Effect of Pulsed Electric Field Pretreatment on the Concentration of Lipophilic and Hydrophilic Compounds in Cold-Pressed Grape Seed Oil Produced from Wine Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Pulsed Electric Field (PEF) Pretreatment of Grape Pomace Seeds
2.4. Oil Extraction by Cold Pressing
2.5. Oil Extraction Yield and Flow Rate
2.6. Determination of Sterols by GC-FID/MS
2.7. Determination of Tocochromanols by HPLC-FLUO
2.8. Spectrophotometric Determination of Total Phenols
2.9. Determination of Individual Phenolic Compounds by HPLC-DAD/MS
2.10. Determination of Fatty Acid Profile by GC-FID
2.11. Determination of Antioxidant Capacity
2.12. Scanning Electron Microscopy (SEM) Analysis
2.13. Data Analysis
3. Results and Discussion
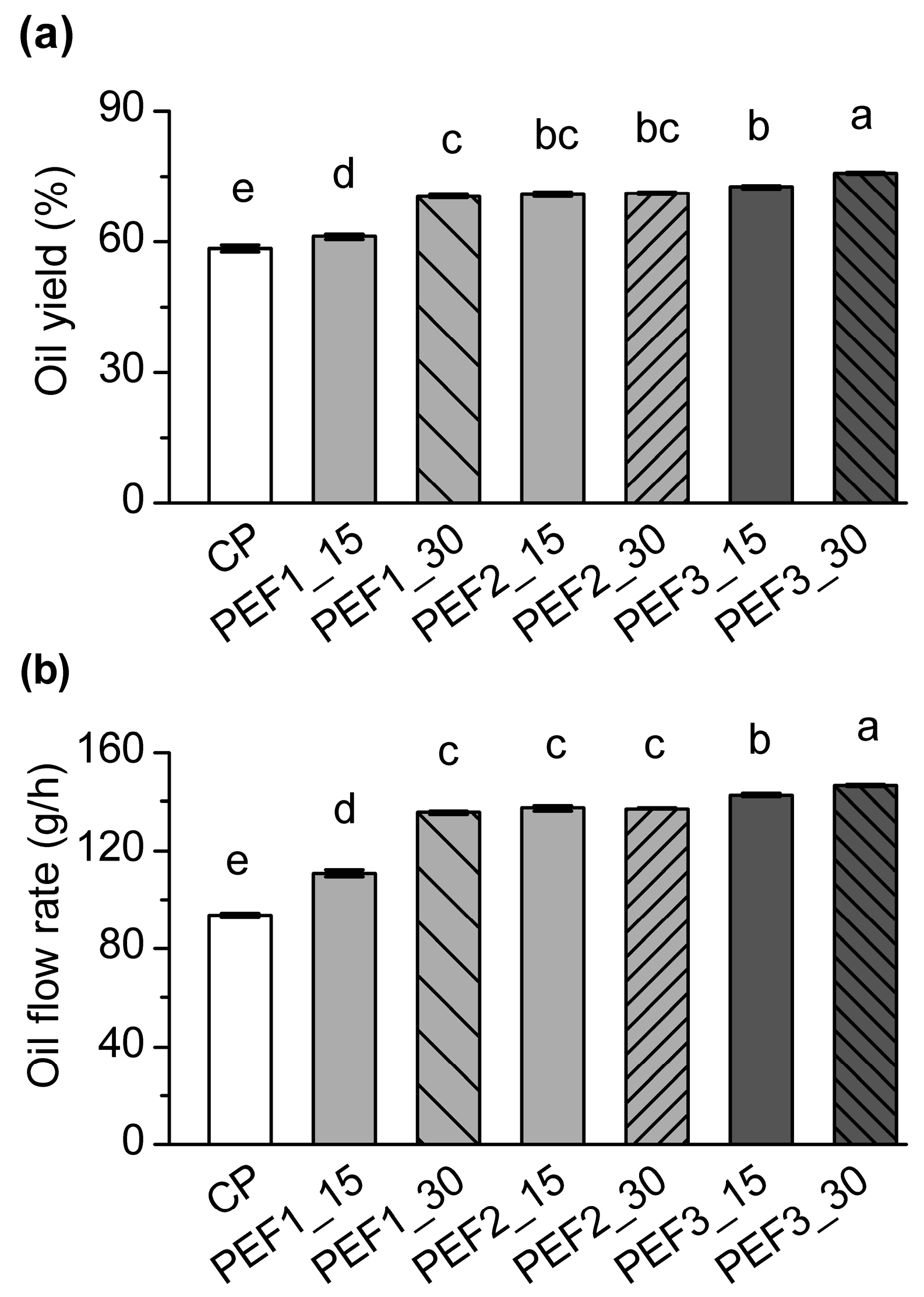
3.1. Oil Yield and Oil Flow Rate
3.2. Sterols
3.3. Tocochromanols
3.4. Phenolic Compounds
3.5. Fatty Acids
3.6. Hydrophilic and Lipophilic Oxygen Radical Absorbance Capacity (H-ORAC and L-ORAC)
3.7. Surface Structure of Grape Pomace Seeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taifouris, M.; El-Halwagi, M.; Martin, M. Evaluation of the Economic, Environmental, and Social Impact of the Valorization of Grape Pomace from the Wine Industry. ACS Sustain. Chem. Eng. 2023, 11, 13718–13728. [Google Scholar] [CrossRef] [PubMed]
- OIV International Organisation of Vine and Wine (OIV) Report on State of the World Vine and Wine Sector in 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2.pdf (accessed on 10 April 2024).
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-De la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B. Virgin Grape Seed Oil: Is It Really a Nutritional Highlight? Eur. J. Lipid Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Quantitation of the Main Constituents of Some Authentic Grape-Seed Oils of Different Origin. J. Agric. Food Chem. 2006, 54, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, F.B.; de Santana, F.C.; Araujo, E.; Purgatto, E.; Mancini-Filho, J. Chemical Composition of Cold Pressed Brazilian Grape Seed Oil. Food Sci. Technol. 2018, 38, 164–171. [Google Scholar] [CrossRef]
- Zhao, L.; Yagiz, Y.; Xu, C.; Fang, X.; Marshall, M.R. Identification and Characterization of Vitamin E Isomers, Phenolic Compounds, Fatty Acid Composition, and Antioxidant Activity in Seed Oils from Different Muscadine Grape Cultivars. J. Food Biochem. 2017, 41, e12384. [Google Scholar] [CrossRef]
