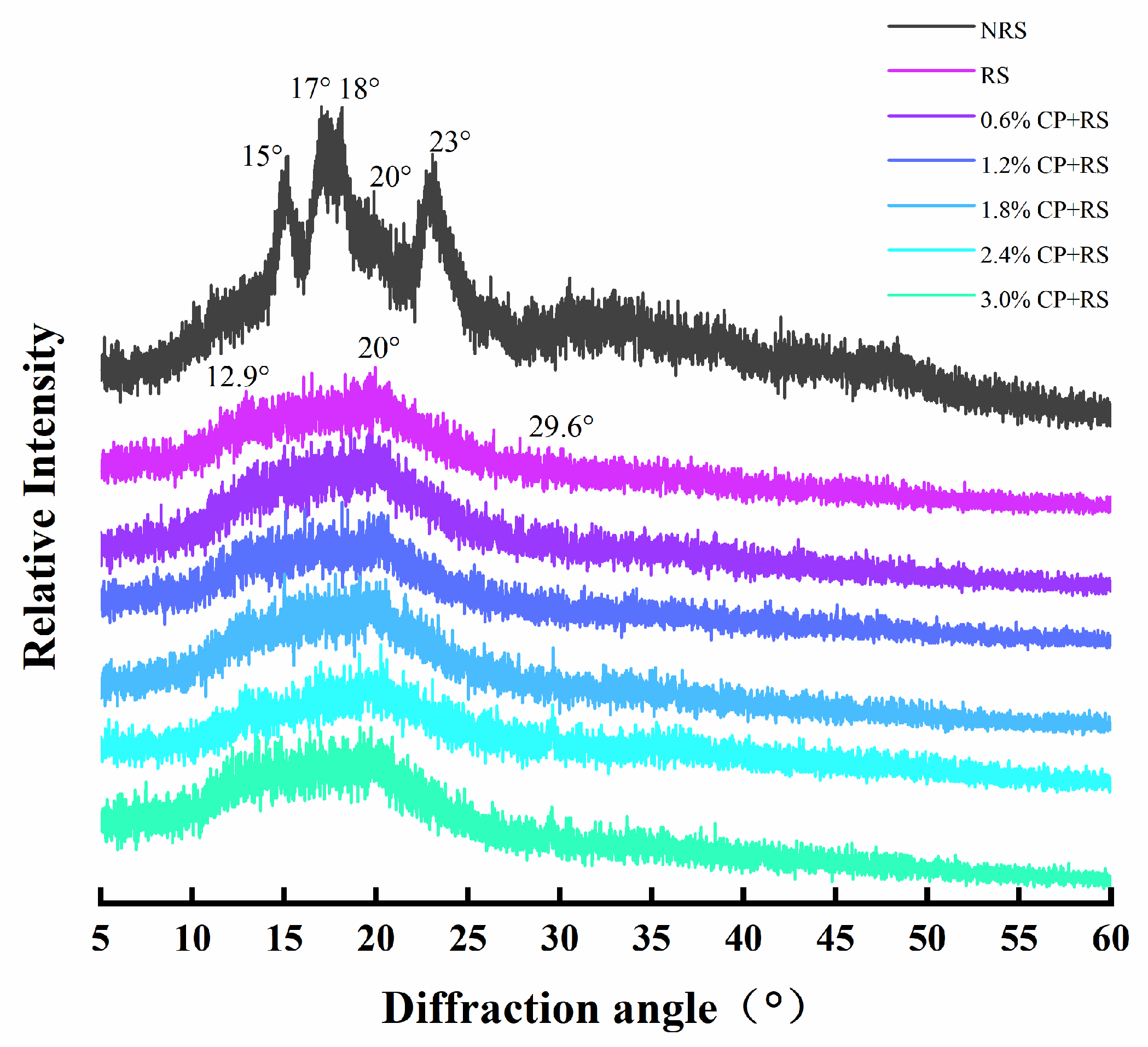
3.5. XRD
3.6. FT-IR
3.7. In Vitro Digestibility
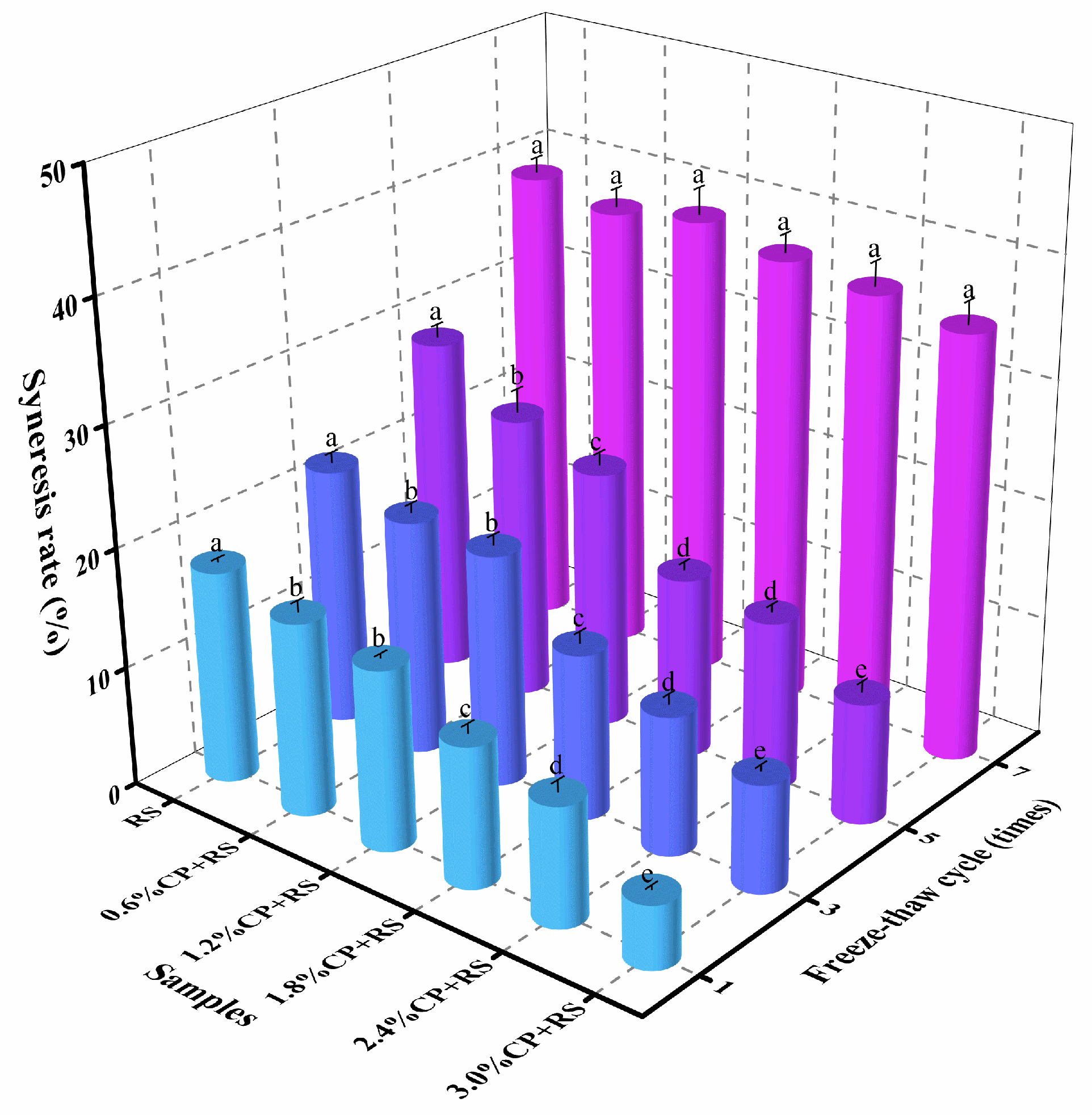
3.8. Freeze–Thaw Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatade, A.A.; Sahoo, A.K. Effect of additives and steaming on quality of air dried noodles. J. Food Sci. Technol. 2015, 52, 8395–8402. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Ilyas, R.A.; Zainudin, E.S. Thermal, flammability, and antimicrobial properties of arrowroot (Maranta arundinacea) fiber reinforced arrowroot starch biopolymer composites for food packaging applications. Int. J. Biol. Macromol. 2022, 213, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Si, F.; Xiong, L.; Chu, L. Effect of dry heating with ionic gums on physicochemical properties of starch. Food Chem. 2013, 136, 1421–1425. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, H.; Shang, W.; Xie, F.; Li, X.; Chen, L.; Zhou, Z. Understanding the digestibility and nutritional functions of rice starch subjected to heat-moisture treatment. J. Funct. Foods 2018, 45, 165–172. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Sun, J.; Zuo, X.B.; Fang, S.; Xu, H.N.; Chen, J.; Meng, Y.C.; Chen, T. Effects of cellulose derivative hydrocolloids on pasting, viscoelastic, and morphological characteristics of rice starch gel. J. Texture Stud. 2017, 48, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, M.; Xiao, Y.; Luo, Y.; Xie, J. Effect of maize, potato, and pea starches with Mesona chinensis polysaccharide on pasting, gelatinization properties, granular morphology and digestion. Food Hydrocoll. 2020, 108, 106047. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, H.; Nie, C.; Zhao, T.; Xia, Y.; Ai, L. Structural characteristics of tamarind seed polysaccharides treated by high-pressure homogenization and their effects on physicochemical properties of corn starch. Carbohydr. Polym. 2021, 262, 117661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.-J.; Liu, Q.-Q.; Qian, J.-Y.; Lim, S.-T. Improvement of pasting and gelling behaviors of waxy maize starch by partial gelatinization and freeze-thawing treatment with xanthan gum. Food Chem. 2022, 375, 131656. [Google Scholar] [CrossRef]
