Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DHA Algal Oil Pickering Emulsion
2.3. Characterization of DHA Algal oil Pickering Emulsion
2.3.1. ζ-potential and Particle Size Distribution
2.3.2. Confocal Laser Scanning Microscopy (CLSM)
2.3.3. Characterization of Rheological Properties
2.4. Stability of DHA Algal Oil Pickering Emulsions
2.4.1. Salt Stability
2.4.2. Thermal Stability
2.4.3. Storage Stability
2.5. Oxidative Stability of Emulsions
2.5.1. Determination of DHA Content
2.5.2. Determination of Hydrogen Peroxide Content
2.5.3. Determination of Malondialdehyde (MDA) Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of DHA Algal Oil Pickering Emulsion
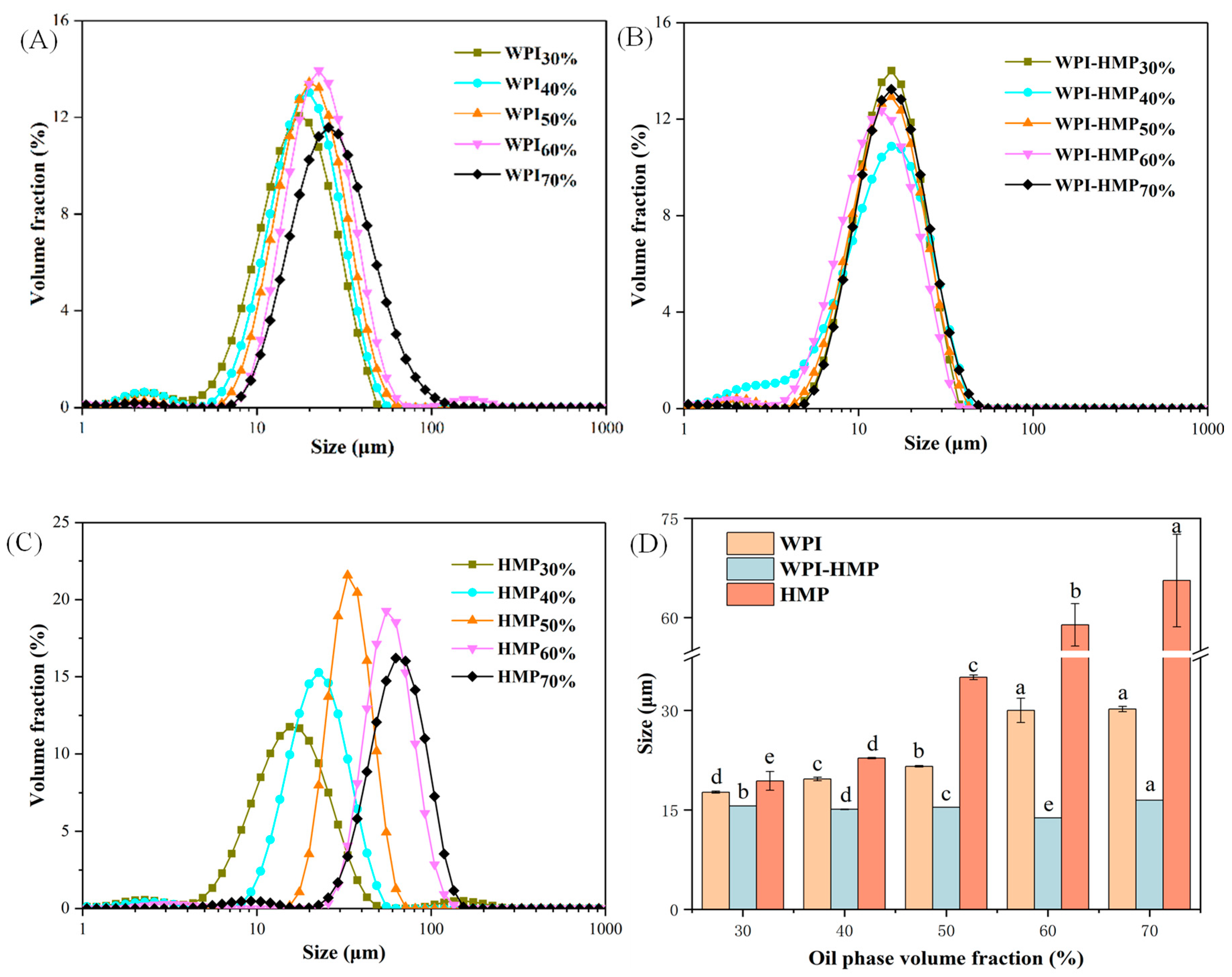
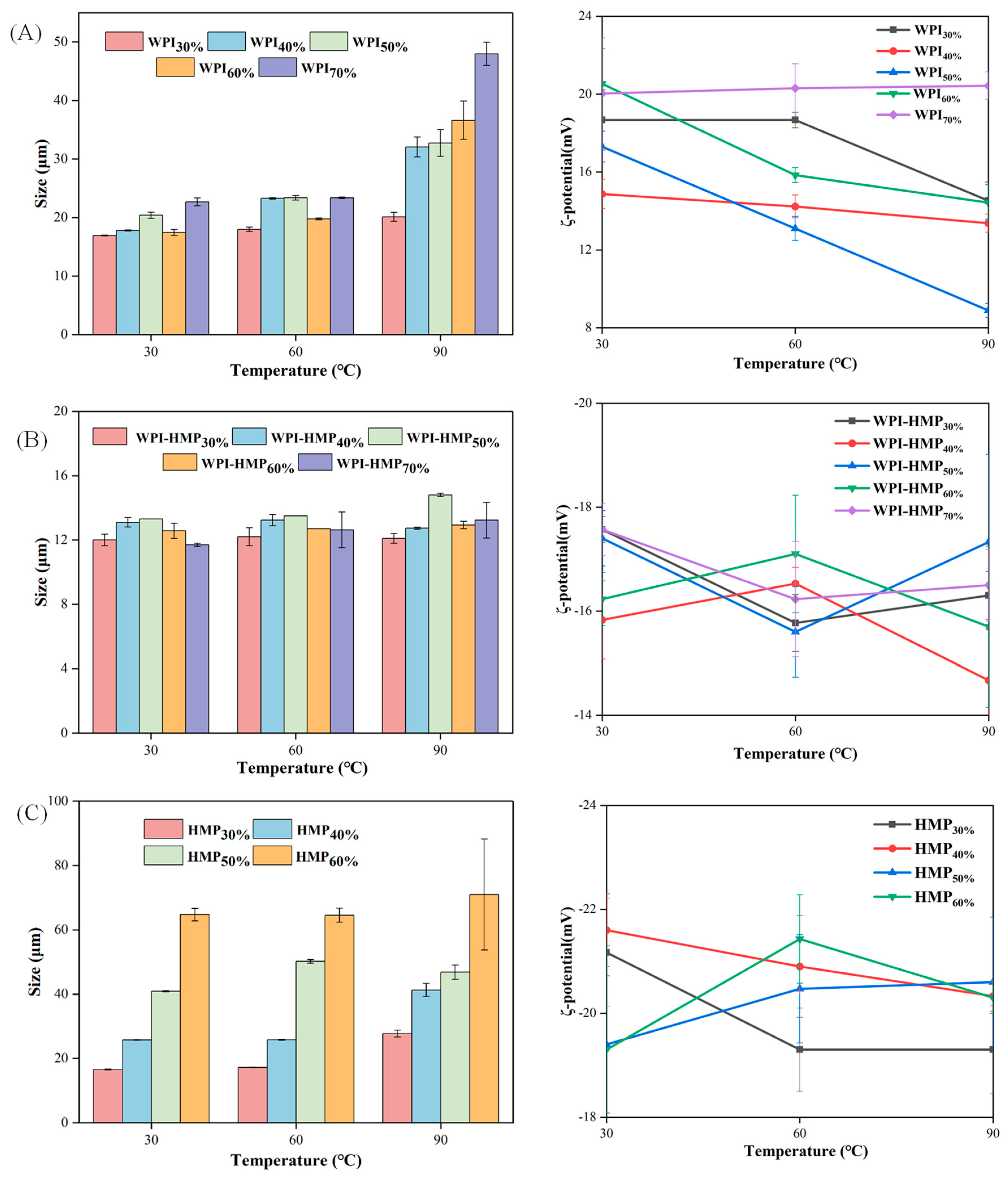
3.1.1. Particle Size Distribution and Average Particle Size
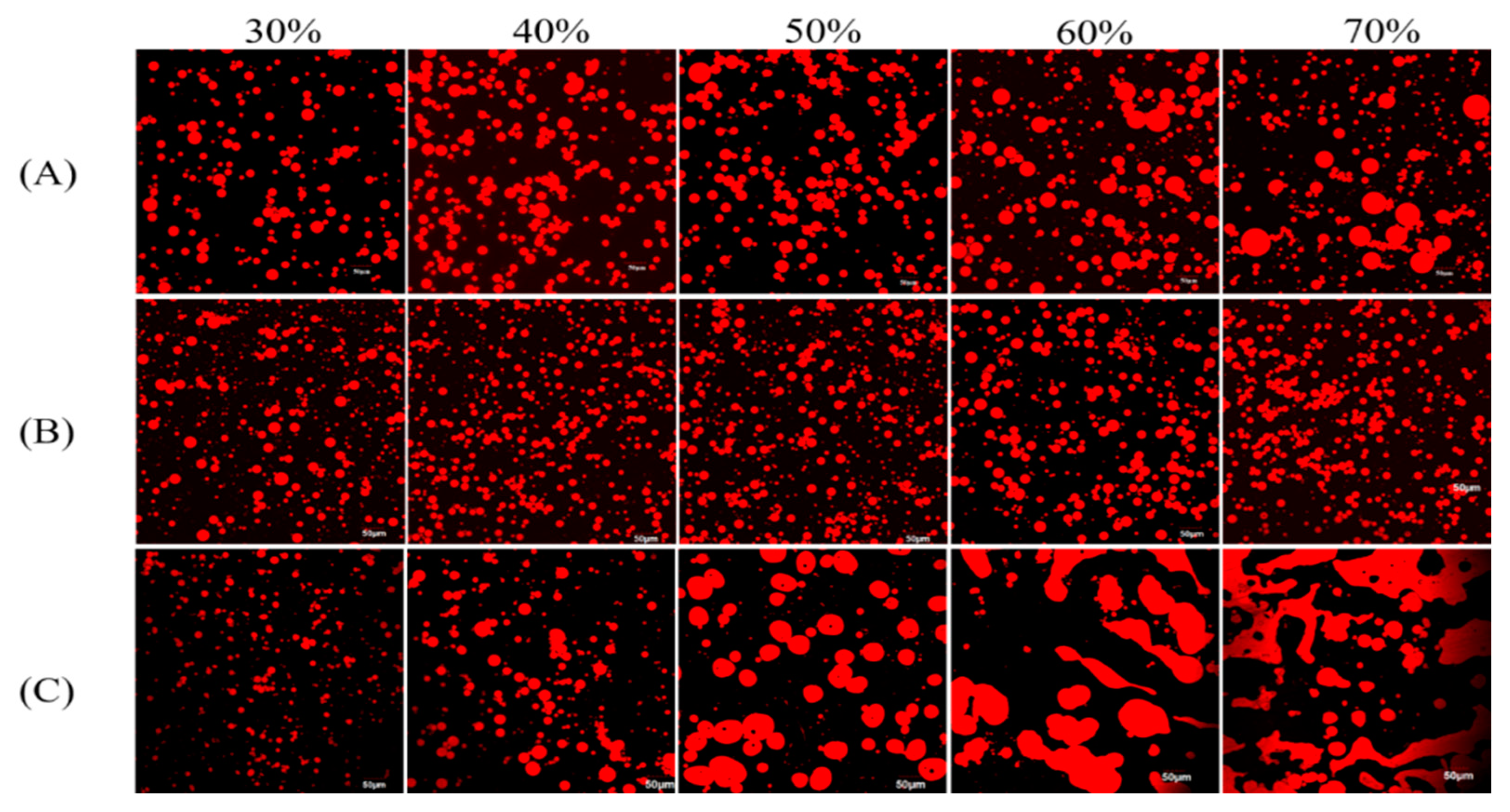
3.1.2. Microstructure of the Various DHA Algal Oil Pickering Emulsions
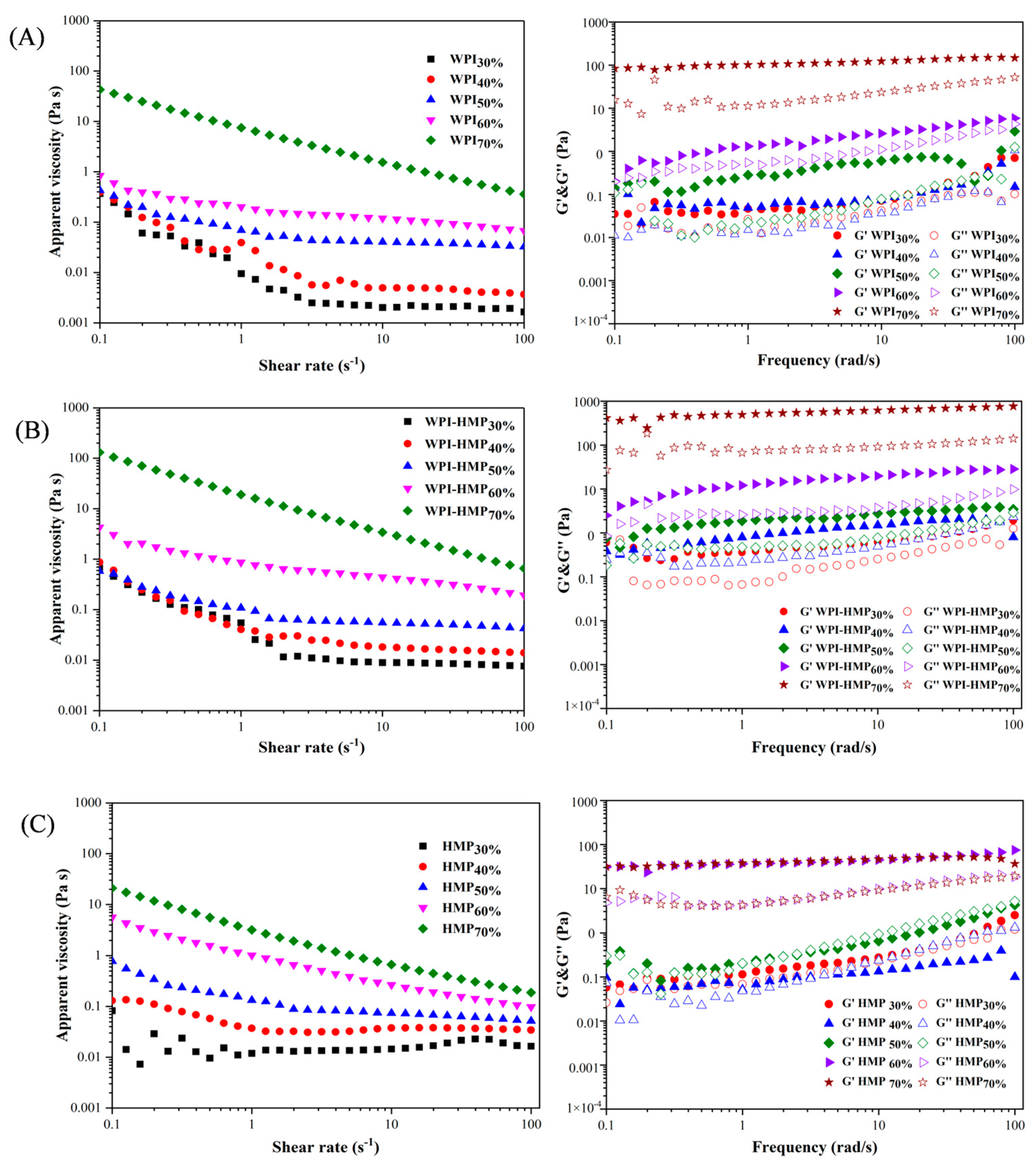
3.1.3. Analysis of Rheological Behavior
3.2. Stability of Different DHA Algal Oil Pickering Emulsions
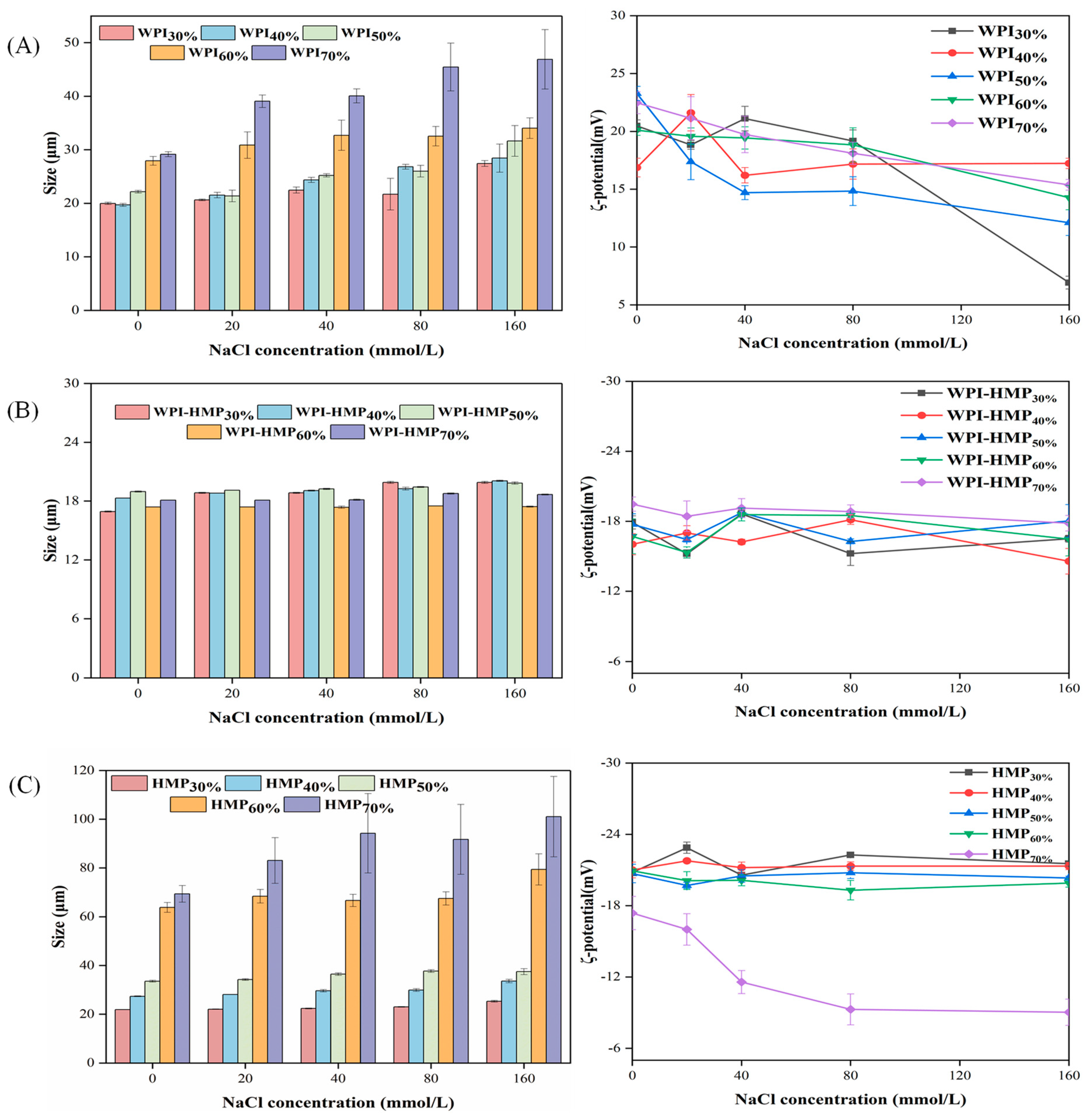
3.2.1. Salt Stability
3.2.2. Thermal Stability
3.3. Storage Stability
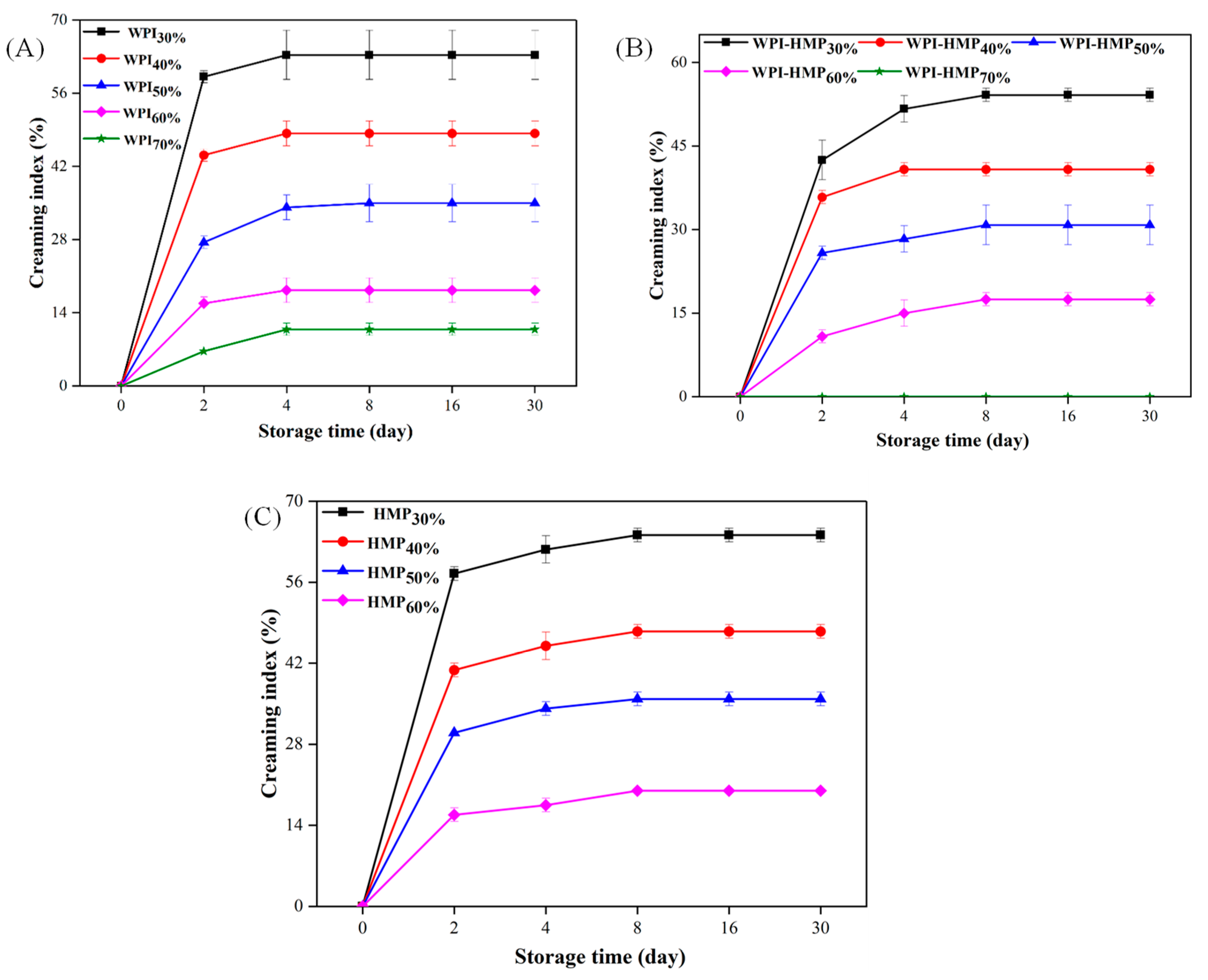
3.3.1. Creaming Index (CI)
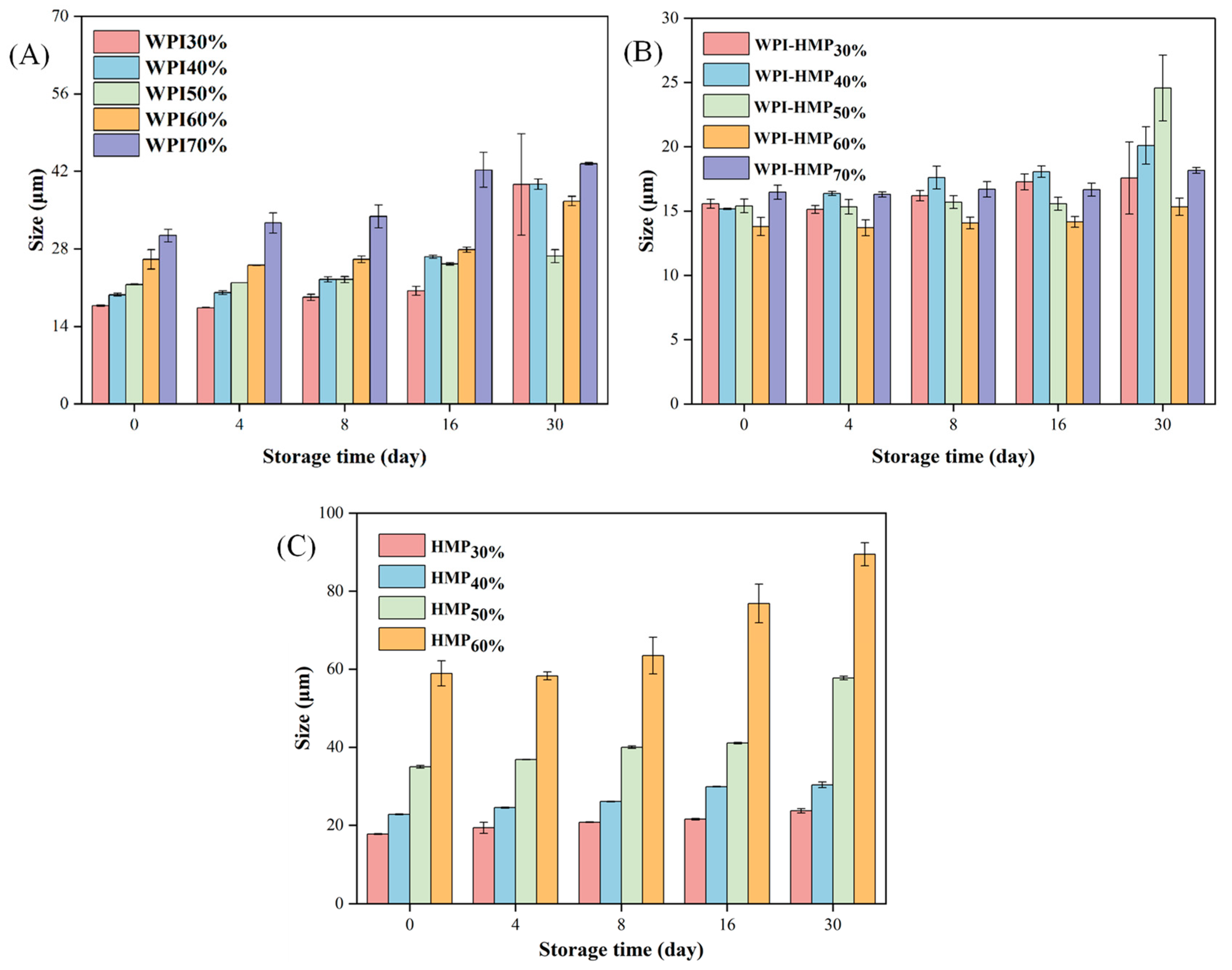
3.3.2. Particle Size Analysis