- Ćurko, N.; Lukić, K.; Tušek, A.J.; Balbino, S.; Vukušić Pavičić, T.; Tomašević, M.; Redovniković, I.R.; Ganić, K.K. Effect of Cold Pressing and Supercritical CO2 Extraction Assisted with Pulsed Electric Fields Pretreatment on Grape Seed Oil Yield, Compo-sition and Antioxidant Characteristics. LWT-Food Sci. Technol. 2023, 184, 114974. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of Various Grape Seed Oils by Volatile Com-pounds, Triacylglycerol Composition, Total Phenols and Antioxidant Capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef]
- Sevindik, O.; Kelebek, H.; Rombolà, A.D.; Selli, S. Grape Seed Oil Volatiles and Odour Activity Values: A Comparison with Turkish and Italian Cultivars and Extraction Methods. J. Food Sci. Technol. 2021, 59, 1968–1981. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M. Effect of Cold Press and Soxhlet Extraction Systems on Fatty Acid, Tocopherol Contents, and Phenolic Compounds of Various Grape Seed Oils. J. Food Process. Preserv. 2018, 42, e13417. [Google Scholar] [CrossRef]
- Kraljić, K.; Škevin, D.; Pospišil, M.; Obranović, M.; Neđeral, S.; Bosolt, T. Quality of Rapeseed Oil Produced by Conditioning Seeds at Modest Temperatures. J. Am. Oil Chem. Soc. 2013, 90, 589–599. [Google Scholar] [CrossRef]
- Mazroei Seydani, L.; Gharachorloo, M.; Asadi, G. Use of Pulsed Electric Field to Extract Rapeseed Oil and Investigation of the Qualitative Properties of Oils. J. Food Process Eng. 2022, 45, e14149. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of Pulsed Electric Fields on Bioactive Compounds in Foods: A Review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Pataro, G.; Ferrari, G. Limitations of Pulsed Electric Field Utilization in Food Industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 283–310. [Google Scholar]
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-Compounds from Fermented Grape Pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Mirzaei, H.O.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M. The Influence of Pulsed Electric Fields and Microwave Pretreatments on Some Selected Physicochemical Properties of Oil Extracted from Black Cumin Seed. Food Sci. Nutr. 2018, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of Pulsed Electric Fields and High Voltage Electrical Discharges for Oil Extraction from Sesame Seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez De Marañón, I. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Shorstkii, I.A.; Zherlicin, A.G.; Li, P. Impact of Pulsed Electric Field and Pulsed Microwave Treatment on Morphological and Structural Characteristics of Sunflower Seed. OCL-Oilseeds Fats Crop. Lipids 2019, 26, 47. [Google Scholar] [CrossRef]