- Fan, X.; Li, X.; Hu, J.; Cheng, Z.; Wang, X.; Hu, X. Physicochemical and in vitro digestibility properties on complexes of fermented wheat starches with konjac gum. Int. J. Biol. Macromol. 2021, 188, 197–206. [Google Scholar] [CrossRef]
- Liu, S.; Lin, L.; Shen, M.; Wang, W.; Xiao, Y.; Xie, J. Effect of Mesona chinensis polysaccharide on the pasting, thermal and rheological properties of wheat starch. Int. J. Biol. Macromol. 2018, 118, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Wang, Y.-S.; Chen, H.-H.; Liu, S.; Li, M. Retardant effect of sodium alginate on the retrogradation properties of normal cornstarch and anti-retrogradation mechanism. Food Hydrocoll. 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, B.; Pan, Y.; Li, X.-M.; Cheng, J.-S.; Chen, H.-Q. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chem. 2018, 264, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, H.; Xia, Y.; Ai, L. Effects of tamarind seed polysaccharide on gelatinization, rheological, and structural properties of corn starch with different amylose/amylopectin ratios. Food Hydrocoll. 2020, 105, 105854. [Google Scholar] [CrossRef]
- Cengiz, E.; Dogan, M. Effect of corn starch–hydrocolloid interactions on the rheological properties of coating batters. J. Food Process. Preserv. 2021, 45, e15250. [Google Scholar] [CrossRef]
- Ren, Y.; Rong, L.; Shen, M.; Liu, W.; Xiao, W.; Luo, Y.; Xie, J. Interaction between rice starch and Mesona chinensis Benth polysaccharide gels: Pasting and gelling properties. Carbohydr. Polym. 2020, 240, 116316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, Y.; Wang, Z.; Liu, K.; Wang, Q.; Bao, H. Effects of Auricularia auricula-judae polysaccharide on pasting, gelatinization, rheology, structural properties and in vitro digestibility of kidney bean starch. Int. J. Biol. Macromol. 2021, 191, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effect of heat-moisture treatment on the in vitro digestibility and physicochemical properties of starch-hydrocolloid complexes. Food Hydrocoll. 2020, 104, 105736. [Google Scholar] [CrossRef]
- Wu, S. Extending shelf-life of fresh-cut potato with cactus Opuntia dillenii polysaccharide-based edible coatings. Int. J. Biol. Macromol. 2019, 130, 640–644. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Koubaa, M.; Ktata, A.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Bouaziz, F.; Driss, D.; Chaabouni, S.E. Water-soluble polysaccharides from Opuntia stricta Haw. fruit peels: Recovery, identification and evaluation of their antioxidant activities. Int. Agrophys. 2015, 29, 299–306. [Google Scholar] [CrossRef]
