Valorization of Saithe (Pollachius virens) Residuals into Protein Hydrolysates—Silaging as Preservation Technology
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Material
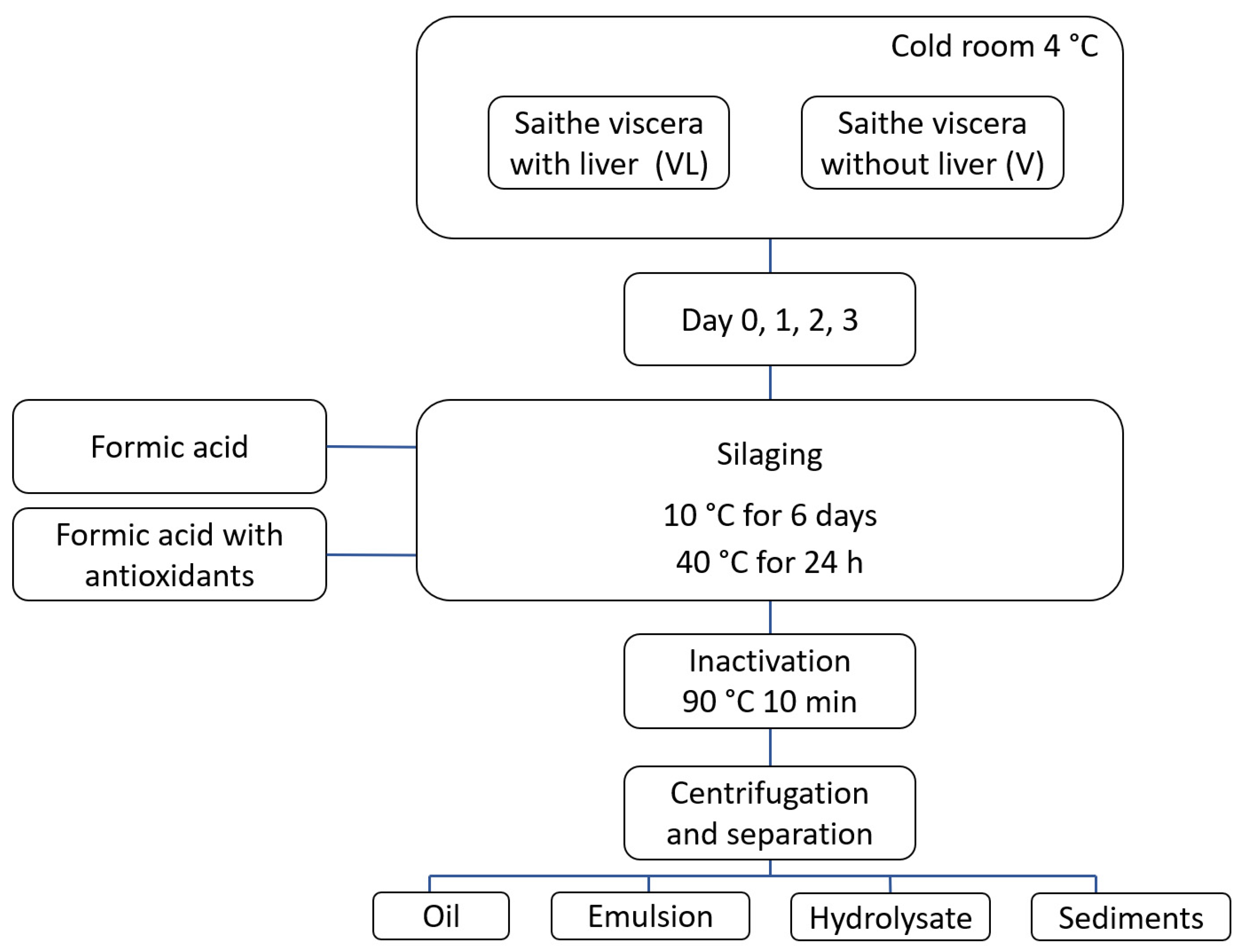
2.2. Experimental Overview
2.3. Dry Matter and Crude Protein
2.4. General Proteolytic Activity and Water-Soluble Proteins
2.5. Bacterial Counts
2.6. Total Volatile Basic Nitrogen
2.7. Degree of Hydrolysis
2.8. Free Amino Acids
2.9. Statistical Analysis
3. Results and Discussion
3.1. Properties and Quality of Raw Material
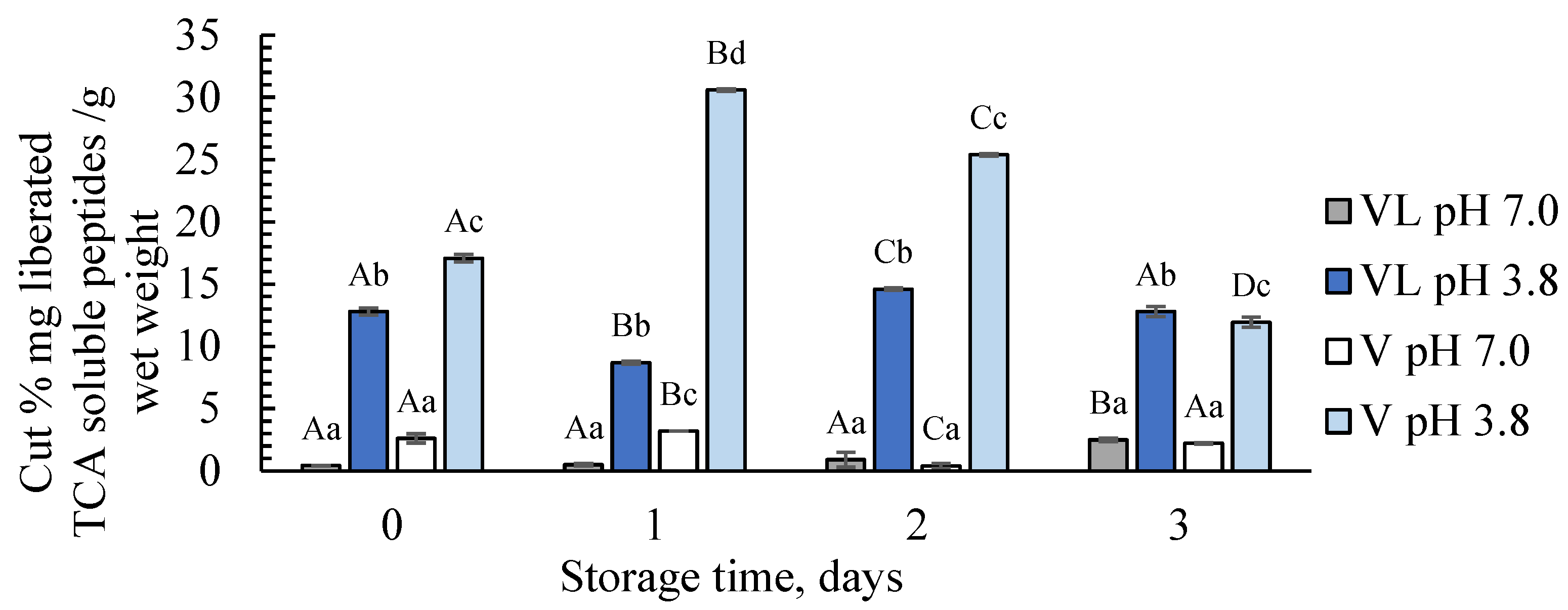
3.1.1. pH, General Proteolytic Activity, and Water-Soluble Proteins
3.1.2. Quality of the Raw Material
3.2. Characteristics of Protein Hydrolysates
