Functional Properties, Rheological Characteristics, Simulated Digestion, and Fermentation by Human Fecal Microbiota of Polysaccharide from Morchella importuna
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. MIP Preparation
2.3. Functional Properties
2.3.1. Water- and Oil-Holding Capacities
2.3.2. Emulsion Properties
2.3.3. Foaming Property
2.4. Rheological Behavior Measurements
2.4.1. Steady Shear Flow Behavior
2.4.2. Oscillatory Shear Measurements
2.5. In Vitro Simulation of Digestion Assay
2.5.1. In Vitro Simulated Saliva–Gastrointestinal Digestion of MIP
2.5.2. Molecular Weight Distribution of MIP and Its Digestion Products
2.6. In Vitro Fermentation Assay
2.6.1. In Vitro Fermentation of MIP
2.6.2. Analysis of In Vitro Fermented MIP Products
Determination of OD600, Total Carbohydrates, CR, pH, and Uronic Acid
Analysis of the Gut Microbiota
Determination of Volatile SCFAs
2.7. Data Analysis
3. Results and Discussion
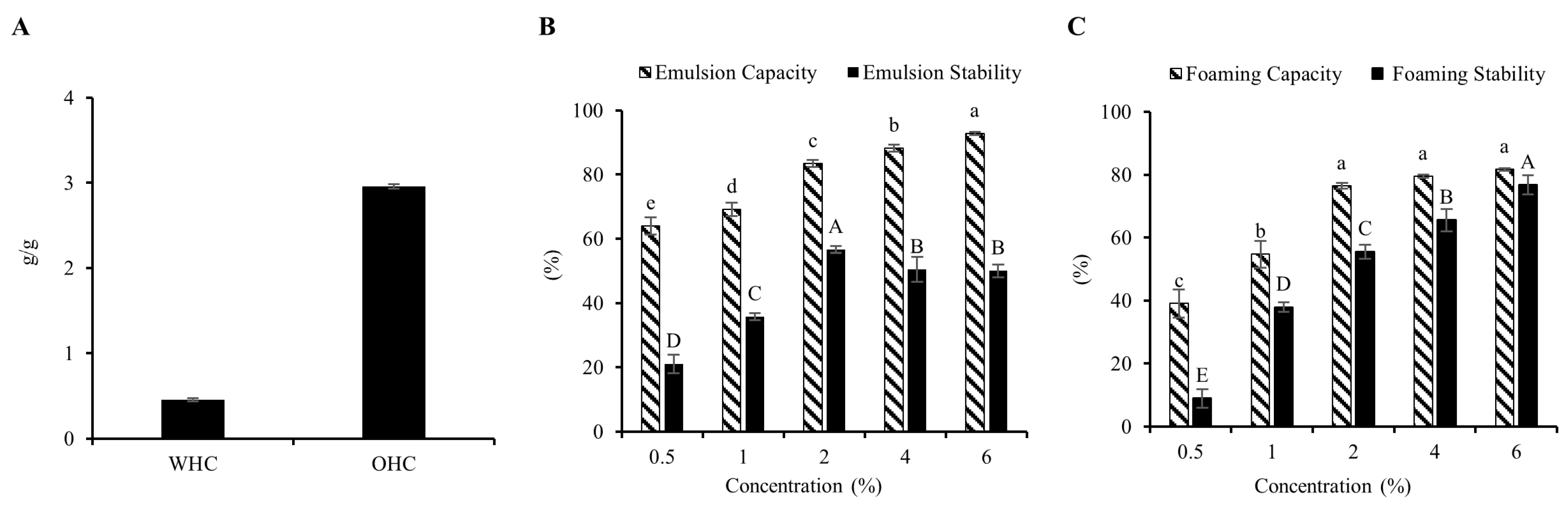
3.1. Functional Properties of MIP
3.1.1. WHC and OHC
3.1.2. Emulsion Properties
3.1.3. Foaming Properties
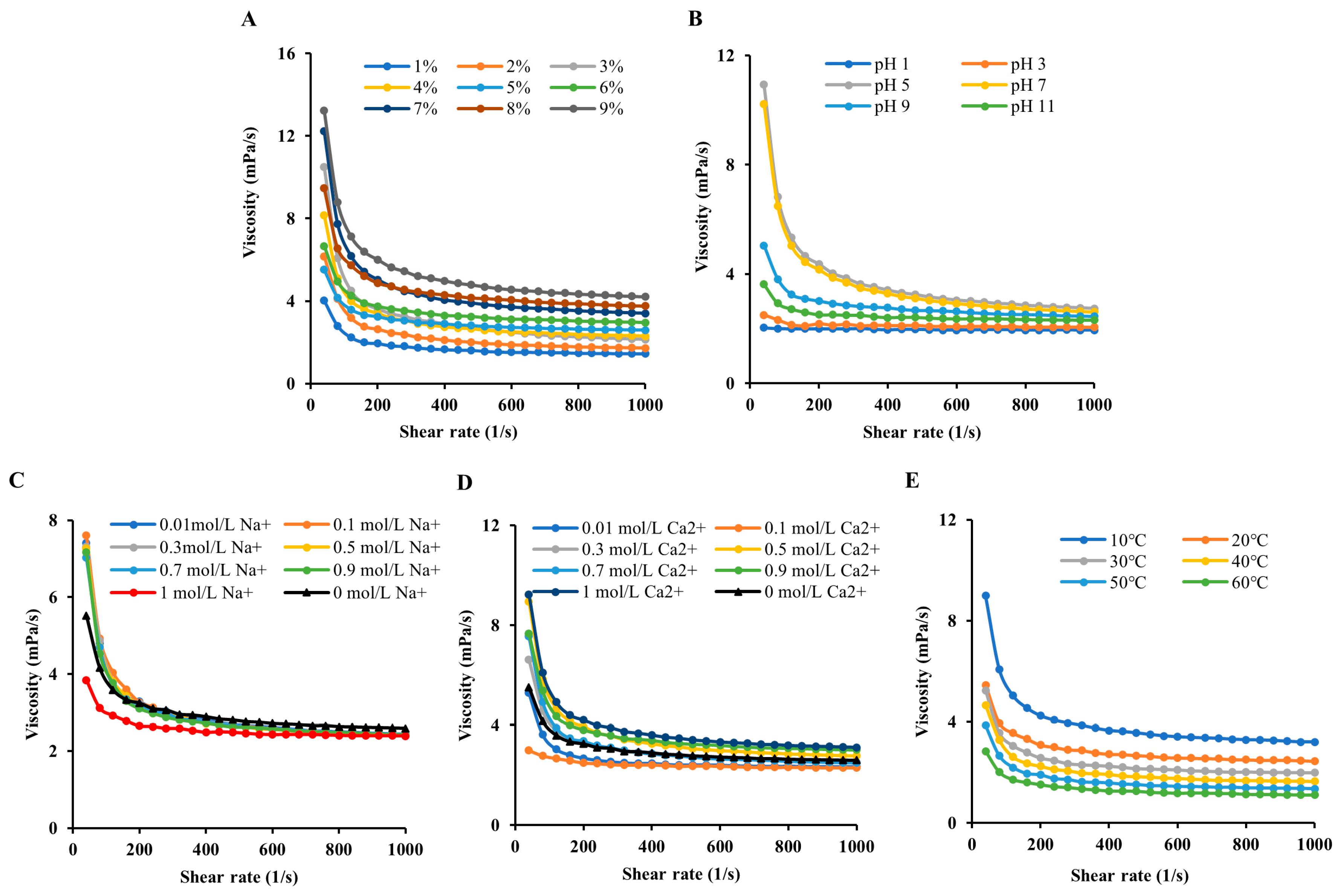
3.2. Steady Shear Flow Behaviors
3.2.1. Influence of the Concentration on the MIP AV
3.2.2. Influence of pH on the MIP AV
3.2.3. Influence of Metal Ions on the MIP AV
3.2.4. Influence of Temperature on the MIP AV
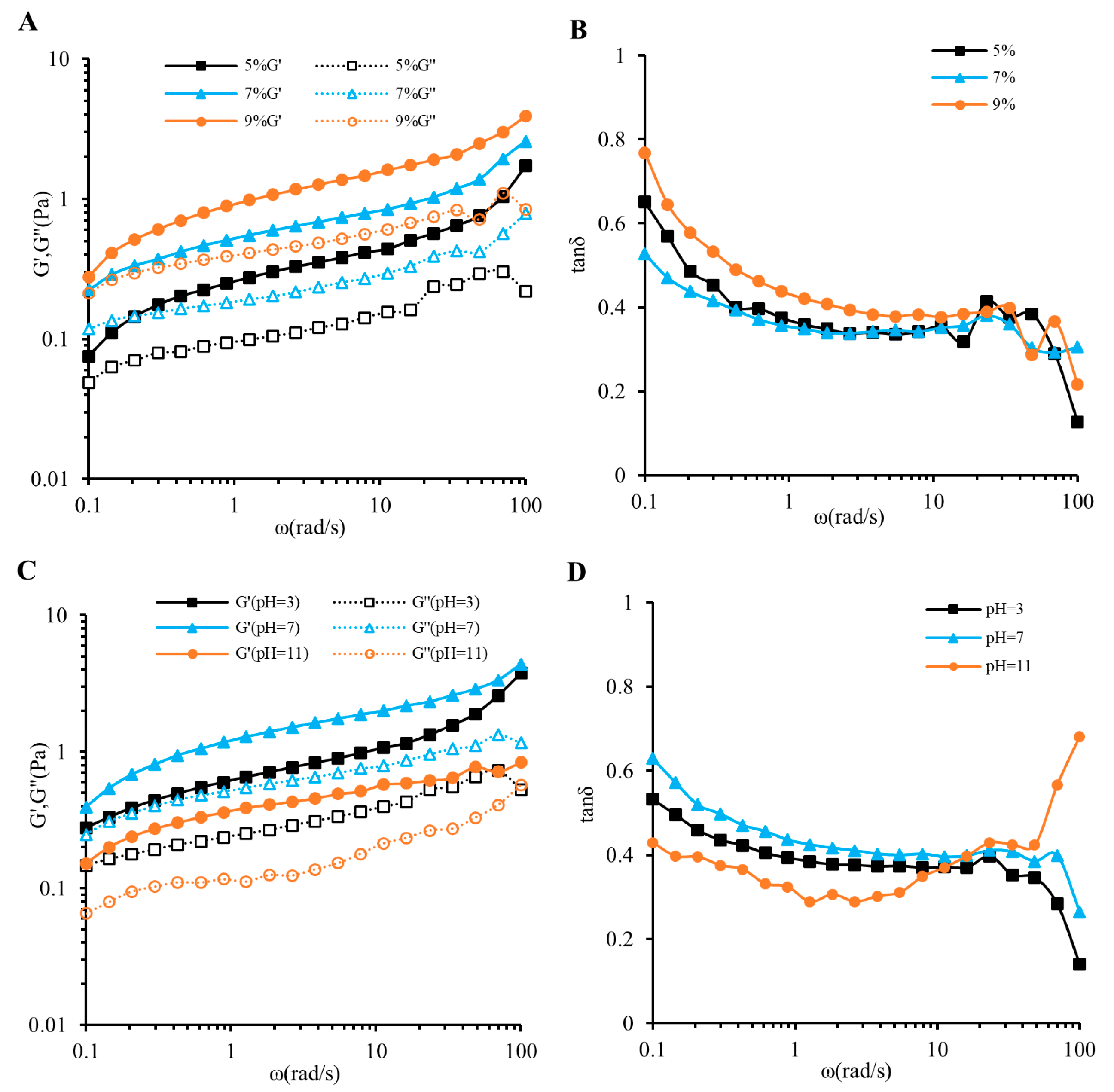
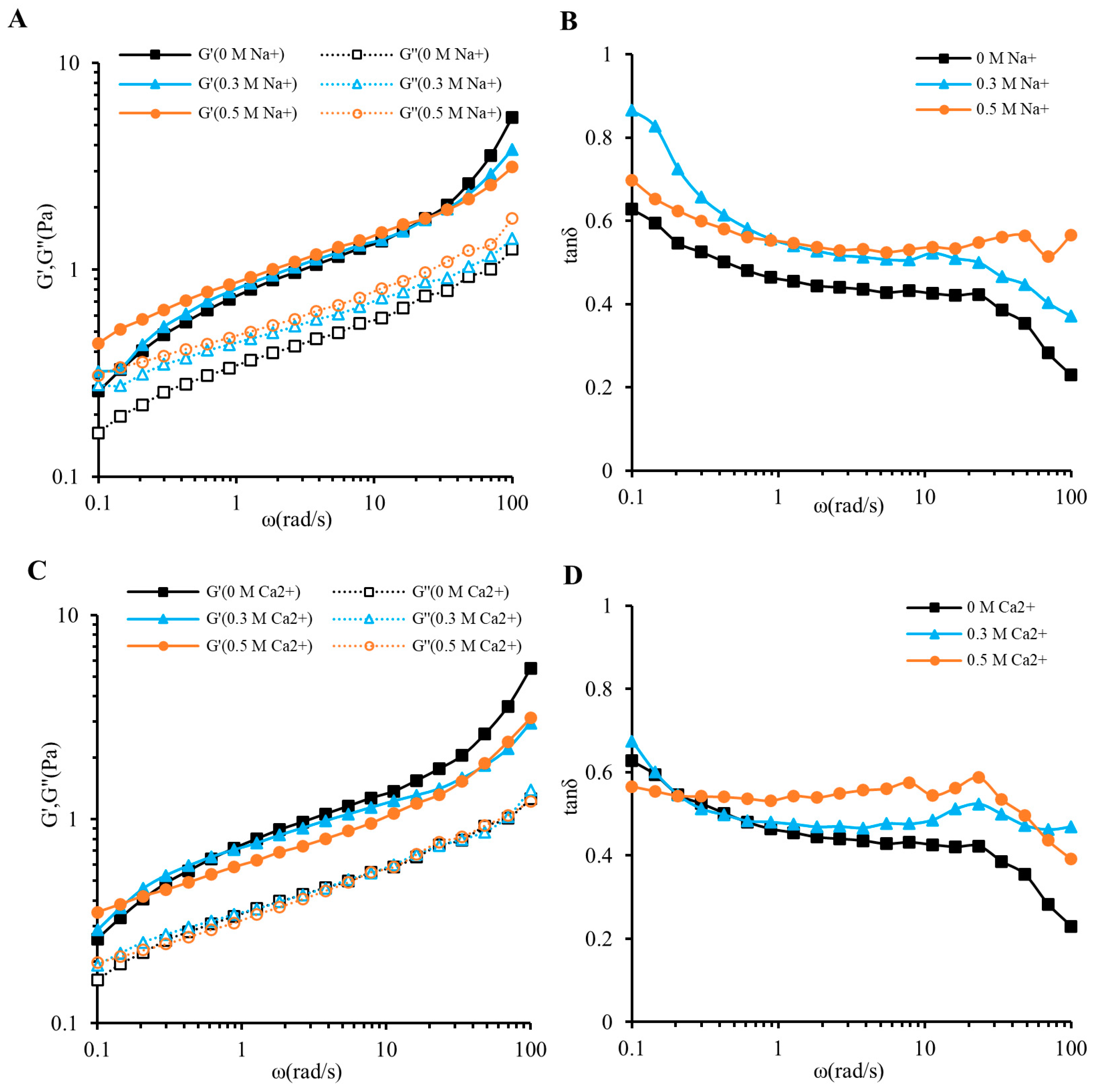
3.3. Oscillatory Shear Measurements
3.4. Analysis of the Molecular Weights of MIP and Its Digestion Products
3.5. In Vitro Fermentation of MIP by the Human Gut Microbiota
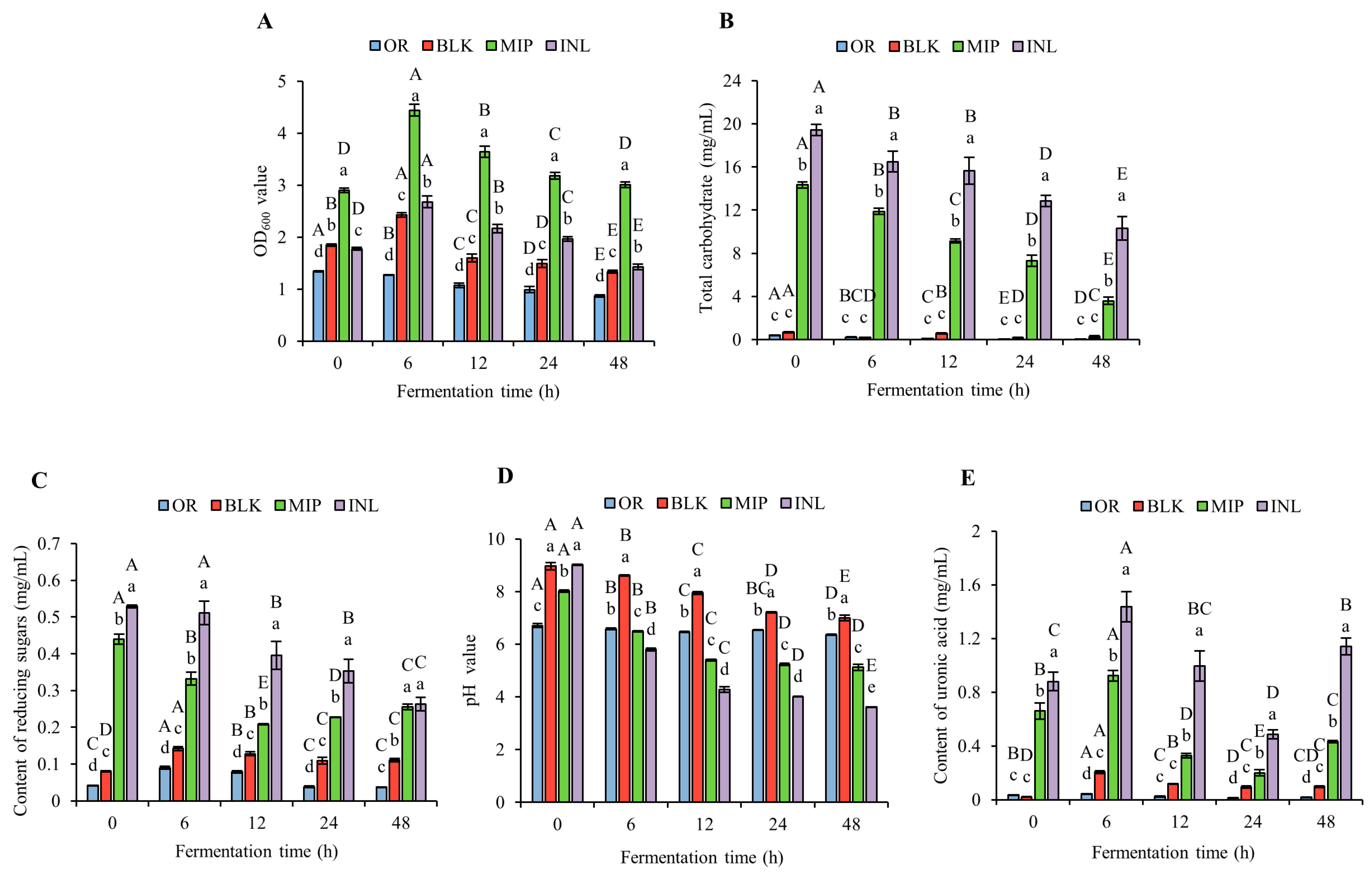
3.5.1. Variations in OD600, Total Carbohydrate, CR, pH, and Uronic Acid Contents after In Vitro Fermentation
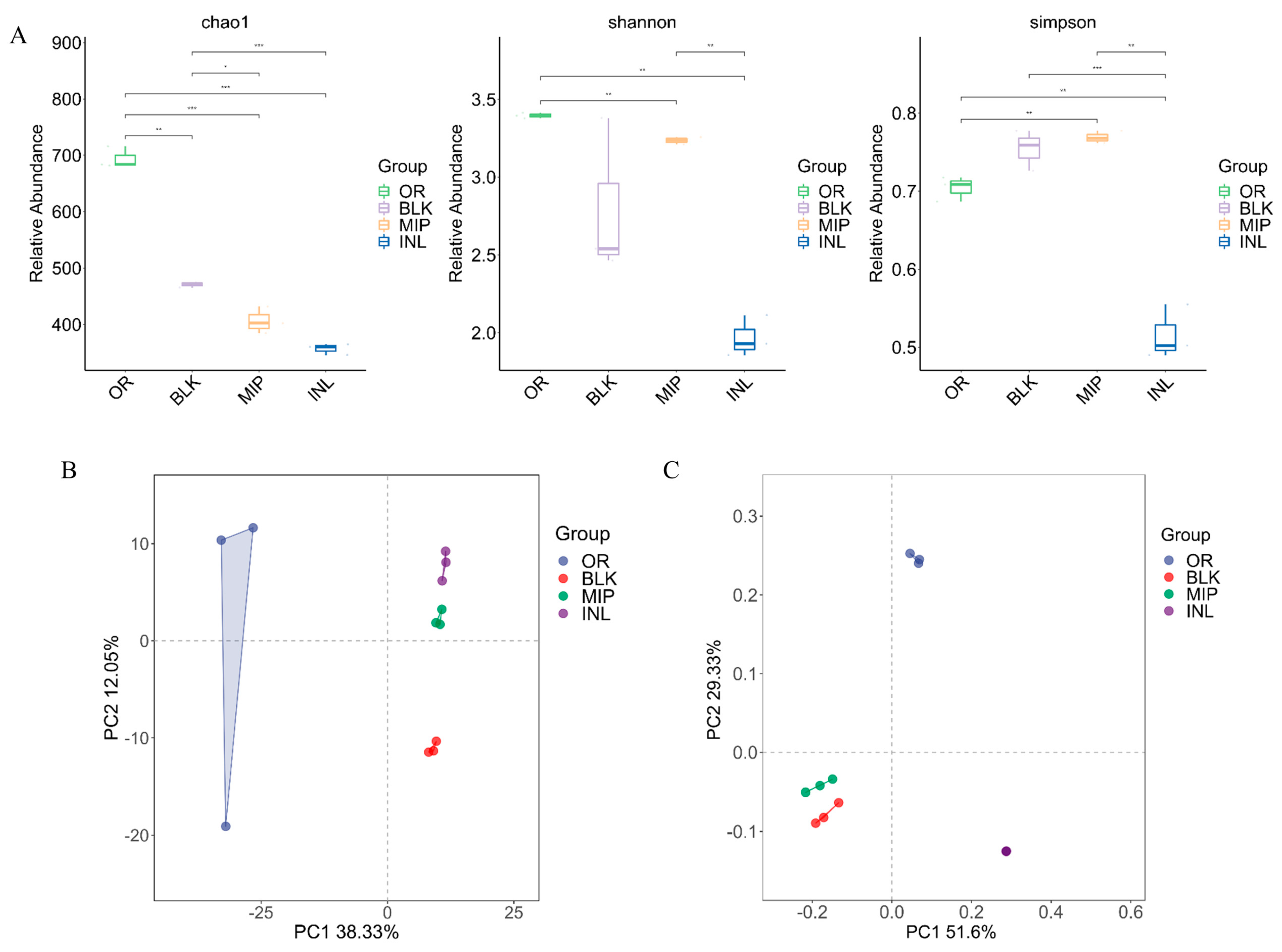
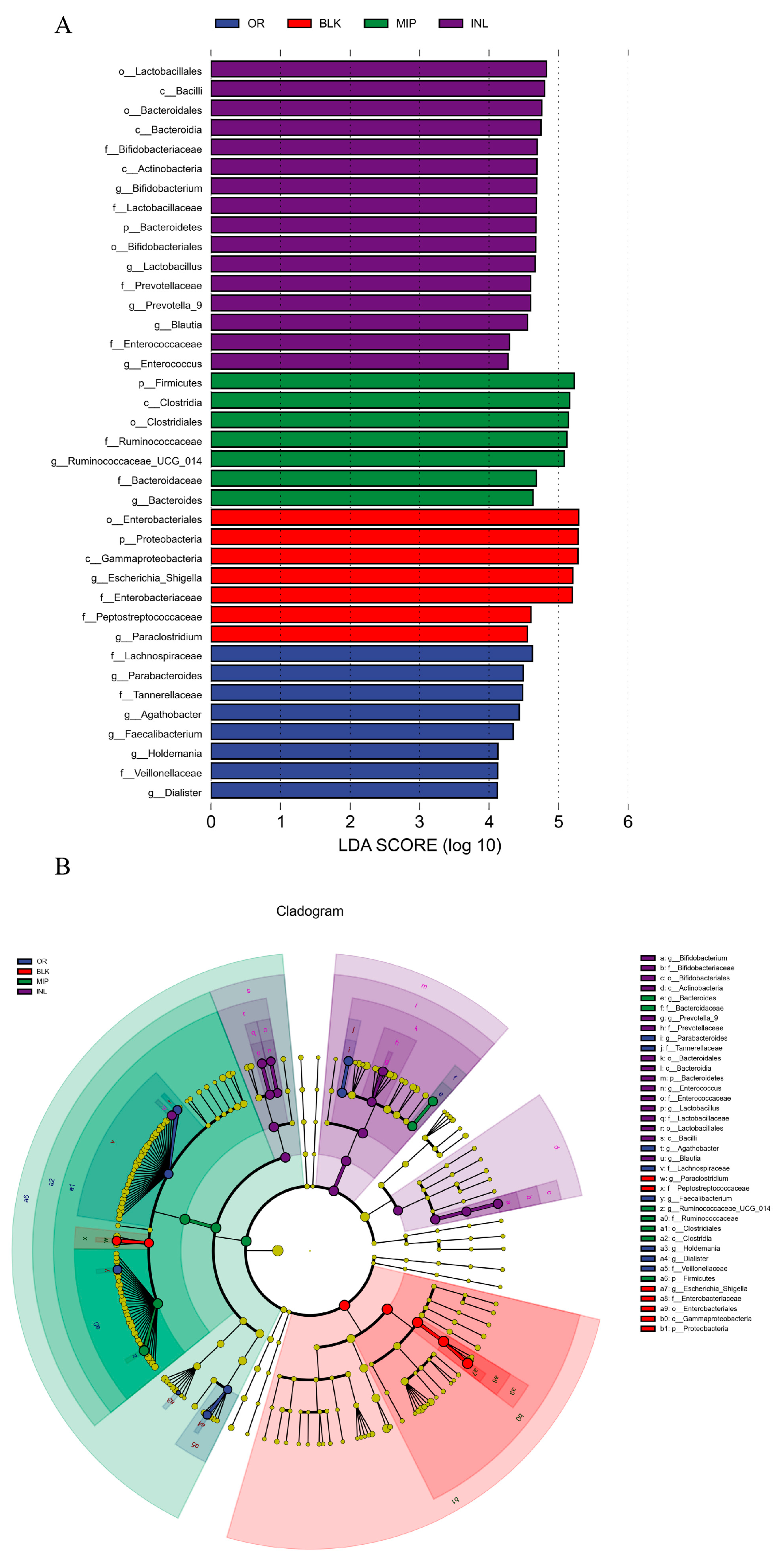
3.5.2. Influences of MIP on the Gut Microbiota
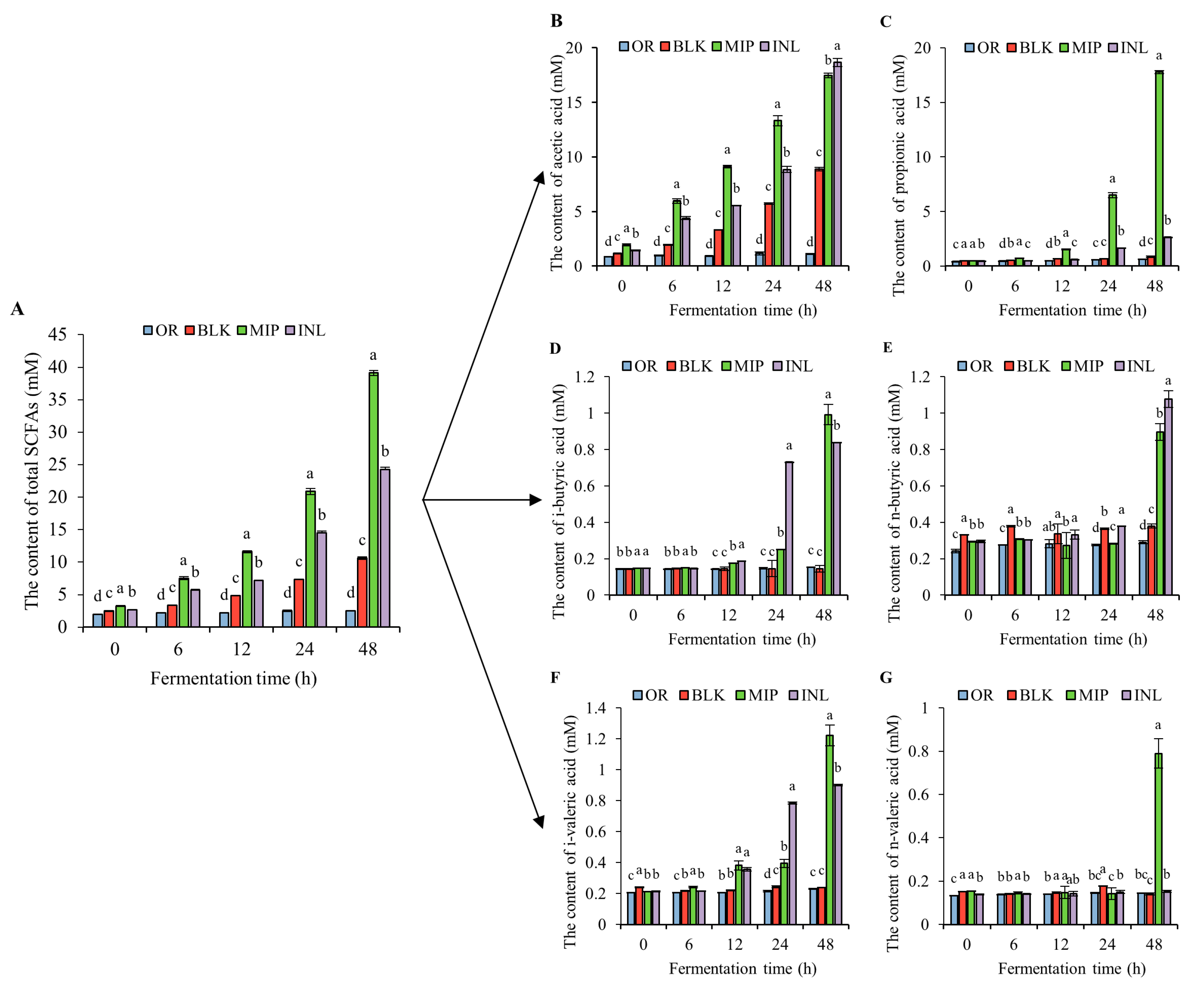