3.4. Oxidation Stability of Various DHA Algal Oil Pickering Emulsions
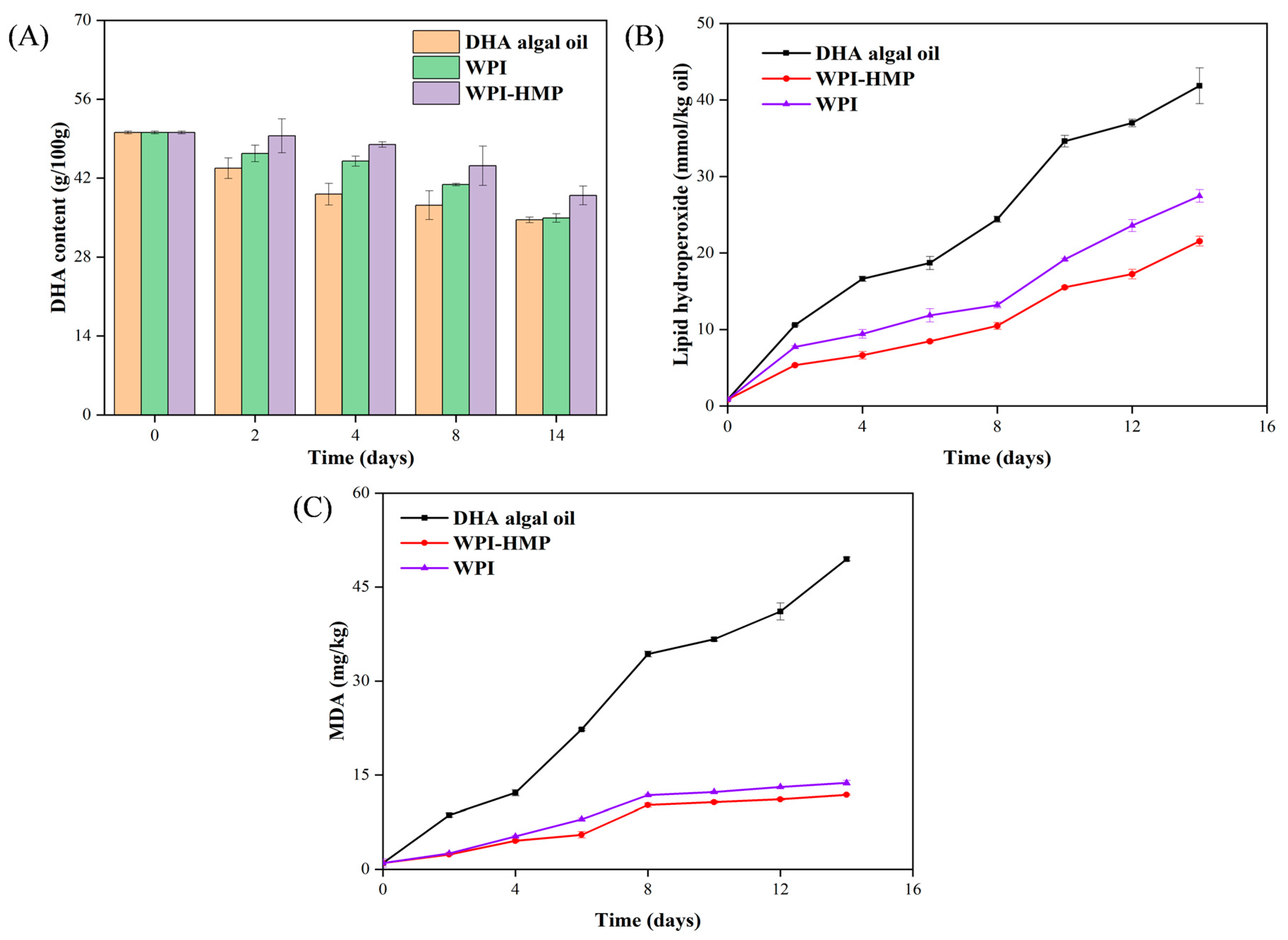
3.4.1. Changes in DHA Content during Accelerated Oxidation
3.4.2. Changes in Hydrogen Peroxide Levels during Accelerated Oxidation
3.4.3. Changes in MDA Content during Accelerated Oxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Y.; Wei, L.; Zhang, H.; Zhang, J.; Zhou, X.; Zhu, S.; Du, Y.; Su, R.; Fang, C.; et al. DHA supplementation and pregnancy complications. J. Int. Transl. Med. 2023, 21, 394. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Katapodis, P.; Sharma, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprospecting of thraustochytrids for omega-3 fatty acids: A sustainable approach to reduce dependency on animal sources. Trends Food Sci. Technol. 2021, 115, 433–444. [Google Scholar] [CrossRef]
- Lv, W.; Xu, D. Docosahexaenoic acid delivery systems, bioavailability, functionality, and Applications: A review. Foods 2022, 11, 2685. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Xie, F.; Zhang, S.; Sun, Y.; Qi, B.; Li, Y. Preparation and digestive characteristics of a novel soybean lipophilic protein-hydroxypropyl methylcellulose-calcium chloride thermosensitive emulsion gel. Food Hydrocoll. 2020, 106, 105891. [Google Scholar] [CrossRef]