- Guderjan, M.; Töpfl, S.; Angersbach, A.; Knorr, D. Impact of Pulsed Electric Field Treatment on the Recovery and Quality of Plant Oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Guderjan, M.; Elez-Martínez, P.; Knorr, D. Application of Pulsed Electric Fields at Oil Yield and Content of Functional Food Ingredients at the Production of Rapeseed Oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62. [Google Scholar] [CrossRef]
- Rábago-Panduro, L.M.; Morales-De La Peña, M.; Romero-Fabregat, M.P.; Martín-Belloso, O.; Welti-Chanes, J. Effect of Pulsed Electric Fields (PEF) on Extraction Yield and Stability of Oil Obtained from Dry Pecan Nuts (Carya illinoinensis (Wangenh. K. Koch)). Foods 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Cadot, Y.; Miñana-Castelló, M.T.; Chevalier, M. Anatomical, Histological, and Histochemical Changes in Grape Seeds from Vitis Vinifera cv. Cabernet Franc during Fruit Development. J. Agric. Food Chem. 2006, 54, 9206–9215. [Google Scholar] [CrossRef] [PubMed]
- Bebek Markovinović, A.; Putnik, P.; Stulić, V.; Batur, L.; Duralija, B.; Pavlić, B.; Vukušić Pavičić, T.; Herceg, Z.; Bursać Kovačević, D. The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness. Foods 2022, 11, 1997. [Google Scholar] [CrossRef]
- ISO 659:2009; Oilseeds-Determination of Oil Content (Reference Method). ISO: Geneva, Switzerland, 2009.
- Zdanowska, P.; Dróżdż, B.; Janakowski, S.; Derewiaka, D. Impact of Preliminary Ultrasound Treatment of Rape Seeds on the Pressing Process and Selected Oil Characteristics. Ind. Crops Prod. 2019, 138, 111572. [Google Scholar] [CrossRef]
- ISO 12228-1:2014; Determination of Individual and Total Sterols Contents. Gas Chromatographic Method. Part 1: Animal and Vegetable Fats and Oils (Reference Method). ISO: Geneva, Switzerland, 2014.
- Balbino, S.; Repajić, M.; Obranović, M.; Medved, A.M.; Tonković, P.; Dragović-Uzelac, V. Characterization of Lipid Fraction of Apiaceae Family Seed Spices: Impact of Species and Extraction Method. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100326. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography (Reference Method). ISO: Geneva, Switzerland, 2016.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 2: Preparation of Methyl Esters of Fatty Acids (Reference Method). ISO: Geneva, Switzerland, 2017.
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determi-nation by Capillary Gas Chromatography (Reference Method). ISO: Geneva, Switzerland, 2015.