3.5.3. Changes in the SCFA Content in the Fermentation Broth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Li, Y.; Dai, X.; Wang, B.; Zhang, J.; Cao, H. Polysaccharides: The Potential Prebiotics for Metabolic Associated Fatty Liver Disease (MAFLD). Nutrients 2023, 15, 3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhang, L.; An, L.; Guo, L.; Huang, L.; Gao, W. Recent progress in plant-derived polysaccharides with prebiotic potential for intestinal health by targeting gut microbiota: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, W.; Jakobsen, L.M.A.; Zachariassen, L.F.; Hansen, A.K.; Rasmussen, M.K.; Bertram, H.C. Inulin Supplementation Modulates the Hepatic Transcriptome, Metabolome, and Ferritin Content in Ovariectomized Rats. Mol. Nutr. Food Res. 2023, 67, e2300372. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O. Acacia gum (Gum Arabic): A nutritional fibre; metabolism and calorific value. Food Addit. Contam. 1998, 15, 251–264. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tao, W.; Zhou, W.; Li, J.; Xing, J.; Luo, M.; Tan, Q. The short-term and long-term effects of Dendrobium officinale leaves polysaccharides on the gut microbiota differ. J. Funct. Foods 2023, 110, 105807. [Google Scholar] [CrossRef]
- Duncan, C.J.G.; Pugh, N.; Pasco, D.S.; Ross, S.A. Isolation of a Galactomannan That Enhances Macrophage Activation from the Edible Fungus Morchella esculenta. J. Agric. Food Chem. 2002, 50, 5683–5685. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wang, Y.; Meng, F.J.; Wang, X.F.; Lu, Y.M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Çayan, G.T.; Çayan, F.; Duru, M.E.; Ahmad, N.; Emwas, A.H.; Jaremko, M. Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Lei, L.; Li, F.; Zhang, Y.; Chen, J.; Zhao, G.; Wu, S.; Yin, R.; Ming, J. Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats. Carbohydr. Polym. 2017, 172, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma Lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M.; Maalej, H.; Slima, S.B.; Nasri, M.; Ben Amara, I.; Ben Salah, R. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Hu, T.; Huang, Q. Rheological properties and critical concentrations of a hyperbranched polysaccharide from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2022, 202, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Grom, L.C.; Coutinho, N.M.; Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Freitas, M.Q.; Duarte, M.C.K.H.; Pimentel, T.C.; Esmerino, E.A.; et al. Probiotic dairy foods and postprandial glycemia: A mini-review. Trends Food Sci. Technol. 2020, 101, 165–171. [Google Scholar] [CrossRef]