- Harrabi, B.; Athmouni, K.; Hamdaoui, L.; Ben Mahmoud, L.; Hakim, A.; El Feki, A.; Zeghal, K.; Ghozzi, H. Polysaccharides extraction from Opuntia stricta and their protective effect against HepG2 cell death and hypolipidaemic effects on hyperlipidaemia rats induced by high-fat diet. Arch. Physiol. Biochem. 2017, 123, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Lan, Q.J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.B.; Liu, H.G.; Cao, J.T. Antitumor effect of polysaccharides from cactus pear fruit in S180-bearing mice. Chin. J. Cancer 2008, 27, 580. [Google Scholar]
- Cheng, D.; Ma, Q.; Zhang, J.; Jiang, K.; Cai, S.; Wang, W.; Wang, J.; Sun, J. Cactus polysaccharides enhance preservative effects of ultrasound treatment on fresh-cut potatoes. Ultrason. Sonochem. 2022, 90, 106205. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Li, S.; Li, C. Effects of cactus Opuntia dillenii polysaccharide-based coatings loaded with glutathione on the preservation of freshly cut Chinese water chestnut. Food Chem. 2023, 401, 134187. [Google Scholar] [CrossRef]
- Yang, F.; Du, Q.; Miao, T.; Zhang, X.; Xu, W.; Jia, D. Interaction between potato starch and Tremella fuciformis polysaccharide. Food Hydrocoll. 2022, 127, 107509. [Google Scholar] [CrossRef]
- Kong, X.-R.; Zhu, Z.-Y.; Zhang, X.-J.; Zhu, Y.-M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Chen, T.; Shen, M.; Yu, Q.; Chen, Y.; Wen, H.; Lu, H.; Chen, S.; Xie, J. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022, 397, 133768. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, Y.; Chen, F.; Huang, Y.; Chen, P. Effects of sucrose on pasting, thermal, rheological and textural properties of native and alcohol-alkali-treated waxy rice starch. Int. J. Biol. Macromol. 2021, 166, 108–116. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Luo, S.; Zou, P.; Guo, B.; Liu, Y.; Chen, J.; Liu, C. Improving instant properties of kudzu powder by extrusion treatment and its related mechanism. Food Hydrocoll. 2020, 101, 105475. [Google Scholar] [CrossRef]
