Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Material
2.2. Alcalase Hydrolysis of Salmon Heads and Hake By-Products
2.3. Subcritical Water Hydrolysis of Salmon Heads
2.4. Hydrolysis and Protein Yields
2.5. Degree of Hydrolysis
2.6. Proximate Composition of Fish Raw Material and FPHs
2.7. Amino Acid Compositions
2.8. Mineral Profile and Contaminants Metals
2.9. Molecular Weight (MW) of FPHs
2.10. Cytotoxicity and Proliferation of FPHs
2.10.1. Cell Line Growth Conditions
2.10.2. Cytotoxicity
2.10.3. Cell Proliferation
2.11. Antioxidant Activity
2.11.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.11.2. 2,2′-Azino-bis(3 ethylbenzthiazoline-6)-sulfonic Acid (ABTS) Radical Scavenging Activity
2.11.3. Reducing Power (RP)
2.12. Metal Chelating Activities
2.12.1. Cu2+ Chelating Activity
2.12.2. Fe2+ Chelation Activity
2.13. ACE Inhibitory Activity
2.14. α-Amylase Inhibitory Activity
2.15. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition, Degree of Hydrolysis, and Protein Yield
3.2. Amino Acid Compositions
3.3. Mineral and Chemical Contaminants
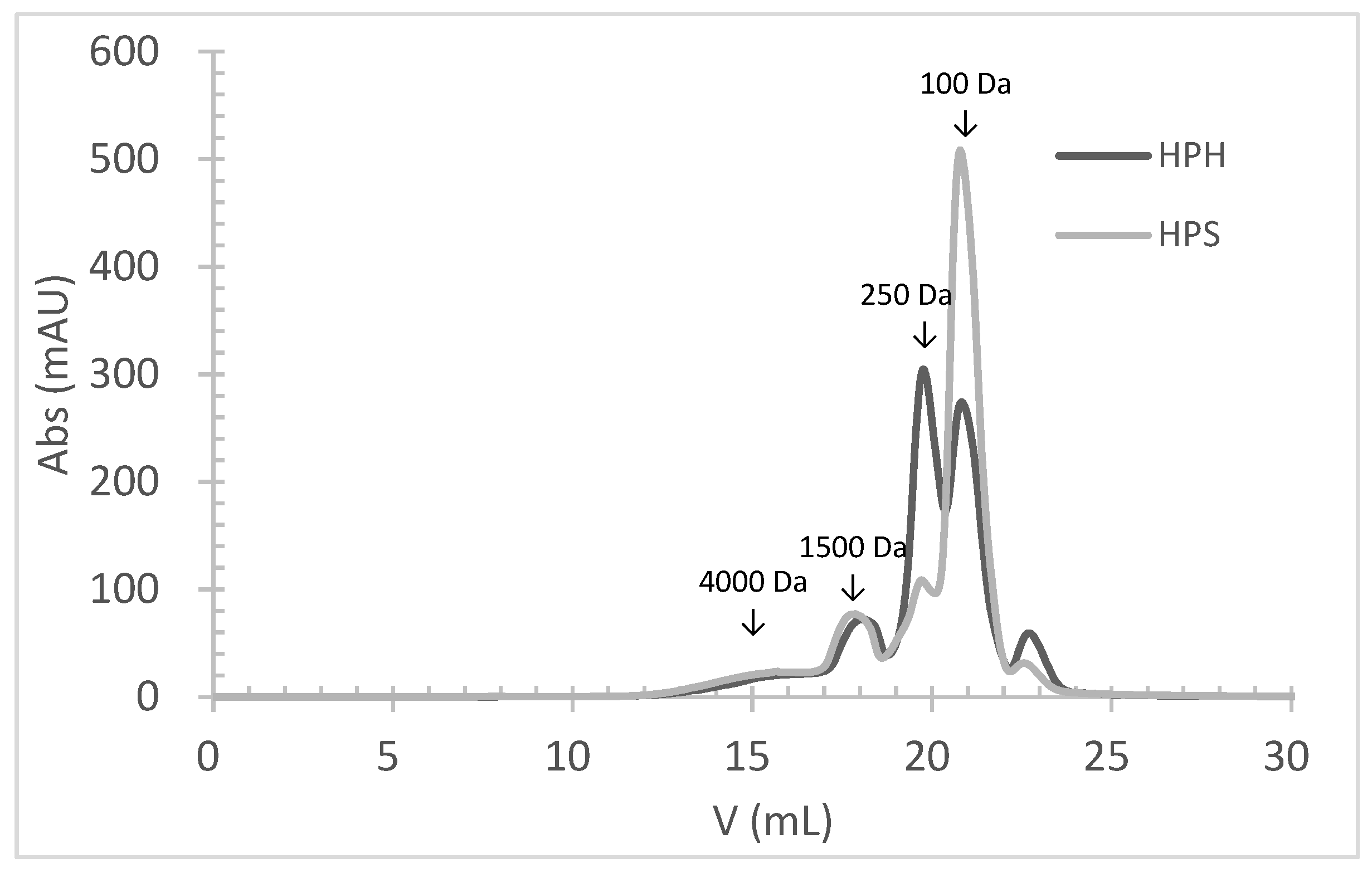
3.4. Molecular Weight Distribution of FPHs
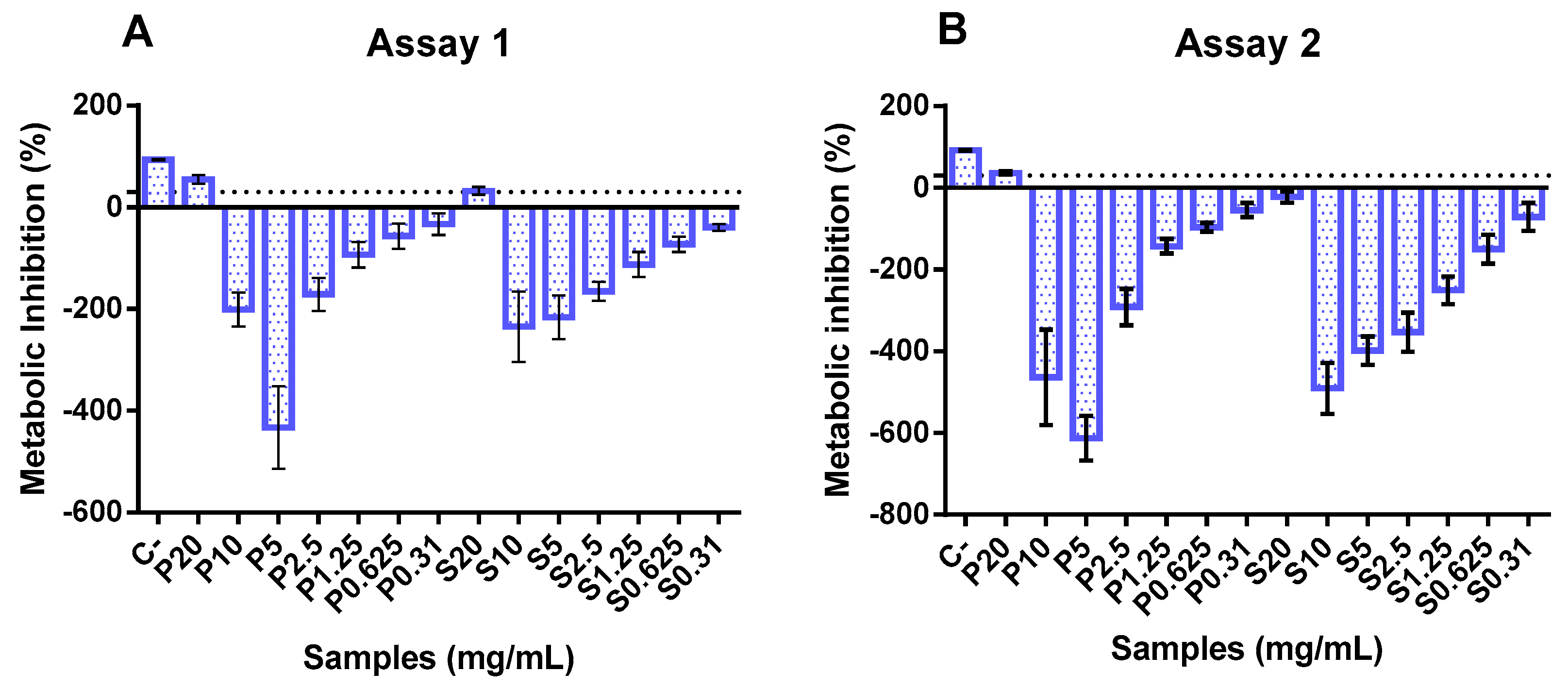
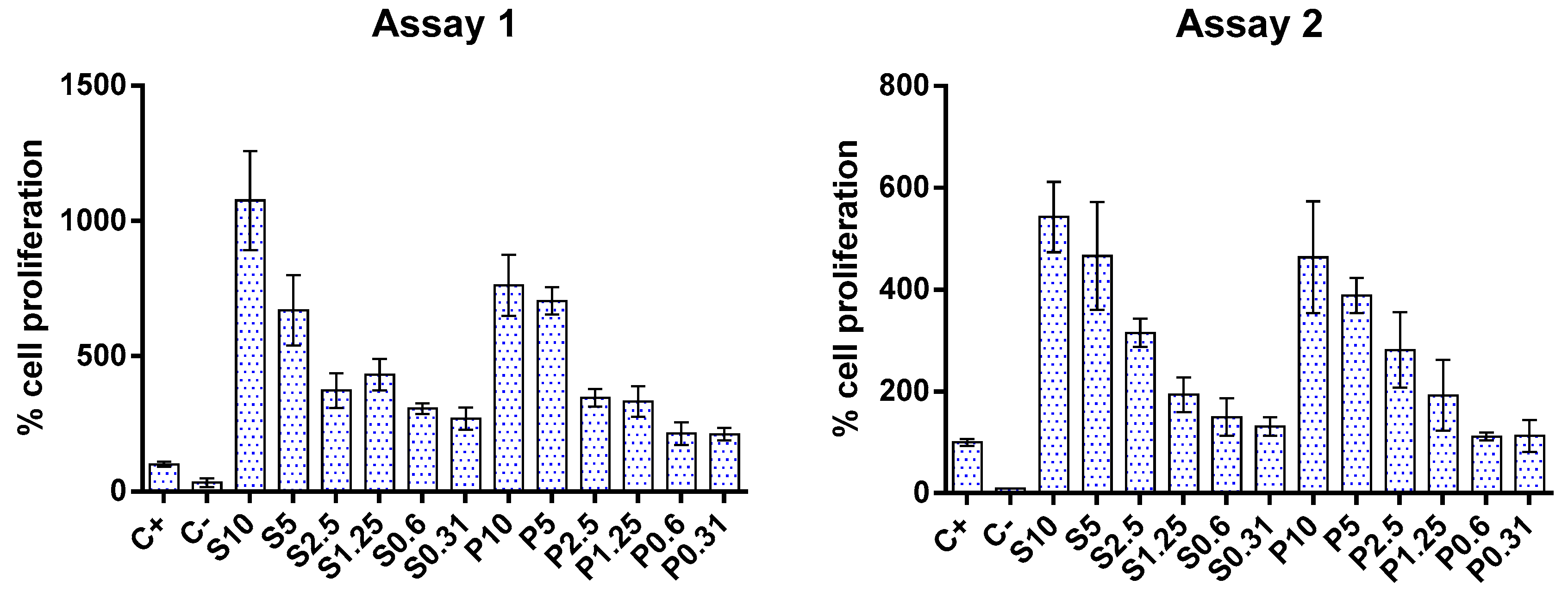
3.5. Cytotoxicity and Proliferation of FPHs
3.6. Antioxidant Activity
3.7. Fe2+ and Cu2+ Chelating Activities
3.8. ACE Inhibitory Activity
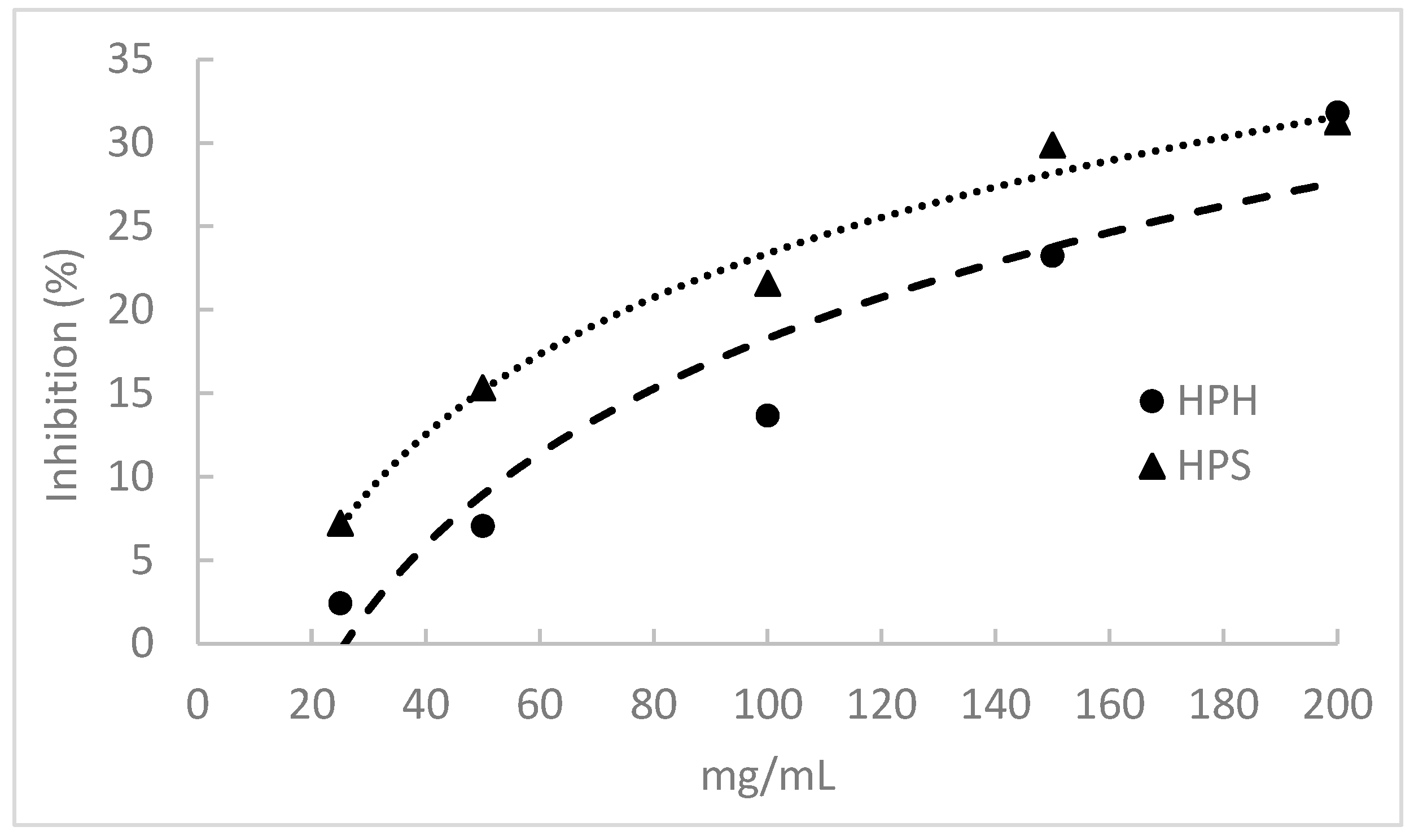
3.9. α-Amylase Inhibitory Activity
3.10. Salmon Heads Protein Hydrolysates Prepared by SWH
3.10.1. Protein Content, Degree of Hydrolysis, and Hydrolysis Yield
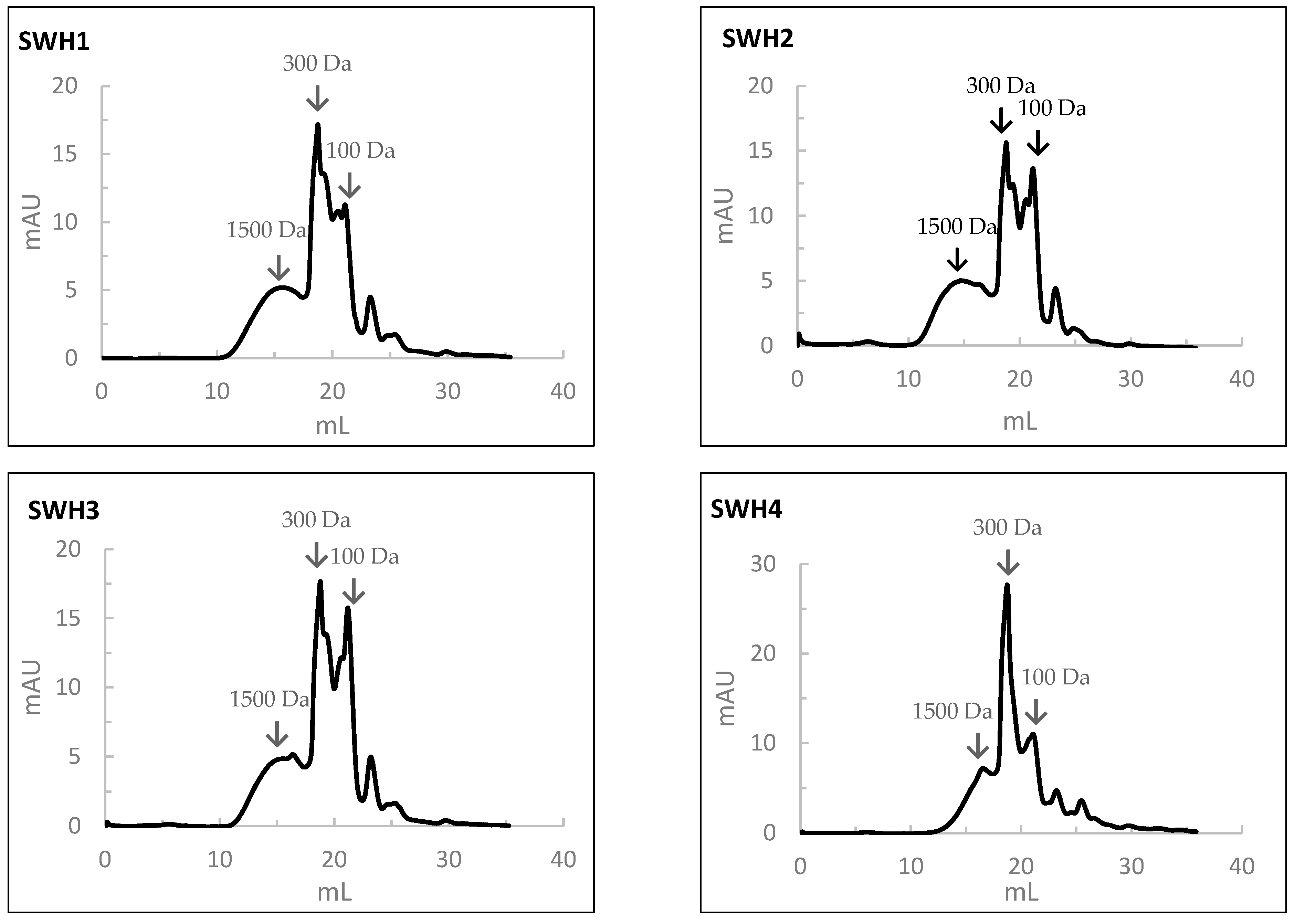
3.10.2. Molecular Weight