- Torlopov, M.; Vaseneva, I.; Mikhaylov, V.; Martakov, I.; Legki, P.; Paderin, N.; Sitnikov, P. Surface, rheopexy, digestive stability and toxicity of olive oil emulsions stabilized by chitin nanocrystals for vitamin d3 delivery. Carbohyd. Polym. 2022, 284, 119162. [Google Scholar] [CrossRef]
- Lin, Y.; Du, H.; Roos, Y.; Miao, S. Transglutaminase crosslinked fish gelatins for emulsion stabilization: From conventional to Pickering emulsions. Food Hydrocoll. 2023, 144, 108979. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Ahn, H.; Lee, J.; Shin, C.; Jang, W.; Kim, B.; Ahn, H. The discrete single-entity electrochemistry of Pickering emulsions. Nanoscale 2022, 14, 6981–6989. [Google Scholar] [CrossRef]
- Zhao, P.; Ji, Y.; Yang, H.; Meng, X.; Liu, B. Soy protein isolate–Chitosan nanoparticle-Stabilized Pickering Emulsions: Stability and in vitro digestion for DHA. Mar. Drugs 2023, 21, 546. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, X.; Zhao, P.; He, Y.; Meng, X.; Liu, B. The effect of octenyl succinic anhydride-modified chitosan coating on DHA-loaded nanoemulsions: Physichemical stability and in vitro digestibility. Food Chem. 2024, 441, 138289. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, Z. Food-grade systems for delivery of DHA and EPA: Opportunities, fabrication, characterization and future perspectives. Crit. Rev. Food Sci. 2021, 63, 2348–2365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Y.; Wei, Z.; Xue, C. Ovalbumin fibril-stabilized oleogel-based Pickering emulsions improve astaxanthin bioaccessibility. Food Res. Int. 2022, 161, 111790. [Google Scholar] [CrossRef] [PubMed]
- do Prado Silva, J.T.; Benetti, J.V.M.; Alexandrino, T.T.d.B.; Assis, O.B.G.; de Ruiter, J.; Schroën, K.; Nicoletti, V.R. Whey protein isolate microgel properties tuned by crosslinking with organic acids to achieve stabilization of Pickering emulsions. Foods 2021, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, B.-K.; Lee, M.H. Effect of small molecular surfactants on physical, turbidimetric, and rheological properties of Pickering nanoemulsions stabilized with whey protein isolate. Food Biosci. 2023, 51, 102214. [Google Scholar] [CrossRef]
- Gu, X.; Li, W.; Jiang, X.; Chang, C.; Wu, J. Pectin-coated whey protein isolate/zein self-aggregated nanoparticles as curcumin delivery vehicles: Effects of heating, pH, and adding sequence. Int. J. Biol. Macromol. 2024, 258, 128892. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, Y.; Zhou, C.; Liu, L.; Wang, L.; Cao, J.; Sun, Y.; He, J.; Pan, D.; Cai, Z.; et al. Tunability of Pickering particle features of whey protein isolate via remodeling partial unfolding during ultrasonication-assisted complexation with chitosan/chitooligosaccharide. Carbohyd. Polym. 