- Takahashi, T.; Fujita, N. Thermal and rheological characteristics of mutant rice starches with widespread variation of amylose content and amylopectin structure. Food Hydrocoll. 2017, 62, 83–93. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, L.; Wang, W.; Xiao, Y.; Liu, S.; Luo, Y.; Shen, M.; Xie, J. Effects of Mesona chinensis Benth polysaccharide on physicochemical and rheological properties of sweet potato starch and its interactions. Food Hydrocoll. 2020, 99, 105371. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, Y.; Kong, X.; Cao, S.; Chen, S.; Liu, D.; Ye, X.; Tian, J. RG-I pectin affects the physicochemical properties and digestibility of potato starch. Food Hydrocoll. 2021, 117, 106687. [Google Scholar] [CrossRef]
- Zhai, Y.; Xing, J.; Luo, X.; Zhang, H.; Yang, K.; Shao, X.; Chen, K.; Li, Y. Effects of Pectin on the Physicochemical Properties and Freeze-Thaw Stability of Waxy Rice Starch. Foods 2021, 10, 2419. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tong, Q.; Ren, F.; Zhu, G. Pasting and rheological properties of rice starch as affected by pullulan. Int. J. Biol. Macromol. 2014, 66, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, Z.; Wang, M.; Wang, Q.; Barba, F.J.; Wan, L.; Wang, X.; Fu, Y. Effects of Laminaria japonica polysaccharides on gelatinization properties and long-term retrogradation of wheat starch. Food Hydrocoll. 2022, 133, 107908. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, S.; Zhao, R.; Wang, N.; Kan, J.; Zhang, F. Effect of four viscous soluble dietary fibers on the physicochemical, structural properties, and in vitro digestibility of rice starch: A comparison study. Food Chem. 2021, 362, 130181. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-j.; Chen, R.-y.; Huang, L.; Liang, R.-h.; Liu, C.-m.; Chen, J. Investigation on the influence of pectin structures on the pasting properties of rice starch by multiple regression. Food Hydrocoll. 2017, 63, 580–584. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Zhou, S.; Wang, L.; Qian, H.; Zhang, H.; Qi, X. Effect of Vaccinium bracteatum Thunb. leaf pigment on the thermal, pasting, and textural properties and microstructure characterization of rice starch. Food Chem. 2017, 228, 435–440. [Google Scholar] [CrossRef]