- Pan, X.; Xu, L.; Meng, J.; Chang, M.; Cheng, Y.; Geng, X.; Guo, D.; Liu, R. Ultrasound-Assisted Deep Eutectic Solvents Extraction of Polysaccharides from Morchella importuna: Optimization, Physicochemical Properties, and Bioactivities. Front. Nutr. 2022, 9, 912014. [Google Scholar] [CrossRef]
- Xiong, G.; Ma, L.; Zhang, H.; Li, Y.; Zou, W.; Wang, X.; Xu, Q.; Xiong, J.; Hu, Y.; Wang, X. Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022, 194, 484–498. [Google Scholar] [CrossRef]
- Liu, C.Y.; Sun, Y.Y.; Jia, Y.Q.; Geng, X.Q.; Pan, L.; Jiang, W.; Xie, B.Y.; Zhu, Z.Y. Effect of steam explosion pretreatment on the structure and bioactivity of Ampelopsis grossedentata polysaccharides. Int. J. Biol. Macromol. 2021, 185, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Meng, J.; Xu, L.; Chang, M.; Feng, C.; Geng, X.; Cheng, Y.; Guo, D.; Liu, R.; Wang, Z.; et al. In-depth investigation of the hypoglycemic mechanism of Morchella importuna polysaccharide via metabonomics combined with 16S rRNA sequencing. Int. J. Biol. Macromol. 2022, 220, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-T2DM by modulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, D.; Gao, L.; Ouyang, Y.; Wen, Y.; Ai, C.; Chen, Y.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, M.; Zhang, Y.; Wang, Z.; Yuan, B.; Zhao, C.; Wang, M. Integrated microbiota and metabolite profiling analysis of prebiotic characteristics of Phellinus linteus polysaccharide in vitro fermentation. Int. J. Biol. Macromol. 2023, 242, 124854. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Models for intestinal fermentation: Association between food components, delivery systems, bioavailability and functional interactions in the gut. Curr. Opin. Biotechnol. 2007, 18, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, X.; Li, D.; Wang, Z.; Tan, L.; Chang, M.; Feng, C.; Cheng, Y.; Geng, X.; Meng, J. Structural characterization, rheological characterization, hypoglycemic and hypolipidemic activities of polysaccharides from Morchella importuna using acidic and alkaline deep eutectic solvents. LWT 2024, 193, 115742. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Xu, J.; Zou, Y.; Guo, L.; Lin, J.; Jiang, Z.; Zheng, Q. Rheological and microstructural properties of polysaccharide obtained from the gelatinous Tremella fuciformis fungus. Int. J. Biol. Macromol. 2023, 228, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, Y.; Hu, Y.; Zhou, T.; Li, J.; He, L.; Zhang, W.; Zeng, X.; Fan, J. Comparison of chemical property and in vitro digestion behavior of polysaccharides from Auricularia polytricha mycelium and fruit body. Food Chem. X 2023, 17, 100570. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Guo, D.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y.; Feng, C.; Meng, J.; Chang, M. Effects of in vitro digestion and fecal fermentation on physico-chemical properties and metabolic behavior of polysaccharides from Clitocybe squamulosa. Food Chem. X 2023, 18, 100644. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lei, J.; He, C.; Peng, Z.; Liu, R.; Pan, X.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; et al. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. Int. J. Biol. Macromol. 2022, 208, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lin, Z.; Tang, H.; Geng, J.; Hu, Y.; Mayo, K.H.; Tai, G.; Zhou, Y. The model polysaccharide potato galactan is actually a mixture of different polysaccharides. Carbohydr. Polym. 2023, 313, 120889. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 2002, 31, 426–428. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhang, X.; Li, F.; Zhao, H. Effects of different drying methods on the physicochemical and antioxidative characteristics of Osmunda japonica Thunb. polysaccharides. J. Food Process. Preserv. 2020, 44, e14742. [Google Scholar] [CrossRef]
- Ai, J.; Yang, Z.; Liu, J.; Schols, H.A.; Battino, M.; Bao, B.; Tian, L.; Bai, W. Structural Characterization and In Vitro Fermentation Characteristics of Enzymatically Extracted Black Mulberry Polysaccharides. J. Agric. Food Chem. 2022, 70, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated Digestion and Fermentation in Vitro by Human Gut Microbiota of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef]