3.10.3. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics—Yearbook 2021; FAO: Rome, Italy, 2024; ISBN 978-92-5-138574-6. [Google Scholar]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Henriques, A.; Vázquez, J.A.; Valcarcel, J.; Mendes, R.; Bandarra, N.M.; Pires, C. Characterization of Protein Hydrolysates from Fish Discards and By-Products from the North-West Spain Fishing Fleet as Potential Sources of Bioactive Peptides. Mar. Drugs 2021, 19, 338. [Google Scholar] [CrossRef]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castaño-Tostado, E.; Castillo-Herrera, G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules 2021, 26, 6655. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Chun, B.-S. Subcritical Water Hydrolysis for the Production of Bioactive Peptides from Tuna Skin Collagen. J. Supercrit. Fluids 2018, 141, 88–96. [Google Scholar] [CrossRef]
- Hao, G.; Cao, W.; Li, T.; Chen, J.; Zhang, J.; Weng, W.; Osako, K.; Ren, H. Effect of Temperature on Chemical Properties and Antioxidant Activities of Abalone Viscera Subcritical Water Extract. J. Supercrit. Fluids 2019, 147, 17–23. [Google Scholar] [CrossRef]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and Subcritical Water Technologies for the Production of Bioactive Extracts from Sardine (Sardina pilchardus) Waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Melgosa, R.; Marques, M.; Paiva, A.; Bernardo, A.; Fernández, N.; Sá-Nogueira, I.; Simões, P. Subcritical Water Extraction and Hydrolysis of Cod (Gadus morhua) Frames to Produce Bioactive Protein Extracts. Foods 2021, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.-L.; Wang, J.-X.; Sun, P.-P.; Nargotra, P.; Kuo, C.-H.; Chen, C.-W.; Dong, C.-D. Extraction of Novel Bioactive Peptides from Fish Protein Hydrolysates by Enzymatic Reactions. Appl. Sci. 2023, 13, 5768. [Google Scholar] [CrossRef]
- Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Mar. Drugs 2021, 19, 377. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M. Bio-functional Properties of Sardine Protein Hydrolysates Obtained by Brewer’s Spent Yeast and Commercial Proteases. J. Sci. Food Agric. 2017, 97, 5414–5422. [Google Scholar] [CrossRef]