- Xu, X.; Ye, S.; Zuo, X.; Fang, S. Impact of Guar Gum and Locust Bean Gum Addition on the Pasting, Rheological Properties, and Freeze–Thaw Stability of Rice Starch Gel. Foods 2022, 11, 2508. [Google Scholar] [CrossRef]
- Qiu, S.; Yadav, M.P.; Chen, H.; Liu, Y.; Tatsumi, E.; Yin, L. Effects of corn fiber gum (CFG) on the pasting and thermal behaviors of maize starch. Carbohydr. Polym. 2015, 115, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Yuris, A.; Goh, K.K.T.; Hardacre, A.K.; Matia-Merino, L. Understanding the interaction between wheat starch and Mesona chinensis polysaccharide. LWT 2017, 84, 212–221. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, rheological, thermal and gel textural properties of pearl millet starch as modified by guar gum and its acid hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Williams, P.A.; Chong, D.; Luo, S.; Chen, J.; Liu, C. The interaction of pectin with wheat starch and its influence on gelatinization and rheology. Food Hydrocoll. 2023, 136, 108288. [Google Scholar] [CrossRef]
- Tang, M.; Hong, Y.; Gu, Z.; Zhang, Y.; Cai, X. The effect of xanthan on short and long-term retrogradation of rice starch. Starch-Stärke 2013, 65, 702–708. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, L.; Li, D.; Xiao, J. Synergistic effects of plant protein hydrolysates and xanthan gum on the short- and long-term retrogradation of rice starch. Int. J. Biol. Macromol. 2020, 144, 967–977. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Ma, S.; Wang, H. Physicochemical characterization of rice, potato, and pea starches, each with different crystalline pattern, when incorporated with Konjac glucomannan. Food Hydrocoll. 2020, 101, 105499. [Google Scholar] [CrossRef]
- Charoenrein, S.; Tatirat, O.; Rengsutthi, K.; Thongngam, M. Effect of konjac glucomannan on syneresis, textural properties and the microstructure of frozen rice starch gels. Carbohydr. Polym. 2011, 83, 291–296. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, H.; Chen, L.; Zeng, M.; Qin, F.; Wang, Z.; He, Z.; Chen, J. Interactions between soluble soybean polysaccharide and starch during the gelatinization and retrogradation: Effects of selected starch varieties. Food Hydrocoll. 2021, 118, 106765. [Google Scholar] [CrossRef]