- Ghanavati, P.A.Z.; Khodadadi, M.; Tadayoni, M. Structural characterization and bioactive and functional properties of the Brown macroalgae (Sargassum illicifolium) polysaccharide. J. Food Meas. Charact. 2022, 16, 1437–1447. [Google Scholar] [CrossRef]
- Mokni Ghribi, A.; Sila, A.; Maklouf Gafsi, I.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015, 75, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–150. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Peng, D.; Shang, W.; Yang, J.; Li, K.; Shen, W.; Wan, C.; Geng, F.; Deng, Q.; Jin, W. Interfacial arrangement of tunable gliadin nanoparticles via electrostatic assembly with pectin: Enhancement of foaming property. Food Hydrocoll. 2023, 143, 108852. [Google Scholar] [CrossRef]
- Li, L.; Liao, B.Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 109, 761–771. [Google Scholar] [CrossRef]
- Lai, L.S.; Tung, J.; Lin, P.S. Solution properties of hsian-tsao (Mesona procumbens Hemsl) leaf gum. Food Hydrocoll. 2000, 14, 287–294. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Hu, F.; Wei, Z.J. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.X.; Shi, J.J.; Zhang, J.G.; Li, L.; Jiang, L.; Wei, Z.J. Thermal, emulsifying and rheological properties of polysaccharides sequentially extracted from Vaccinium bracteatum Thunb leaves. Int. J. Biol. Macromol. 2016, 93, 1240–1252. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Ma, Y.L.; Thakur, K.; Wang, C.H.; Wang, H.; Ren, Y.F.; Zhang, J.G.; Wei, Z.J. Effects of extraction methods on the rheological properties of polysaccharides from onion (Allium cepa L.). Int. J. Biol. Macromol. 2018, 112, 22–32. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, R.; Yin, J.Y.; Wang, Y.X.; Ma, L.Y.; Nie, S.P.; Xiong, T.; Xie, M.Y. Physicochemical, structural and rheological properties of alkali-extracted polysaccharide from fruiting body of Hericium erinaceus. LWT 2019, 115, 108330. [Google Scholar] [CrossRef]
- Cai, W.; Hu, T.; Huang, Q. A polysaccharide from Lignosus rhinocerotis sclerotia: Self-healing properties and the effect of temperature on its rheological behavior. Carbohydr. Polym. 2021, 267, 118223. [Google Scholar] [CrossRef] [PubMed]