- Vieira, E.F.; das Neves, J.; Ferreira, I.M.P.L.V.O. Bioactive Protein Hydrolysate Obtained from Canned Sardine and Brewing By-Products: Impact of Gastrointestinal Digestion and Transepithelial Absorption. Waste Biomass Valorization 2021, 12, 1281–1292. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological Activity of Peptides Purified from Fish Skin Hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein Hydrolysate from Salmon Frames: Production, Characteristics and Antioxidative Activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef]
- Cheng, X.L.; Oslan, S.N.H.; Ikhlas, B.; Huda, N. Bioactive Angiotensin Converting Enzyme Inhibitory Activity and Antihypertensive Activity Derived from Fish Protein Hydrolysate: A Systematic Review. Food Res. 2023, 7, 308–330. [Google Scholar] [CrossRef]
- Korczek, K.; Tkaczewska, J.; MigdaŁ, W. Antioxidant and Antihypertensive Protein Hydrolysates in Fish Products—A Review. Czech J. Food Sci. 2018, 36, 195–207. [Google Scholar] [CrossRef]
- Wan, P.; Cai, B.; Chen, H.; Chen, D.; Zhao, X.; Yuan, H.; Huang, J.; Chen, X.; Luo, L.; Pan, J. Antidiabetic Effects of Protein Hydrolysates from Trachinotus Ovatus and Identification and Screening of Peptides with α-Amylase and DPP-IV Inhibitory Activities. Curr. Res. Food Sci. 2023, 6, 100446. [Google Scholar] [CrossRef]
- Amini Sarteshnizi, R.; Sahari, M.A.; Ahmadi Gavlighi, H.; Regenstein, J.M.; Nikoo, M.; Udenigwe, C.C. Influence of Fish Protein Hydrolysate-Pistachio Green Hull Extract Interactions on Antioxidant Activity and Inhibition of α-Glucosidase, α-Amylase, and DPP-IV Enzymes. LWT 2021, 142, 111019. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Fusang, K.; Pripatnanont, P.; Benjakul, S. Properties and Characteristics of Acid-Soluble Collagen from Salmon Skin Defatted with the Aid of Ultrasonication. Fishes 2022, 7, 51. [Google Scholar] [CrossRef]