- Sheng, L.; Li, P.; Wu, H.; Liu, Y.; Han, K.; Gouda, M.; Tong, Q.; Ma, M.; Jin, Y. Tapioca starch-pullulan interaction during gelation and retrogradation. LWT 2018, 96, 432–438. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Xu, Z.; Pan, W.; Shen, M.; Han, J.; Sun, X.; Zhang, Y.; Xie, J.; Zhang, X.; et al. Cross-linked corn bran arabinoxylan improves the pasting, rheological, gelling properties of corn starch and reduces its in vitro digestibility. Food Hydrocoll. 2022, 126, 107440. [Google Scholar] [CrossRef]
- Sun, M.; Feng, X.; Chen, L.; Hua, W.; Chen, X. Purification of letinous edodes polysaccharide and its effect on gel properties of rice starch. IOP Conf. Ser. Earth Environ. Sci. 2020, 615, 012091. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Zhang, F.; Fang, C.; Liu, C.; Luo, S. Freeze-thaw stability of rice starch modified by Improved Extrusion Cooking Technology. Carbohydr. Polym. 2016, 151, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yadav, M.P.; Liu, Y.; Chen, H.; Tatsumi, E.; Yin, L. Effects of corn fiber gum with different molecular weights on the gelatinization behaviors of corn and wheat starch. Food Hydrocoll. 2016, 53, 180–186. [Google Scholar] [CrossRef]
- Teng, L.Y.; Chin, N.L.; Yusof, Y.A. Rheological and textural studies of fresh and freeze-thawed native sago starch-sugar gels. II. Comparisons with other starch sources and reheating effects. Food Hydrocoll. 2013, 31, 156–165. [Google Scholar] [CrossRef]
- Arunyanart, T.; Charoenrein, S. In effects of sucrose, xylitol and konjac clucomannan on stability of frozen rice starch gels. In Proceedings of the 34th Congress on Science and Technology of Thailand, Bangkok, Thailand, 31 October–2 November 2008. [Google Scholar]


| Smaple | RS (g) | CP (g) |
|---|---|---|
| 0.6% CP–RS | 100 | 0.6 |
| 1.2% CP–RS | 100 | 1.2 |
| 1.8% CP–RS | 100 | 1.8 |
| 2.4% CP–RS | 100 | 2.4 |
| 3.0% CP–RS | 100 | 3.0 |
| Samples | PV (mPa·s) | TV (mPa·s) | BV (mPa·s) | FV (mPa·s) | SV (mPa·s) | PT (°C) |
|---|---|---|---|---|---|---|
| RS | 2774.33 ± 69.06 a | 2035.33 ± 25.93 a | 739.00 ± 77.93 a | 3319.33 ± 59.48 a | 1284.00 ± 65.09 a | 90.03 ± 0.03 a |
| 0.6% CP + RS | 2620.00 ± 33.15 b | 2035.00 ± 38.16 a | 585.00 ± 13.53 b | 3087.00 ± 26.91 b | 1052.00 ± 15.87 b | 89.78 ± 0.38 a |