- Wee, M.S.M.; Sims, I.M.; Goh, K.K.T.; Matia-Merino, L. Molecular, rheological and physicochemical characterisation of puka gum, an arabinogalactan-protein extracted from the Meryta sinclairii tree. Carbohydr. Polym. 2019, 220, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qin, L.; Chen, T.; Yu, Q.; Chen, Y.; Xiao, W.; Ji, X.; Xie, J. Modification of starch by polysaccharides in pasting, rheology, texture and in vitro digestion: A review. Int. J. Biol. Macromol. 2022, 207, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Norziah, M.H.; Kong, S.S.; Abd Karim, A.; Seow, C.C. Pectin–sucrose–Ca2+ interactions: Effects on rheological properties. Food Hydrocoll. 2001, 15, 491–498. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part III—Steady and dynamic shear rheology. Food Hydrocoll. 2017, 67, 243–250. [Google Scholar] [CrossRef]
- Han, M.; Du, C.; Xu, Z.Y.; Qian, H.; Zhang, W.G. Rheological properties of phosphorylated exopolysaccharide produced by Sporidiobolus pararoseus JD-2. Int. J. Biol. Macromol. 2016, 88, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Ghanbarzadeh, B.; Karimi, S.; Ebrahimi, B.; Hosseini, M. Gelling and rheological properties of a polysaccharide extracted from Ocimum album L. seed. Int. J. Biol. Macromol. 2023, 246, 125603. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Y.; Zhang, R.; Bai, Y.; Dong, L.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. Int. J. Biol. Macromol. 2019, 140, 965–972. [Google Scholar] [CrossRef]
- Tao, W.D.; Rui, N.X.; You, G.R.; Huan, G.; Yuan, F.; Qin, Y.; Qing, Z.; Wen, Q. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2020, 114, 106577. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.; Zeng, M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Res. Int. 2021, 149, 110646. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Ray, P.; Aich, P. A comparative analysis of gut microbial dysbiosis by select antibiotics and DSS to understand the effects of perturbation on the host immunity and metabolism. Life Sci. 2023, 312, 121212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Aweya, J.J.; Huang, Z.X.; Kang, Z.Y.; Bai, Z.H.; Li, K.H.; He, X.T.; Liu, Y.; Chen, X.Q.; Cheong, K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stott, K.; et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 