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Vorobiev, E. Application of Pulsed Electric Energies for Oil and Polyphenol Extraction from Sesame Cake and Sesame Seeds. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer Nature: Berlin, Germany, 2017; Volume 4, pp. 2699–2712. [Google Scholar]
- Hartmann, M.A. Plant Sterols and the Membrane Environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Delsart, C.; Cholet, C.; Ghidossi, R.; Grimi, N.; Gontier, E.; Gény, L.; Vorobiev, E.; Mietton-Peuchot, M. Effects of Pulsed Electric Fields on Cabernet Sauvignon Grape Berries and on the Characteristics of Wines. Food Bioprocess Technol. 2014, 7, 424–436. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Shi, X.; Pan, K.; Zhang, S.; Zhang, W.; Hua, X. Oxidation of Oleic Acid under Pulsed Electric Field Pro-cessing. Food Res. Int. 2011, 44, 1463–1467. [Google Scholar] [CrossRef]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Merah, O. Sterol Concentration and Distribution in Sunflower Seeds (Helianthus annuus L.) during Seed Development. Food Chem. 2010, 119, 1451–1456. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Monica, H.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Accumulation of Tocopherols and Tocotrienols during Seed Development of Grape (Vitis vinifera L. cv. Albert Lavallée). Plant Physiol. Biochem. 2006, 44, 724–731. [Google Scholar] [CrossRef]
- De Souza, R.D.C.; Souza Machado, B.A.; de Abreu Barreto, G.; Leal, I.L.; dos Anjos, J.P.; Umsza-Guez, M.A. Effect of Experimental Parameters on the Extraction of Grape Seed Oil Obtained by Low Pressure and Supercritical Fluid Extraction. Molecules 2020, 25, 1634. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Novel Seeds Pretreatment Techniques: Effect on Oil Quality and Antioxidant Properties: A Review. J. Food Sci. Technol. 2021, 58, 4451–4464. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Wang, J.; Lu, J. Extraction, Distribution and Characterisation of Phenolic Compounds and Oil in Grapeseeds. Food Chem. 2010, 122, 688–694. [Google Scholar] [CrossRef]


| Sample Name | PEF Pretreatment of Grape Pomace Seeds | Oil Production Method | ||
|---|---|---|---|---|
| Pulse Power (kV) | Electric Field Intensity (kV/cm) | Time (min) | ||
| CP | – | – | – | Cold pressing |
| PEF1_15 | 40 | 12.5 | 15 | |
| PEF1_30 | 40 | 12.5 | 30 | |
| PEF2_15 | 45 | 14.0 | 15 | |
| PEF2_30 | 45 | 14.0 | 30 | |
| PEF3_15 | 50 | 15.6 | 15 | |