| 1.2% CP + RS | 2507.67 ± 10.02 c | 1957.67 ± 11.06 b | 550.00 ± 17.35 b | 2975.67 ± 9.61 c | 1018.00 ± 10.82 b | 89.68 ± 0.42 a |
| 1.8% CP + RS | 2429.00 ± 51.96 cd | 1864.67 ± 5.13 c | 564.33 ± 53.35 b | 2873.67 ± 37.75 d | 1009.00 ± 42.04 b | 89.67 ± 0.49 a |
| 2.4% CP + RS | 2421.33 ± 19.43 cd | 1812.67 ± 42.00 c | 608.67 ± 23.46 b | 2798.33 ± 24.79 de | 985.67 ± 32.47 bc | 89.35 ± 0.48 a |
| 3.0% CP + RS | 2365.67 ± 68.04 d | 1821.00 ± 45.21 c | 544.67 ± 34.43 b | 2752.67 ± 69.62 e | 931.67 ± 28.29 c | 89.12 ± 0.83 a |
| Samples | Upward Curve | Downward Curve | S (Pa/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| τ0 (Pa) | K (Pa·sn) | n | R2 | τ0 (Pa) | K (Pa·sn) | n | R2 | ||
| RS | 71.58 ± 4.13 a | 43.26 ± 2.59 a | 0.421 ± 0.01 b | 0.999 | 58.28 ± 3.48 a | 19.08 ± 1.59 a | 0.552 ± 0.014 a | 0.998 | 19,480 |
| 0.6% CP + RS | 52.21 ± 5.57 c | 44.59 ± 3.7 a | 0.392 ± 0.013 c | 0.998 | 49.07 ± 2.8 b | 15.23 ± 1.23 b | 0.564 ± 0.014 a | 0.998 | 18,732 |
| 1.2% CP + RS | 55.73 ± 5.56 bc | 40.24 ± 3.49 a | 0.419 ± 0.014 b | 0.998 | 50.69 ± 2.85 b | 17.1 ± 1.29 ab | 0.555 ± 0.013 a | 0.998 | 17,159 |
| 1.8% CP + RS | 57.75 ± 4.65 bc | 39.82 ± 2.9 a | 0.422 ± 0.012 b | 0.998 | 51.33 ± 2.84 b | 17.2 ± 1.28 ab | 0.557 ± 0.013 a | 0.998 | 16,783 |
| 2.4% CP + RS | 54.71 ± 4.43 c | 39.76 ± 2.8 a | 0.417 ± 0.011 b | 0.999 | 49.6 ± 2.63 b | 16.06 ± 1.16 b | 0.564 ± 0.012 a | 0.999 | 16,030 |
| 3.0% CP + RS | 63.94 ± 3.6 ab | 32.42 ± 2.13 b | 0.446 ± 0.011 a | 0.999 | 47.9 ± 2.57 b | 17.7 ± 1.19 ab | 0.547 ± 0.011 a | 0.999 | 13,045 |
| Samples | Gelatinization | Retrogradation | ||||||
|---|---|---|---|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH0 (J/g) | R1(%)/ΔHr1 (J/g) | R3(%)/ΔHr3 (J/g) | R5(%)/ΔHr5 (J/g) | R7/(%)ΔHr7 (J/g) | |
| RS | 63.62 ± 0.15 c | 67.87 ± 0.05 c | 74.29 ± 0.10 b | 11.66 ± 0.09 a | 13.38 ± 0.02 b/1.56 ± 0.01 a | 17.75 ± 0.81 a/2.07 ± 0.11 a | 24.78 ± 1.95 a/2.89 ± 0.25 a | 34.89 ± 2.30 a/4.07 ± 0.30 a |
| 0.6% CP + RS | 63.65 ± 0.06 c | 67.94 ± 0.19 bc | 74.73 ± 0.34 b | 10.95 ± 0.18 b | 14.26 ± 0.04 a/1.51 ± 0.03 a | 17.38 ± 0.01 a/1.84 ± 0.03 b | 21.05 ± 0.40 b/2.23 ± 0.08 b | 32.57 ± 0.86 b/3.45 ± 0.15 b |
| 1.2% CP + RS | 63.72 ± 0.11 c | 67.96 ± 0.31 bc | 74.73 ± 0.25 b | 10.94 ± 0.24 b | 12.61 ± 0.36 c/1.38 ± 0.07 b | 16.54 ± 0.00 b/1.81 ± 0.04 b | 19.20 ± 0.24 c/2.10 ± 0.02 b | 28.33 ± 0.11 c/3.10 ± 0.08 c |