- Pires, C.; Clemente, T.; Batista, I. Functional and Antioxidative Properties of Protein Hydrolysates from Cape Hake By-products Prepared by Three Different Methodologies. J. Sci. Food Agric. 2013, 93, 771–780. [Google Scholar] [CrossRef]
- Lee, H.-J.; Saravana, P.S.; Cho, Y.-N.; Haq, M.; Chun, B.-S. Extraction of Bioactive Compounds from Oyster (Crassostrea gigas) by Pressurized Hot Water Extraction. J. Supercrit. Fluids 2018, 141, 120–127. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Saint-Denis, T.; Goupy, J. Optimization of a Nitrogen Analyser Based on the Dumas Method. Anal. Chim. Acta 2004, 515, 191–198. [Google Scholar] [CrossRef]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid. Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids; Agilent Technologies: Santa Clara, CA, USA, 2000. [Google Scholar]
- Jorhem, L.; Afthan, G.; Cumont, G.; Dypdahl, H.P.; Gadd, K.; Havre, G.N.; Julshamn, K.; Kåverud, K.; Lind, B.; Loimaranta, J.; et al. Determination of Metals in Foods by Atomic Absorption Spectrometry after Dry Ashing: NMKL1 Collaborative Study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- BS EN 14084:2003; Foodstuffs—Determination of Trace Elements Determination of Lead, Cadmium, Zinc, Copper and Iron by Atomic Absorption Spectrometry (AAS) after Microwave Digestion. CEN-European Committee for Standardization: Brussels, Belgium, 2003; pp. 1–20.
- EPA Test Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation and Atomic Absorption Spectrometry; SW-846; Environment Protection Agency: Washington, DC, USA, 1998; pp. 1–17.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2007; pp. 1–11.