| PEF3_30 | 50 | 15.6 | 30 | |
| Sterols (mg/kg) | Sample Name | ||||||
|---|---|---|---|---|---|---|---|
| CP | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | |
| Campesterol | 301.7 ± 0.3 e | 325.6 ± 0.8 c | 369.8 ± 0.1 a | 311.7 ± 0.6 d | 331.9 ± 0.3 b | 326.9 ± 0.1 c | 313.0 ± 0.5 d |
| Campestanol | 11.2 ± 0.0 e | 18.0 ± 0.1 b | 11.9 ± 0.7 de | 13.7 ± 0.2 c | 18.6 ± 0.3 ab | 20.2 ± 0.1 a | 13.4 ± 0.7 cd |
| Stigmasterol | 434.0 ± 0.1 e | 445.0 ± 0.1 d | 457.8 ± 0.5 a | 457.5 ± 0.1 a | 447.7 ± 0.4 c | 429.9 ± 0.6 f | 454.0 ± 0.7 b |
| β-Sitosterol | 2599.2 ± 1.6 g | 2962.8 ± 1.1 c | 3368.4 ± 2.7 a | 2809.1 ± 0.1 e | 3039.0 ± 1.3 b | 2943.5 ± 0.6 d | 2718.5 ± 1.3 f |
| Sitostanol | 11.2 ± 0.3 d | 15.9 ± 0.1 b | 12.9 ± 0.0 c | 18.9 ± 0.2 a | 12.8 ± 0.0 c | 12.3 ± 0.3 cd | 15.7 ± 0.6 b |
| Δ5-Avenasterol | 71.3 ± 0.3 d | 84.0 ± 0.4 b | 80.0 ± 0.6 c | 78.2 ± 0.3 c | 87.5 ± 0.8 a | 85.0 ± 0.3 b | 78.5 ± 0.1 c |
| Δ5,24-Stigmastadienol | 3.5 ± 0.1 e | 11.1 ± 0.0 b | 7.4 ± 0.0 d | 12.4 ± 0.3 a | 10.3 ± 0.0 c | 11.6 ± 0.1 b | 11.3 ± 0.1 b |
| Δ7-Stigmastenol | 49.6 ± 0.1 e | 89.2 ± 0.5 b | 61.8 ± 1.0 d | 69.6 ± 0.1 c | 93.9 ± 1.3 a | 87.0 ± 0.1 b | 61.7 ± 1.6 d |
| Δ7-Avenasterol | 29.8 ± 0.1 f | 38.7 ± 0.1 b | 35.2 ± 0.0 d | 32.9 ± 0.3 e | 43.2 ± 0.1 a | 38.8 ± 0.1 b | 36.5 ± 0.3 c |
| ∑ Sterols | 3511.6 ± 1.0 g | 3999.2 ± 0.6 c | 4405.3 ± 1.0 a | 3791.6 ± 0.6 e | 4095.1 ± 0.3 b | 3982.9 ± 0.7 d | 3696.4 ± 2.0 f |
| Tocochromanols (mg/kg) | Sample Name | ||||||
|---|---|---|---|---|---|---|---|
| CP | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | |
| α-Tocopherol | 44.3 ± 0.4 e | 45.2 ± 0.4 e | 54.0 ± 1.0 c | 50.8 ± 0.5 d | 56.6 ± 0.8 b | 51.1 ± 0.2 d | 65.3 ± 0.4 a |
| γ-Tocopherol | 18.5 ± 0.1 c | 19.0 ± 0.1 bc | 19.3 ± 0.2 bc | 19.5 ± 0.5 b | 19.7 ± 0.4 ab | 19.7 ± 0.5 ab | 20.6 ± 0.4 a |
| α-Tocotrienol | 102.8 ± 0.4 e | 103.0 ± 0.8 e | 119.4 ± 0.1 b | 111.0 ± 0.5 d | 118.5 ± 1.3 bc | 117.4 ± 0.7 c | 133.4 ± 0.3 a |
| γ-Tocotrienol | 138.7 ± 0.2 d | 139.8 ± 0.7 d | 144.4 ± 1.0 c | 148.2 ± 1.3 b | 150.2 ± 1.2 b | 150.1 ± 0.3 b | 153.3 ± 0.7 a |
| Plastochromanol-8 | 9.3 ± 0.0 c | 9.5 ± 0.1 bc | 9.6 ± 0.1 bc | 9.8 ± 0.2 b | 9.9 ± 0.2 ab | 9.8 ± 0.3 ab | 10.3 ± 0.2 a |
| ∑ Tocochromanols | 313.6 ± 0.8 e | 316.5 ± 0.4 e | 346.6 ± 0.9 c | 339.3 ± 1.7 d | 354.8 ± 2.6 b | 348.2 ± 1.7 c | 382.8 ± 1.8 a |
| Phenolic Compounds | Sample Name | ||||||
|---|---|---|---|---|---|---|---|