| 1.8% CP + RS | 63.99 ± 0.01 b | 68.33 ± 0.22 ab | 75.38 ± 0.54 a | 10.31 ± 0.06 c | 10.67 ± 0.13 d/1.10 ± 0.02 c | 13.48 ± 0.21 c/1.39 ± 0.03 c | 17.26 ± 0.00 d/1.78 ± 0.01 c | 28.71 ± 0.71 c/2.96 ± 0.09 c |
| 2.4% CP + RS | 64.16 ± 0.20 b | 68.56 ± 0.17 a | 75.60 ± 0.37 a | 10.30 ± 0.04 c | 7.28 ± 0.46 e/0.75 ± 0.05 d | 13.11 ± 0.53 c/1.35 ± 0.06 c | 15.05 ± 0.52 e/1.55 ± 0.06 d | 23.88 ± 0.09 d/2.46 ± 0.00 d |
| 3.0% CP + RS | 64.43 ± 0.14 a | 68.73 ± 0.33 a | 75.86 ± 0.16 a | 9.93 ± 0.26 d | 4.23 ± 0.01 f/0.42 ± 0.01 e | 8.86 ± 0.03 d/0.88 ± 0.02 d | 11.88 ± 0.01 f/1.18 ± 0.03 e | 20.33 ± 0.88 e/2.02 ± 0.14 e |
| Sample | RDS (%) | SDS (%) | Resistant Starch (%) |
|---|---|---|---|
| RS | 38.83 ± 0.85 a | 27.03 ± 0.91 d | 34.14 ± 0.75 b |
| 0.6% CP + RS | 35.41 ± 0.80 b | 28.97 ± 0.24 c | 35.62 ± 0.43 ab |
| 1.2% CP + RS | 32.38 ± 0.48 c | 29.77 ± 0.19 bc | 37.91 ± 0.78 ab |
| 1.8% CP + RS | 29.79 ± 0.02 d | 30.61 ± 0.65 b | 39.60 ± 0.66 ab |
| 2.4% CP + RS | 28.62 ± 0.30 e | 31.71 ± 0.77 a | 39.67 ± 0.54 a |
| 3.0% CP + RS | 28.27 ± 0.26 e | 32.04 ± 0.97 a | 39.69 ± 0.27 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Dong, C.; Chi, F.; Gu, X.; Liu, L.; Yang, L. Effects of Cactus Polysaccharide on Pasting, Rheology, Structural Properties, In Vitro Digestibility, and Freeze–Thaw Stability of Rice Starch. Foods 2024, 13, 2420. https://doi.org/10.3390/foods13152420
Zhu Y, Dong C, Chi F, Gu X, Liu L, Yang L. Effects of Cactus Polysaccharide on Pasting, Rheology, Structural Properties, In Vitro Digestibility, and Freeze–Thaw Stability of Rice Starch. Foods. 2024; 13(15):2420. https://doi.org/10.3390/foods13152420
Chicago/Turabian StyleZhu, Yahui, Chuang Dong, Fumin Chi, Xuedong Gu, Lei Liu, and Lin Yang. 2024. "Effects of Cactus Polysaccharide on Pasting, Rheology, Structural Properties, In Vitro Digestibility, and Freeze–Thaw Stability of Rice Starch" Foods 13, no. 15: 2420. https://doi.org/10.3390/foods13152420
APA StyleZhu, Y., Dong, C., Chi, F., Gu, X., Liu, L., & Yang, L. (2024). Effects of Cactus Polysaccharide on Pasting, Rheology, Structural Properties, In Vitro Digestibility, and Freeze–Thaw Stability of Rice Starch. Foods, 13(15), 2420. https://doi.org/10.3390/foods13152420