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Antioxidative Activities of Browning Products of Glucosamine Fractionated by Organic Solvent and Thin-Layer Chromatography. Nippon Shokuhin Kogyo Gakkaishi 1988, 35, 771–775. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity Purification and Characterisation of Chelating Peptides from Chickpea Protein Hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Pires, C.; Teixeira, B.; Cardoso, C.; Mendes, R.; Nunes, M.L.; Batista, I. Cape Hake Protein Hydrolysates Prepared from Alkaline Solubilised Proteins Pre-Treated with Citric Acid and Calcium Ions: Functional Properties and ACE Inhibitory Activity. Process Biochem. 2015, 50, 1006–1015. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Alpha-Amylase Inhibitors from Roselle (Hibiscus sabdariffa Linn.) Tea. Biosci. Biotechnol. Biochem. 2000, 64, 1041–1043. [Google Scholar] [CrossRef]
- Ramakrishnan, V.V.; Hossain, A.; Dave, D.; Shahidi, F. Salmon Processing Discards: A Potential Source of Bioactive Peptides—A Review. Food Prod. Process. Nutr. 2024, 6, 22. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of Bioprocesses for the Integral Valorisation of Fish Discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Iñarra, B.; Bald, C.; Gutierrez, M.; San Martin, D.; Zufía, J.; Ibarruri, J. Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process. Mar. Drugs 2023, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Regenstein, J.M.; Yasemi, M. Protein Hydrolysates from Fishery Processing By-Products: Production, Characteristics, Food Applications, and Challenges. Foods 2023, 12, 4470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, Z.; Zhang, Y.; Dong, Y.; Hu, X. Structural Characteristics and Stability of Salmon Skin Protein Hydrolysates Obtained with Different Proteases. LWT 2022, 153, 112460. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic Salmon (Salmo salar) Co-Product-Derived Protein Hydrolysates: A Source of Antidiabetic Peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Elango, R. Tolerable Upper Intake Level for Individual Amino Acids in Humans: A Narrative Review of Recent Clinical Studies. Adv. Nutr. 2023, 14, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K.; Oshima, I.; Fukuda, Z.; Kuramochi, Y.; Nagai, Y.; Izumo, N.; Nakano, M. Safety Assessment of L-Lysine Oral Intake: A Systematic Review. Amino Acids 2019, 51, 647–659. [Google Scholar] [CrossRef]
- Lourenço, H.M.; Afonso, C.; Anacleto, P.; Martins, M.F.; Nunes, M.L.; Lino, A.R. Elemental Composition of Four Farmed Fish Produced in Portugal. Int. J. Food Sci. Nutr. 2012, 63, 853–859. [Google Scholar] [CrossRef]
- de la Fuente, B.; Aspevik, T.; Barba, F.J.; Kousoulaki, K.; Berrada, H. Mineral Bioaccessibility and Antioxidant Capacity of Protein Hydrolysates from Salmon (Salmo salar) and Mackerel (Scomber scombrus) Backbones and Heads. Mar. Drugs 2023, 21, 294. [Google Scholar] [CrossRef]
- Jensen, M.B.; Jakobsen, J.; Jacobsen, C.; Sloth, J.J.; Ibarruri, J.; Bald, C.; Iñarra, B.; Bøknæs, N.; Sørensen, A.-D.M. Content and Bioaccessibility of Minerals and Proteins in Fish-Bone Containing Side-Streams from Seafood Industries. Mar. Drugs 2024, 22, 162. [Google Scholar] [CrossRef]
- Arisekar, U.; Shalini, R.; Shakila, R.J.; Sundhar, S.; Afrin Banu, A.M.; Iburahim, S.A.; Umamaheshwari, T. Trace Metals in Commercial Seafood Products (Canned, Pickled and Smoked): Comparison, Exposure and Health Risk Assessment. Food Res. Int. 2024, 178, 113969. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-69933-0. [Google Scholar]
- IOM. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academic Press: Washington, DC, USA, 2005; ISBN 0-309-08537-3. [Google Scholar]
- European Union Regulation. Commission Regulation (EC) No 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, L 119, 103–157. [Google Scholar]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-Vitro Antioxidant Capacity and Cytoprotective/Cytotoxic Effects upon Caco-2 Cells of Red Tilapia (Oreochromis spp.) Viscera Hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Barba, F.J.; Ruiz, M.-J. Enhancement of the Antioxidant Effect of Natural Products on the Proliferation of Caco-2 Cells Produced by Fish Protein Hydrolysates and Collagen. Int. J. Mol. Sci. 2023, 24, 6871. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Aspevik, T.; Kousoulaki, K.; Barba, F.J.; Ruiz, M.-J. Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells. Antioxidants 2021, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.J. Reparative Properties of a Commercial Fish Protein Hydrolysate Preparation. Gut 2005, 54, 775–781. [Google Scholar] [CrossRef]
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from Fish and Crustacean By-Products Hydrolysates Stimulate Cholecystokinin Release in STC-1 Cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Marín, R.; Abad, C.; Rojas, D.; Chiarello, D.I.; Alejandro, T.-G. Biomarkers of Oxidative Stress and Reproductive Complications. Adv. Clin. Chem. 2023, 113, 157–233. [Google Scholar]
- Vázquez, J.A.; Menduíña, A.; Nogueira, M.; Durán, A.I.; Sanz, N.; Valcarcel, J. Optimal Production of Protein Hydrolysates from Monkfish By-Products: Chemical Features and Associated Biological Activities. Molecules 2020, 25, 4068. [Google Scholar] [CrossRef]
- Nurdiani, R.; Dissanayake, M.; Street, W.E.; Donkor, O.N.; Singh, T.K.; Vasiljevic, T. In Vitro Study of Selected Physiological and Physicochemical Properties of Fish Protein Hydrolysates from 4 Australian Fish Species. Int. Food Res. J. 2016, 23, 2029–2040. [Google Scholar]