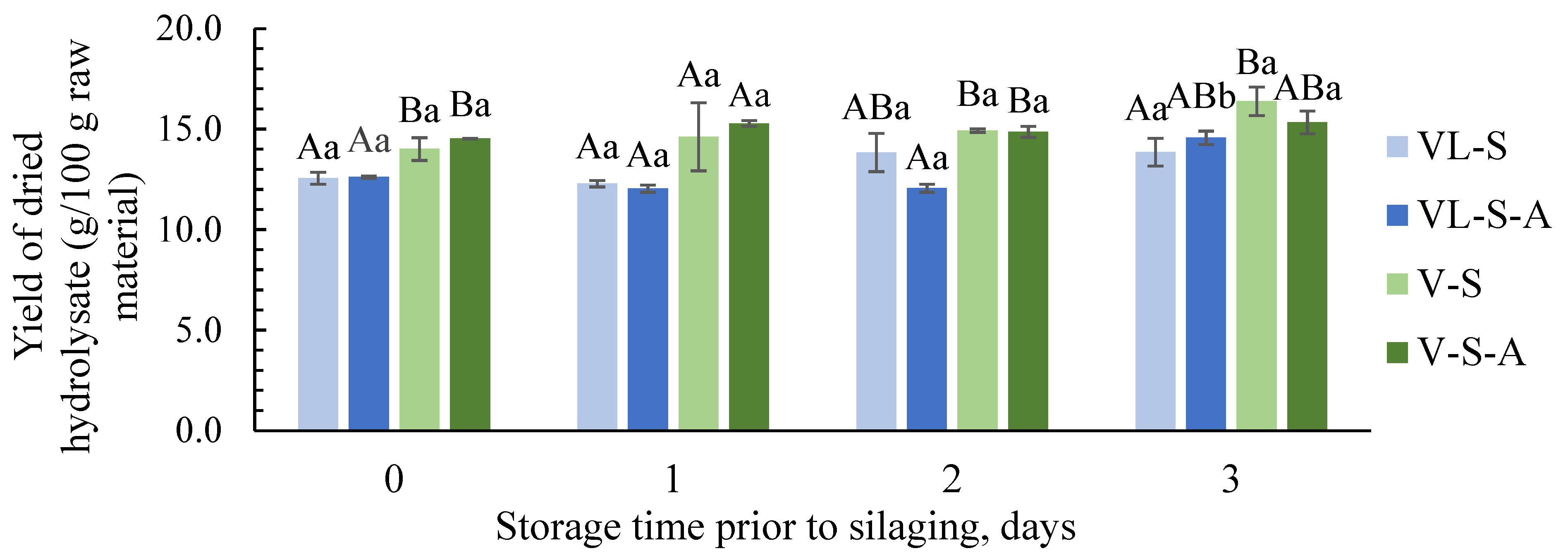
3.2.1. Yield and Protein Content
3.2.2. Degree of Hydrolysis
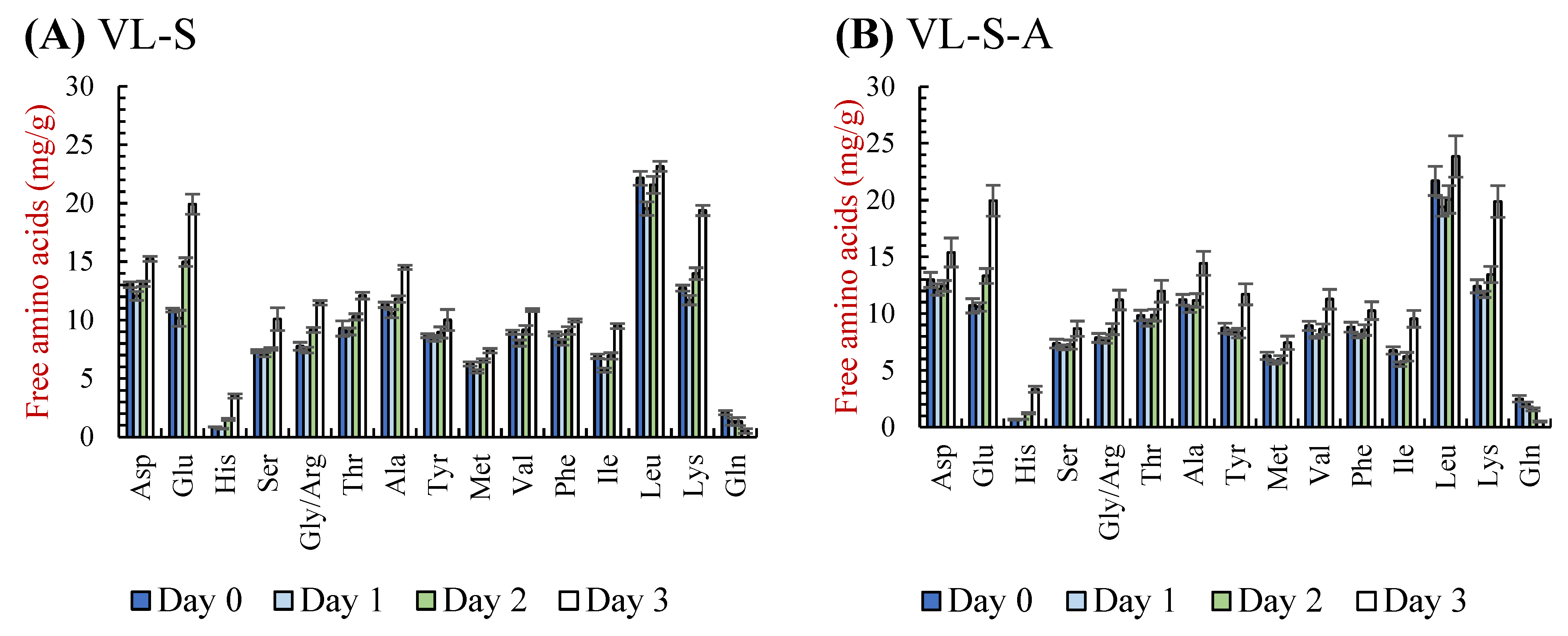
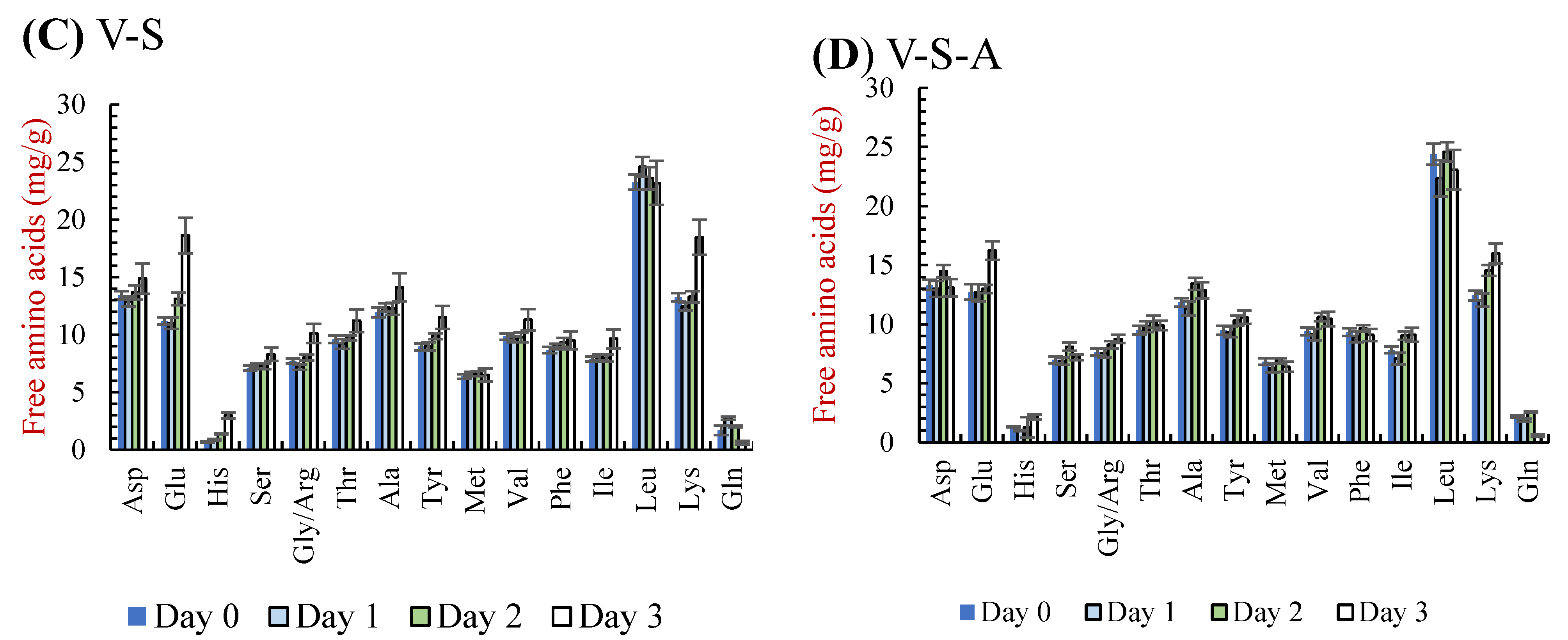
3.2.3. Free Amino Acids
3.2.4. Total Volatile Basic Nitrogen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food and Agriculture 2020. Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar]
- Myhre, M.S.; Richardsen, R.; Nystøyl, R.; Strandheim, G. Analyse Marint Restråstoff 2022. Tilgjengelighet og Anvendelse av Marint Restråstoff i Fra Norsk Fiskeri- og Havbruksnæring; 2021:00633; SINTEF Ocean AS: Trondheim, Norway, 2023. [Google Scholar]