| CP | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | |
| Nonflavonoids (µg/kg) | |||||||
| Gallic acid | 147.3± 5.4 e | 151.5 ± 1.7 e | 250.2 ± 4.3 c | 217.9 ± 2.2 d | 275.1 ± 3.0 b | 278.7 ± 1.7 ab | 285.4 ± 0.8 a |
| Hydroxybenzoic acid | 322.5 ± 3.6 d | 328.0 ± 1.6 d | 377.9 ± 2.3 b | 364.1 ± 3.0 c | 383.6 ± 1.2 a | 387.6 ± 4.4 a | 387.1 ± 2.8 a |
| p-Coumaric acid | 170.0 ± 0.9 f | 191.4 ± 0.5 e | 220.5 ± 1.1 d | 223.6 ± 0.4 c | 225.0 ± 2.3 c | 323.5 ± 1.1 b | 346.2 ± 0.4 a |
| Ferulic acid | 111.3 ± 0.9 f | 111.5 ± 0.9 f | 121.9 ± 1.0 e | 125.4 ± 1.4 d | 130.1 ± 1.2 c | 139.4 ± 0.7 b | 159.2 ± 2.0 a |
| ∑ Nonflavonoids | 751.3 ± 8.7 g | 782.4 ± 2.9 f | 970.5 ± 2.7 e | 931.0 ± 5.7 d | 1013.8 ± 5.3 c | 1129.1 ± 4.6 b | 1177.9 ± 4.8 a |
| Flavonoids (µg/kg) | |||||||
| (+)-Catechin | 637.3 ± 3.3 d | 640.8 ± 2.7 d | 639.4 ± 2.0 d | 639.1 ± 2.0 d | 653.4 ± 3.7 c | 959.4 ± 4.3 b | 1000.0 ± 2.3 a |
| (−)-Epicatechin | 200.2 ± 4.1 e | 213.0 ± 9.4 d | 333.4 ± 5.0 c | 417.2 ± 1.4 b | 419.1 ± 1.4 ab | 421.0 ± 1.3 ab | 428.5 ± 3.4 a |
| Procyanidin dimer B1 | 1388.1 ± 2.7 e | 1470.9 ± 2.7 d | 1570.0 ± 11.7 c | 1579.0 ± 5.7 c | 1582.3 ± 1.5 c | 2435.5 ± 10.8 b | 2540.1 ± 10.9 a |
| Quercetin | 62.7 ± 2.0 e | 104.5 ± 3.5 d | 127.1 ± 1.2 b | 107.6 ± 1.7 cd | 123.2 ± 1.6 b | 112.4 ± 2.6 c | 140.8 ± 1.9 a |
| Myricetin | 66.2 ± 1.3 c | 155.2 ± 0.7 b | 156.8 ± 0.6 b | 163.0 ± 2.4 a | 162.0 ± 2.4 a | 162.8 ± 2.1 a | 164.3 ± 2.0 a |
| ∑ Flavonoids | 2354.4 ± 8.2 g | 2584.4 ± 12.1 f | 2826.7 ± 17.1 e | 2906.0 ± 5.6 d | 2940.0 ± 5.8 c | 4081.1 ± 23.9 b | 4273.7 ± 7.1 a |
| Total phenols (mg/kg) | |||||||
| 30.3 ± 0.1 e | 30.3 ± 0.2 e | 32.5 ± 0.4 d | 32.6 ± 0.1 d | 33.4 ± 0.3 c | 34.3 ± 0.3 b | 37.8 ± 0.2 a | |
| Fatty Acids (% of Total) | Sample Name | ||||||
|---|---|---|---|---|---|---|---|
| CP | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | PEF1_15 | PEF1_30 | |
| Myristic acid (C14:0) | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a |
| Palmitic acid (C16:0) | 7.25 ± 0.01 a | 7.10 ± 0.02 b | 7.08 ± 0.04 b | 7.10 ± 0.07 b | 7.08 ± 0.01 b | 7.09 ± 0.01 b | 7.09 ± 0.05 b |
| Palmitoleic acid (C16:1) | 0.20 ± 0.00 a | 0.20 ± 0.01 a | 0.20 ± 0.00 a | 0.20 ± 0.01 a | 0.19 ± 0.00 a | 0.20 ± 0.01 a | 0.20 ± 0.01 a |
| Stearic acid (C18:0) | 4.50 ± 0.02 b | 4.54 ± 0.01 ab | 4.55 ± 0.01 a | 4.53 ± 0.02 ab | 4.56 ± 0.01 a | 4.53 ± 0.01 ab | 4.55 ± 0.01 a |