- Yaghoubzadeh, Z.; Peyravii Ghadikolaii, F.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant Activity and Anticancer Effect of Bioactive Peptides from Rainbow Trout (Oncorhynchus mykiss) Skin Hydrolysate. Int. J. Pept. Res. Ther. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- Hasani, K.; Ariaii, P.; Ahmadi, M. Antimicrobial, Antioxidant and Anti-Cancer Properties of Protein Hydrolysates from Indian Mackerel (Rastrelliger kanagurta) Waste Prepared Using Commercial Enzyme. Int. J. Pept. Res. Ther. 2022, 28, 86. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant Activity and Functional Properties of Enzymatic Protein Hydrolysates from Common Carp (Cyprinus carpio) Roe (Egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative Activity and Functional Properties of Protein Hydrolysate of Yellow Stripe Trevally (Selaroides leptolepis) as Influenced by the Degree of Hydrolysis and Enzyme Type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.; Pérez-Martín, R.; Sotelo, C. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Pires, C.; Nunes, M.L.; Batista, I. Effect of in Vitro Gastrointestinal Digestion on the Antioxidant Activity of Protein Hydrolysates Prepared from Cape Hake By-products. Int. J. Food Sci. Technol. 2016, 51, 2528–2536. [Google Scholar] [CrossRef]
- Chai, T.-T.; Tong, S.-R.; Law, Y.-C.; Ismail, N.; Manan, F.; Wong, F.-C. Anti-Oxidative, Metal Chelating and Radical Scavenging Effects of Protein Hydrolysates from Blue-Spotted Stingray. Trop. J. Pharm. Res. 2015, 14, 1349. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- Shahosseini, S.R.; Javadian, S.R.; Safari, R. Effects of Molecular Weights -Assisted Enzymatic Hydrolysis on Antioxidant and Anticancer Activities of Liza Abu Muscle Protein Hydrolysates. Int. J. Pept. Res. Ther. 2022, 28, 72. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of Fish Byproducts: Sources to End-product Applications of Bioactive Protein Hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef] [PubMed]
- Phadke, G.G.; Rathod, N.B.; Ozogul, F.; Elavarasan, K.; Karthikeyan, M.; Shin, K.-H.; Kim, S.-K. Exploiting of Secondary Raw Materials from Fish Processing Industry as a Source of Bioactive Peptide-Rich Protein Hydrolysates. Mar. Drugs 2021, 19, 480. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Regenstein, J.M.; Noori, F.; Piri Gheshlaghi, S. Autolysis of Rainbow Trout (Oncorhynchus mykiss) by-Products: Enzymatic Activities, Lipid and Protein Oxidation, and Antioxidant Activity of Protein Hydrolysates. LWT 2021, 140, 110702. [Google Scholar] [CrossRef]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez-Alvarez, O.; Bougatef, A. Characterization, Surface Properties and Biological Activities of Protein Hydrolysates Obtained from Hake (Merluccius merluccius) Heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant Activity of Protein Hydrolysates Obtained from Discarded Mediterranean Fish Species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Chen, X.X.; Hu, X.; Li, L.H.; Yang, X.Q.; Wu, Y.Y.; Lin, W.L.; Zhao, Y.Q.; Ma, H.X.; Wei, Y. Antioxidant Properties of Tilapia Component Protein Hydrolysates and the Membrane Ultrafiltration Fractions. Adv. Mater. Res. 2014, 1073–1076, 1812–1817. [Google Scholar] [CrossRef]
- Taheri, A.; Bakhshizadeh, G.A. Antioxidant and ACE Inhibitory Activities of Kawakawa (Euthynnus affinis) Protein Hydrolysate Produced by Skipjack Tuna Pepsin. J. Aquat. Food Prod. Technol. 2020, 29, 148–166. [Google Scholar] [CrossRef]
- Viji, P.; Phannendra, T.S.; Jesmi, D.; Madhusudana Rao, B.; Dhiju Das, P.H.; George, N. Functional and Antioxidant Properties of Gelatin Hydrolysates Prepared from Skin and Scale of Sole Fish. J. Aquat. Food Prod. Technol. 2019, 28, 976–986. [Google Scholar] [CrossRef]
- Lassoued, I.; Elgaoud, I.; Hamed, F.; Nasri, R.; Nasri, M.; Barkia, A. Evaluation of Four Fish Protein Hydrolysates as a Source of Antioxidants and Amino Acids. Curr. Top. Pept. Protein Res. 2022, 22, 132–144. [Google Scholar]
- Sripokar, P.; Benjakul, S.; Klomklao, S. Antioxidant and Functional Properties of Protein Hydrolysates Obtained from Starry Triggerfish Muscle Using Trypsin from Albacore Tuna Liver. Biocatal. Agric. Biotechnol. 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and Antihypertensive Protein Hydrolysates Produced from Tuna Liver by Enzymatic Hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Wu, J.; Visessanguan, W. Isolation of Antioxidative and ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack (Katsuwana pelamis) Roe. J. Funct. Foods 2013, 5, 1854–1862. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Li, Z.; Tian, Y.; Zhang, L.; Zheng, B. Stability of Antioxidant Peptides Prepared from Large Yellow Croaker (Pseudosciaena crocea). Curr. Top. Nutraceutical Res. 2016, 14, 37–48. [Google Scholar]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, Radical Scavenging, and Chelation Properties of in Vitro Digests of Alcalase-Treated Zein Hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive Peptides from Atlantic Salmon (Salmo salar) with Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory, and Antioxidant Activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and Characterization of Angiotensin-Converting Enzyme-Inhibitory Peptides from Nile Tilapia (Oreochromis niloticus) Skin Gelatine Produced by an Enzymatic Membrane Reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Batista, I.; Ramos, C.; Montero, P. Enhancement of ACE and Prolyl Oligopeptidase Inhibitory Potency of Protein Hydrolysates from Sardine and Tuna By-Products by Simulated Gastrointestinal Digestion. Food Funct. 2016, 7, 2066–2073. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined Biocatalytic Conversion of Smooth Hound Viscera: Protein Hydrolysates Elaboration and Assessment of Their Antioxidant, Anti-ACE and Antibacterial Activities. Food Res. Int. 