2024, 325, 121583. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-N.; Zhou, F.-Z.; Yang, T.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Fabrication and characterization of Pickering High Internal Phase Emulsions (HIPEs) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocoll. 2019, 93, 34–45. [Google Scholar] [CrossRef]
- Ye, Z.; Cheng, L.; Zhou, L.; Hong, K.Q.; He, D.P.; Lei, F.F. Preparation and properties of whey protein-pectin complex. Grain Oil 2024, 5, 113–119. (In Chinese) [Google Scholar]
- Wang, L.; Hu, Y.; Yin, S.; Yang, X.; Lai, F.; Wang, S. Fabrication and characterization of antioxidant Pickering emulsions stabilized by zein/Chitosan complex particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef]
- Shi, M.; Wang, F.; Jiang, H.; Qian, W.; Xie, Y.; Wei, X.; Zhou, T. Effect of enzymatic degraded polysaccharides from Enteromorpha prolifera on the physical and oxidative stability of fish oil-in-water emulsions. Food Chem. 2020, 322, 126774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, C.; Liu, M.; Yang, H.; Zhang, F.; Liu, B.; Meng, X. The formation, stability of DHA/EPA nanoemulsion prepared by emulsion phase inversion method and its application in apple juice. Food Res. Int. 2020, 133, 109132. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Characterizations of novel konjac glucomannan emulsion films incorporated with high internal phase Pickering emulsions. Food Hydrocoll. 2020, 109, 106088. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering emulsions stabilized by ovotransferrin–gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.; Liu, B. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohyd. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Xu, D.; Liu, G.; Xiao, J.; Cao, Y.; Sun, B. Influence of unabsorbed emulsifiers on the rheological properties and structure of heteroaggregate of whey protein isolate (WPI) coated droplets and flaxseed gum (FG) coated droplets. Food Hydrocoll. 2018, 80, 42–52. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of Pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; He, Q.; Fan, Y. Protection of menhaden oil from oxidation in Pickering emulsion-based delivery systems with α-lactalbumin-chitosan colloidal nanoparticle. Food Funct. 2021, 12, 11366–11377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 106211. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Wang, Y.; Zhang, Y.; Luo, Y. Low density lipoprotein-pectin complexes stabilized high internal phase Pickering emulsions: The effects of pH conditions and mass ratios. Food Hydrocoll. 2023, 134, 108004. [Google Scholar] [CrossRef]
- Liang, W.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zou, J.; Wang, M.; Huang, R.; Qian, Y.; Zeng, M.; Li, F. A new strategy for maintaining the thermal stability of phycocyanin under acidic conditions: PH-induced whey protein isolate-phycocyanin coprecipitation forms composite with chitosan. Food Hydrocoll. 2024, 148, 109468. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Liu, P.; Hu, Z.; Qin, X.; Liu, G. A glycated whey protein isolate–epigallocatechin gallate nanocomplex enhances the stability of emulsion delivery of β-carotene during simulated digestion. Food Funct. 2019, 10, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, L.; Lu, K.; Qi, B.; Du, X.; Liu, G.; Tang, Y.; Zhang, S.; Li, Y. Whey protein isolate–phytosterols nanoparticles: Preparation, characterization, and stabilized food-grade Pickering emulsions. Food Chem. 2022, 384, 132486. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2020, 340, 127893. [Google Scholar] [CrossRef]
- Gayoso, L.; Ansorena, D.; Astiasarán, I. DHA rich algae oil delivered by O/W or gelled emulsions: Strategies to increase its bioaccessibility. J. Sci. Food Agric. 2019, 99, 2251–2258. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Li, M.; Guo, Y.; Chen, Y.; Qian, H.; Yao, W. Evaluation on the formation of lipid free radicals in the oxidation process of peanut oil. LWT 2019, 104, 24–29. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C.; Gao, X.; Zhao, X.; Wei, H.; Luo, J.; You, X.; Jiang, H.; Zhang, X.; Yuan, C. DHA-mediated milk protein treated by ultrasound-assisted pH-shifting for enhanced astaxanthin delivery and processed cheese application. J. Dairy Sci. 2024, 107, 4161–4173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Zhou, L.; Chen, Z.; Chen, L.; Hong, K.; He, D.; Lei, F. Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex. Foods 2024, 13, 2159. https://doi.org/10.3390/foods13132159
Yu Z, Zhou L, Chen Z, Chen L, Hong K, He D, Lei F. Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex. Foods. 2024; 13(13):2159. https://doi.org/10.3390/foods13132159
Chicago/Turabian StyleYu, Zhe, Li Zhou, Zhe Chen, Ling Chen, Kunqiang Hong, Dongping He, and Fenfen Lei. 2024. "Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex" Foods 13, no. 13: 2159. https://doi.org/10.3390/foods13132159
APA StyleYu, Z., Zhou, L., Chen, Z., Chen, L., Hong, K., He, D., & Lei, F. (2024). Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex. Foods, 13(13), 2159. https://doi.org/10.3390/foods13132159