| Oleic acid(C18:1) | 20.30 ± 0.04 a | 20.43 ± 0.07 a | 20.39 ± 0.00 a | 20.35 ± 0.06 a | 20.33 ± 0.02 a | 20.35 ± 0.01 a | 20.31 ± 0.09 a |
| Linoleic acid(C18:2) | 67.09 ± 0.02 b | 67.11 ± 0.05 b | 67.18 ± 0.01 ab | 67.18 ± 0.04 ab | 67.20 ± 0.01 ab | 67.21 ± 0.02 a | 67.22 ± 0.01 a |
| Linolenic acid (C18:3) | 0.43 ± 0.00 a | 0.41 ± 0.01 a | 0.41 ± 0.00 a | 0.42 ± 0.02 a | 0.41 ± 0.01 a | 0.41 ± 0.01 a | 0.41 ± 0.02 a |
| Arachidic acid (C20:0) | 0.16 ± 0.00 a | 0.16 ± 0.00 a | 0.16 ± 0.01 a | 0.17 ± 0.00 a | 0.17 ± 0.00 a | 0.16 ± 0.00 a | 0.16 ± 0.00 a |
| ∑ SFA | 11.97 ± 0.01 a | 11.85 ± 0.02 b | 11.85 ± 0.04 b | 11.85 ± 0.06 b | 11.86 ± 0.00 ab | 11.84 ± 0.02 b | 11.86 ± 0.06 ab |
| ∑ MUFA | 20.51 ± 0.03 a | 20.62 ± 0.07 a | 20.59 ± 0.01 a | 20.55 ± 0.05 a | 20.54 ± 0.02 a | 20.55 ± 0.02 a | 20.52 ± 0.02 a |
| ∑ PUFA | 67.52 ± 0.02 ab | 67.52 ± 0.06 ab | 67.59 ± 0.01 ab | 67.60 ± 0.02 ab | 67.61 ± 0.01 ab | 67.62 ± 0.02 a | 67.64 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćurko, N.; Perić, K.; Vukušić Pavičić, T.; Balbino, S.; Tomašević, M.; Iveković, D.; Radojčić Redovniković, I.; Kovačević Ganić, K. Effect of Pulsed Electric Field Pretreatment on the Concentration of Lipophilic and Hydrophilic Compounds in Cold-Pressed Grape Seed Oil Produced from Wine Waste. Foods 2024, 13, 2299. https://doi.org/10.3390/foods13142299
Ćurko N, Perić K, Vukušić Pavičić T, Balbino S, Tomašević M, Iveković D, Radojčić Redovniković I, Kovačević Ganić K. Effect of Pulsed Electric Field Pretreatment on the Concentration of Lipophilic and Hydrophilic Compounds in Cold-Pressed Grape Seed Oil Produced from Wine Waste. Foods. 2024; 13(14):2299. https://doi.org/10.3390/foods13142299
Chicago/Turabian StyleĆurko, Natka, Katarina Perić, Tomislava Vukušić Pavičić, Sandra Balbino, Marina Tomašević, Damir Iveković, Ivana Radojčić Redovniković, and Karin Kovačević Ganić. 2024. "Effect of Pulsed Electric Field Pretreatment on the Concentration of Lipophilic and Hydrophilic Compounds in Cold-Pressed Grape Seed Oil Produced from Wine Waste" Foods 13, no. 14: 2299. https://doi.org/10.3390/foods13142299
APA StyleĆurko, N., Perić, K., Vukušić Pavičić, T., Balbino, S., Tomašević, M., Iveković, D., Radojčić Redovniković, I., & Kovačević Ganić, K. (2024). Effect of Pulsed Electric Field Pretreatment on the Concentration of Lipophilic and Hydrophilic Compounds in Cold-Pressed Grape Seed Oil Produced from Wine Waste. Foods, 13(14), 2299. https://doi.org/10.3390/foods13142299