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Ishani, B.; Iddya, K.; Mamatha, B.S. Antihypertensive Activity of Fish Protein Hydrolysates and Its Peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological Activities and Potential Health Benefits of Sulfated Polysaccharides Derived from Marine Algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Purification and Characterization of Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Alaska Pollack (Theragra chalcogramma) Skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin- I- Converting Enzyme (ACE) Inhibitory Peptides from Pacific Cod Skin Gelatin Using Ultrafiltration Membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Fahmi, A.; Morimura, S.; Guo, H.C.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of Angiotensin I Converting Enzyme Inhibitory Peptides from Sea Bream Scales. Process Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Kumar, L.V.; Shakila, R.J.; Jeyasekaran, G. In Vitro Anti-Cancer, Anti-Diabetic, Anti-Inflammation and Wound Healing Properties of Collagen Peptides Derived from Unicorn Leatherjacket (Aluterus monoceros) at Different Hydrolysis. Turk. J. Fish. Aquat. Sci. 2018, 19, 551–560. [Google Scholar] [CrossRef]
- Ben Slama-Ben Salem, R.; Ktari, N.; Bkhairia, I.; Nasri, R.; Mora, L.; Kallel, R.; Hamdi, S.; Jamoussi, K.; Boudaouara, T.; El-Feki, A.; et al. In Vitro and in Vivo Anti-Diabetic and Anti-Hyperlipidemic Effects of Protein Hydrolysates from Octopus Vulgaris in Alloxanic Rats. Food Res. Int. 2018, 106, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Siala, R.; Chu, A.K.; Bourguiba, H.; Tunisie, M.; Lassoued, I.; Trigui, M.; Ghlissi, Z.; Nasri, R.; Jamoussi, K.; Kessis, M.; et al. Functional and Antioxidant Properties of Protein Hydrolysates from Grey Triggerfish Muscle and in Vivo Evaluation of Hypoglycemic and Hypolipidemic Activities Evaluation of Hypocholesterolemic Effect and Antioxidant Activity of Boops Boops Proteins in Cholesterol-Fed Rats. J. Appl. Environ. Microbiol. 2016, 4, 105–119. [Google Scholar]
- Farias, T.C.; de Souza, T.S.P.; Fai, A.E.C.; Koblitz, M.G.B. Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach. Nutrients 2022, 14, 4275. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Moon, H.E.; Roh, M.K.; Ha, Y.M.; Lee, B.B.; Cho, K.K.; Choi, I.S. Physiological Properties of Scomber Japonicus Meat Hydrolysate Prepared by Subcritical Water Hydrolysis. J. Environ. Biol. 2016, 37, 57–63. [Google Scholar]
- Jeong, Y.-R.; Park, J.-S.; Nkurunziza, D.; Cho, Y.-J.; Chun, B.-S. Valorization of Blue Mussel for the Recovery of Free Amino Acids Rich Products by Subcritical Water Hydrolysis. J. Supercrit. Fluids 2021, 169, 105135. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Haq, M.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Physicochemical and Biofunctional Properties of Shrimp (Penaeus Japonicus) Hydrolysates Obtained from Hot-Compressed Water Treatment. J. Supercrit. Fluids 2019, 147, 322–328. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Chun, B.-S. Recovery of Functional Materials with Thermally Stable Antioxidative Properties in Squid Muscle Hydrolyzates by Subcritical Water. J. Food Sci. Technol. 2015, 52, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of Antioxidants in Glycation Model Systems Treated under Subcritical Water Extraction Conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A.; Barea, P.; Illera, A.E.; Melgosa, R.; Beltrán, S.; Sanz, M.T. Potential of Subcritical Water Hydrolysis to Valorize Low-Valued Ray-Finned Fish (Labeobarbus nedgia): Effects of Hydrolysis Temperature and Pressurization Agent. Foods 2024, 13, 1462. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Haq, M.; Chun, B.-S. Characterization of Pepsin-Solubilised Collagen Recovered from Mackerel (Scomber japonicus) Bone and Skin Using Subcritical Water Hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef]

| Protein (%) | Moisture (%) | Ash (%) | Fat (%) | DH (%) | Protein Yield (%) | Hydrolysis Yield (%) | |
|---|---|---|---|---|---|---|---|
| Salmon heads | 13.8 ± 0.9 A | 61.2 ± 0.5 A | 2.39 ± 0.19 A | 19.8 ± 0.2 B | --- | --- | |
| Hake by-products | 14.3 ± 1.4 A | 81.6 ± 0.0 B | 2.76 ± 0.13 B | 0.5 ± 0.0 A | --- | --- | |
| HPS | 75.6 ± 0.9 a | 7.6 ± 0.5 b | 13.45 ± 0.31 a | 2.1 ± 0.1 a | 26.2 ± 0.2 b | 68.1 ± 2.3 a | 32.4 ± 2.3 a |
| HPH | 76.8 ± 0.5 a | 4.4 ± 0.1 a | 16.69 ± 0.16 b | 2.7 ± 0.0 b | 20.3 ± 0.0 a | 75.5 ± 3.4 b | 76.4 ± 3.4 b |
| EAA | Hake By-Products | Salmon Heads | HPH | HPS |
|---|---|---|---|---|
| HIS | 1.33 ± 0.10 b (1.70 ± 0.13) A | 0.58 ± 0.10 a (1.63 ± 0.27) A | 0.75 ± 0.15 a,b (0.97 ± 0.20) A | 1.06 ± 0.37 a,b (1.40 ± 0.48) A |
| LYS | 5.68 ± 1.67 a (7.30 ± 2.15) A | 3.38 ± 0.46 a (9.51 ± 1.30) A | 6.24 ± 0.61 a (8.12 ± 0.80) A | 6.09 ± 0.92 a (8.05 ± 1.22) A |
| LEU | 5.95 ± 0.05 d (7.64 ± 0.06) A | 2.22 ± 0.25 a (6.25 ± 0.70) B | 5.42 ± 0.11 c (7.05 ± 0.14) A,B | 4.60 ± 0.02 b (6.08 ± 0.02) B |
| VAL | 3.75 ± 0.22 c (4.81 ± 0.28) A | 1.53 ± 0.12 a (4.30 ± 0.33) A | 3.43 ± 0.03 b,c (4.46 ± 0.04) A | 3.13 ± 0.03 b (4.13 ± 0.04) A |
| MET | 1.90 ± 0.50 b (2.44 ± 0.64) A | 0.47 ± 0.08 a (1.31 ± 0.21) A | 1.56 ± 0.08 b (2.02 ± 0.10) A | 1.44 ± 0.11 b (1.91 ± 0.15) A |
| CYS | 0.79 ± 0.07 b (1.01 ± 0.08) B | 0.51 ± 0.06 a (1.44 ± 1.18) A | 0.89 ± 0.03 b (1.16 ± 0.04) A,B | 1.05 ± 0.01 c (1.39 ± 0.02) A |
| PHE | 3.13 ± 0.17 c (4.02 ± 0.21) A | 1.21 ± 0.13 a (3.39 ± 0.36) A,B | 2.89 ± 0.06 c (3.76 ± 0.07) A,B | 2.47 ± 0.06 b (3.27 ± 0.08) B |
| TYR | 2.69 ± 0.16 c (3.45 ± 0.21) A | 1.05 ± 0.10 a (2.94 ± 0.29) A,B | 2.42 ± 0.04 c (3.15 ± 0.05) A,B | 2.06 ± 0.06 b (2.73 ± 0.08) B |