2018, 3, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ding, Q.; Wei, Y.; Gou, X.; Tian, J.; Li, M.; Tong, X. Effect of traditional Chinese medicine on gut microbiota in adults with type 2 diabetes: A systematic review and meta-analysis. Phytomedicine 2021, 88, 153455. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2017, 8, e71108. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.M.; Deng, Y.L.; Wan, J.J.; Wang, Y.J.; Jin, P. Dysbiosis of gut microbiota and decreased propionic acid associated with metabolic abnormality in Cushing’s syndrome. Front. Endocrinol. 2022, 13, 3656. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Liu, R.; Zhang, F.; Wen, S.a.; Liu, Y.; Ren, W.; Zhang, X.; Shang, Y.; Gao, M.; et al. Predominance of Escherichia-Shigella in Gut Microbiome and Its Potential Correlation with Elevated Level of Plasma Tumor Necrosis Factor Alpha in Patients with Tuberculous Meningitis. Microbiol. Spectr. 2022, 10, e01922–e01926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, M.; Ning, X.; Qin, Y.; Wang, Y.; Yu, Z.; Dong, R.; Zhang, Y.; Sun, S. Expansion of Escherichia-Shigella in Gut Is Associated with the Onset and Response to Immunosuppressive Therapy of IgA Nephropathy. J. Am. Soc. Nephrol. 2022, 33, 2276–2292. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, P.; Srikanth, P.; Seshadri, K.G.; Selvarajan, S.; Pitani, R.S.; Kumar, T.D.; Janarthanan, R. Gut Microbiota in Type 2 Diabetes Individuals and Correlation with Monocyte Chemoattractant Protein1 and Interferon Gamma from Patients Attending a Tertiary Care Centre in Chennai, India. Indian J. Endocrinol. Metab. 2016, 20, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Gao, Z.; Zhao, L.; Wang, H.; Luo, Z.; Vandeputte, D.; He, L.; Li, M.; Di, S.; Liu, Y.; et al. Multiomics Analyses With Stool-Type Stratification in Patient Cohorts and Blautia Identification as a Potential Bacterial Modulator in Type 2 Diabetes Mellitus. Diabetes 2023, 73, 511–527. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Huang, X.; Luo, K. Intestinal microbiology and metabolomics of streptozotocin-induced type 2 diabetes mice by polysaccharide from Cardamine violifolia. J. Funct. Foods 2022, 97, 105251. [Google Scholar] [CrossRef]