| ILE | 3.40 ± 0.24 c (4.37 ± 0.30) A | 1.28 ± 0.12 a (3.58 ± 0.34) B | 2.93 ± 0.07 b (3.81 ± 0.10) A,B | 2.60 ± 0.02 b (3.44 ± 0.03) B |
| THR | 4.03 ± 0.24 b (5.18 ± 0.31) A | 1.93 ± 0.20 a (5.41 ± 0.57) A | 4.02 ± 0.01 b (5.23 ± 0.01) A | 3.53 ± 0.13 b (4.67 ± 0.18) A |
| NEAA | ||||
| ASP | 8.16 ± 0.33 c (10.48 ± 0.42) A | 3.22 ± 0.34 a (9.05 ± 0.95) A | 7.57 ± 0.15 b,c (9.86 ± 0.20) A | 6.80 ± 0.09 b (8.98 ± 0.12) A |
| GLU | 12.96 ± 0.55 c (16.64 ± 0.70) A | 4.79 ± 0.50 a (13.44 ± 1.40) B | 12.11 ± 0.22 c (15.76 ± 0.28) A,B | 10.12 ± 0.12 b (13.38 ± 0.17) B |
| ASN | <QL (<QL) | <QL (<QL) | <QL (<QL) | <QL (<QL) |
| SER | 3.52 ± 0.15 b (4.52 ± 0.10) A | 1.49 ± 0.19 a (4.17 ± 0.54) A | 3.50 ± 0.07 b (4.55 ± 0.09) A | 3.14 ± 0.10 b (4.15 ± 0.13) A |
| GLN | <QL (<QL) | <QL (<QL) | <QL (<QL) | <QL (<QL) |
| GLY | 4.14 ± 0.05 a (5.31 ± 0.07) C | 3.27 ± 0.53 a (9.17 ± 1.49) A,B | 5.47 ± 0.12 b (7.12 ± 0.16) B,C | 7.31 ± 0.15 c (9.66 ± 0.20) A |
| ARG | 4.86 ± 0.16 b (6.24 ± 0.20) A | 2.21 ± 0.26 a (6.22 ± 0.74) A | 4.74 ± 0.12 b (6.17 ± 0.15) A | 4.58 ± 0.14 b (6.05 ± 0.19) A |
| ALA | 4.98 ± 0.19 b (6.39 ± 0.25) A | 2.42 ± 0.25 a (6.80 ± 0.70) A | 5.16 ± 0.03 b (6.72 ± 0.04) A | 5.25 ± 0.11 b (6.94 ± 0.15) A |
| Tau | <QL (<QL) | <QL (<QL) | <QL (<QL) | <QL (<QL) |
| PRO | 3.11 ± 0.41 b (3.99 ± 0.53) A | 1.80 ± 0.22 a (5.05 ± 0.61) A | 3.49 ± 0.10 b (4.54 ± 0.13) A | 3.91 ± 0.36 b (5.17 ± 0.48) A |
| HYP | <QL (<QL) | 0.42 ± 0.07 a (1.19 ± 0.19) A | <QL (<QL) | 1.00 ± 0.03 b (1.32 ± 0.04) A |
| Total AA (g/100 g) | 74.38 | 33.77 | 72.57 | 70.14 |
| Minerals (mg/kg) | DRI (mg/Day) | Cape Hake By-Products | Salmon Heads | HPH | HPS | ||
|---|---|---|---|---|---|---|---|
| mg/kg | DRI (%) | mg/kg | DRI (%) | ||||
| Na | 1500 | 6985± 1349 a | 5425 ± 582 a | 58,075 ± 3722 b | 387.2 | 49,295 ± 1493 b | 328.6 |
| K | 4700 | 53,845 ± 1008 a | 10,721 ± 1405 b | 15,315 ± 622 c | 32.6 | 12,107 ± 104 b | 25.8 |
| Mg | 420 | 1657 ± 104 c | 1445 ± 317 c | 335 ± 8 b | 8.0 | 29 ± 2 a | 0.7 |
| Fe | 18 | 13.8 ± 3.4 b | 22.9 ± 1.6 c | 3.0 ± 0.1 a | 1.7 | 18.0 ± 0.1 b,c | 10.0 |
| Zn | 11 | 9.7 ± 0.1 b | 24.2 ± 2.1 c | 3.1 ± 0.0 a | 2.8 | 7.0 ± 0.1 a,b | 6.4 |
| Mn # | - | 2.7 ± 0.4 a | 8.7 ± 0.9 b | <DL | - | <DL | - |
| Cr ## | - | 0.576 ± 0.08 a | 0.713 ± 0.07 a | <DL | - | <DL | - |
| Ni | - | 0.056 ± 0.002 a | 0.113 ± 0.011 a | 0.111 ± 0.002 a | - | 0.154 ± 0.07 a | - |
| Cu | 0.9 | 0.838 ± 0.05 a | 2.25 ± 0.35 b | 0.57 ± 0.01 a | 6.3 | 0.53 ± 0.01 a | 5.9 |
| Contaminant metals (mg/kg) | |||||||
| Pb * | 0.002 (<DL) | 0.002 (<DL) | 0.04 (<QL) | 0.07 ± 0.00 | |||
| Cd ** | 0.003 (<QL) | 0.001 (<DL) | 0.004 (<QL) | 0.002 (DL) | |||
| Hg *** | 0.43 ± 0.16 | 0.009 (<QL) | 0.18 ± 0.00 | 0.013 ± 0.000 | |||
| Sample | EC50/A0.5 * (mg/mL) | |||||
|---|---|---|---|---|---|---|
| ABTS | DPPH | RP * | Fe2+ | Cu2+ | ACE | |
| HPS | 2.4 ± 0.01 a | 10.5 ± 0.04 b | 25.5 ± 0.16 b | 0.45 ± 0.04 a | n.a. | 2.2 ± 0.13 b |
| HPH | 2.1 ± 0.05 a | 9.5 ± 0.10 a | 20.1 ± 0.35 a | 0.52 ± 0.02 b | 0.64 ± 0.01 | 0.86 ± 0.05 a |
| FPH | P (%) | DH (%) | Hydrolysis Yield (%) |
|---|---|---|---|
| SWH1 (200 °C, 100 bar, 30 min) | 84.8 ± 0.5 c | 10.7 ± 0.13 a | 83.5 ± 0.53 d |
| SWH2 (200 °C, 100 bar, 10 min) | 86.2 ± 0.8 c | 11.6 ± 0.02 a | 93.3 ± 0.78 e |
| SWH3 (200 °C, 50 bar, 30 min) | 88.7 ± 0.6 c | 11.7 ± 0.29 a | 94.3 ± 0.42 e |
| SWH4 (250 °C, 100 bar, 30 min) | 75.8 ± 0.4 b | 33.8 ± 1.02 b | 70.3 ± 0.23 c |
| SWH5 (250 °C, 100 bar, 10 min) | 70.4 ± 0.4 a | 36.4 ± 0.62 c | 47.0 ± 0.21 a |
| SWH6 (250 °C, 50 bar, 30 min) | 76.8 ± 0.6 b | 32.4 ± 0.15 b | 63.8 ± 0.08 b |
| Antioxidant Activity (%) | Chelating Activity (%) | ||||
|---|---|---|---|---|---|
| DPPH | ABTS | RP | Cu2+ | Fe2+ | |
| SWH1 (200 °C, 100 bar, 30 min) | 17.9 ± 0.53 c | 2.50 ± 0.17 a,b | 0.061 ± 0.006 a | 9.7 ± 0.4 a | 1.7 ± 0.40 a |
| SWH2 (200 °C, 100 bar, 10 min) | 15.2 ± 0.83 c | 2.40 ± 0.13 a,b | 0.058 ± 0.000 a | 12.8 ± 2.7 a,b | 0.37 ± 0.08 a |
| SWH3 (200 °C, 50 bar, 30 min) | 9.6 ± 0.09 b | 1.89 ± 0.63 a | 0.061 ± 0.000 a | 12.0 ± 1.7 a,b | 1.4 ± 0.40 a |
| SWH4 (250 °C, 100 bar, 30 min) | 22.9 ± 0.28 d | 3.10 ± 0.07 b | 0.064 ± 0.002 a | 11.2 ± 1.1 a | 13.0 ± 0.8 d |
| SWH5 (250 °C, 100 bar, 10 min) | 18.8 ± 0.80 c,d | 2.78 ± 0.17 a,b | 0.054 ± 0.009 a | 13.0 ± 0.4 a,b | 6.5 ± 0.8 c |
| SWH6 (250 °C, 0 bar, 30 min) | 14.9 ± 2.2 c | 2.87 ± 0.01 a,b | 0.058 ± 0.001 a | 16.7 ± 1.7 c | 2.5 ± 0.10 a,b |
| HPS (60 °C, pH 8.5, 3 h, 1% Alcalase) | 0.83 ± 0.08 a | 5.45 ± 0.48 c | 0.051 ± 0.002 a | nd | 3.1 ± 0.62 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, C.; Leitão, M.; Sapatinha, M.; Gonçalves, A.; Oliveira, H.; Nunes, M.L.; Teixeira, B.; Mendes, R.; Camacho, C.; Machado, M.; et al. Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties. Foods 2024, 13, 2418. https://doi.org/10.3390/foods13152418
Pires C, Leitão M, Sapatinha M, Gonçalves A, Oliveira H, Nunes ML, Teixeira B, Mendes R, Camacho C, Machado M, et al. Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties. Foods. 2024; 13(15):2418. https://doi.org/10.3390/foods13152418
Chicago/Turabian StylePires, Carla, Matilde Leitão, Maria Sapatinha, Amparo Gonçalves, Helena Oliveira, Maria Leonor Nunes, Bárbara Teixeira, Rogério Mendes, Carolina Camacho, Manuela Machado, and et al. 2024. "Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties" Foods 13, no. 15: 2418. https://doi.org/10.3390/foods13152418
APA StylePires, C., Leitão, M., Sapatinha, M., Gonçalves, A., Oliveira, H., Nunes, M. L., Teixeira, B., Mendes, R., Camacho, C., Machado, M., Pintado, M., Ribeiro, A. R., Vieira, E. F., Delerue-Matos, C., Lourenço, H. M., & Marques, A. (2024). Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties. Foods, 13(15), 2418. https://doi.org/10.3390/foods13152418