- Iyiola, O.O.; Preeya, D.; Chutha, T.Y.; Santad, W. Gut microbiota metabolism of functional carbohydrates and phenolic compounds from soaked and germinated purple rice. J. Funct. Foods 2020, 66, 103787. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.; Qiang, H.; Xiong, F.; Hai, L.R. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Funct. 2016, 7, 4637–4643. [Google Scholar] [CrossRef] [PubMed]

| Sample | Fraction | Mw (kDa) | Mn (kDa) | Mw/Mn |
|---|---|---|---|---|
| MIP | 1 | 398.2 | 388.7 | 1.024 |
| 2 | 21.5 | 20.7 | 1.035 | |
| MIP-S | 1 | 32.3 | 23.8 | 1.357 |
| MIP-G | 1 | 19.1 | 14.9 | 1.286 |
| 2 | 11.6 | 8.5 | 1.369 | |
| MIP-I | 1 | 21.9 | 16.8 | 1.304 |
| 2 | 11.7 | 8.5 | 1.369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, D.; Li, G.; Duan, N.; He, C.; Meng, J.; Cheng, Y.; Geng, X.; Hou, L.; Chang, M.; et al. Functional Properties, Rheological Characteristics, Simulated Digestion, and Fermentation by Human Fecal Microbiota of Polysaccharide from Morchella importuna. Foods 2024, 13, 2148. https://doi.org/10.3390/foods13132148
Wang S, Li D, Li G, Duan N, He C, Meng J, Cheng Y, Geng X, Hou L, Chang M, et al. Functional Properties, Rheological Characteristics, Simulated Digestion, and Fermentation by Human Fecal Microbiota of Polysaccharide from Morchella importuna. Foods. 2024; 13(13):2148. https://doi.org/10.3390/foods13132148
Chicago/Turabian StyleWang, Shurong, Dongjie Li, Guangle Li, Naixin Duan, Chang He, Junlong Meng, Yanfen Cheng, Xueran Geng, Ludan Hou, Mingchang Chang, and et al. 2024. "Functional Properties, Rheological Characteristics, Simulated Digestion, and Fermentation by Human Fecal Microbiota of Polysaccharide from Morchella importuna" Foods 13, no. 13: 2148. https://doi.org/10.3390/foods13132148
APA StyleWang, S., Li, D., Li, G., Duan, N., He, C., Meng, J., Cheng, Y., Geng, X., Hou, L., Chang, M., & Xu, L. (2024). Functional Properties, Rheological Characteristics, Simulated Digestion, and Fermentation by Human Fecal Microbiota of Polysaccharide from Morchella importuna. Foods, 13(13), 2148. https://doi.org/10.3390/foods13132148