- Nærings- og Fiskeridepartementet. Regjeringa Sin Strategi for Auka Verdiskaping Frå Marint Restråstoff; Nærings-og Fiskeridepartementet: Oslo, Norway, 2019. [Google Scholar]
- Kerry, J.; Murphy, S. Physical and chemical properties of lipid by-products from seafood waste. In Maximising the Value of Marine by-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 22–46. [Google Scholar]
- Rustad, T.; Hayes, M. Marine bioactive peptides and protein hydrolysates: Generation, isolation procedures, and biological and chemical characterizations. In Marine Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–113. [Google Scholar]
- Rustad, T. Utilisation of marine by-products. Electron. J. Environ. Agric. Food Chem. 2003, 2, 458–463. [Google Scholar]
- Falch, E.; Rustad, T.; Aursand, M. By-products from gadiform species as raw material for production of marine lipids as ingredients in food or feed. Process Biochem. 2006, 41, 666–674. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Moreira, M.M.; Delerue-Matos, C.; Gomes, A.M.; Freitas, A.C.; Grosso, C. Valorization of lipid by-products. In Lipids and Edible Oils; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 133–174. [Google Scholar]
- Meidell, L.S.; Carvajal, A.K.; Rustad, T.; Falch, E. Upgrading Marine Oils from Cod (Gadus morhua) On-Board the Deep-Sea Vessels—From Waste to Value. Foods 2023, 12, 1659. [Google Scholar] [CrossRef] [PubMed]
- Falch, E.; Sandbakk, M.; Aursand, M. On-board handling of marine by-products to prevent microbial spoilage, enzymatic reactions and lipid oxidation. In Maximising the Value of Marine by-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 47–64. [Google Scholar]
- Tatterson, I.N.; Windsor, M.L. Fish silage. J. Sci. Food Agric. 1974, 25, 369–379. [Google Scholar] [CrossRef]
- Bekkevold, S.; Olafsen, T. Marine Biprodukter: Råstoff Med Muligheter; Stiftelsen RUBIN: Trondheim, Norge, 2007. [Google Scholar]
- Olsen, R.L.; Toppe, J. Fish silage hydrolysates: Not only a feed nutrient, but also a useful feed additive. Trends Food Sci. Technol. 2017, 66, 93–97. [Google Scholar] [CrossRef]
- Sajib, M.; Trigo, J.P.; Abdollahi, M.; Undeland, I. Pilot-Scale Ensilaging of Herring Filleting Co-Products and Subsequent Separation of Fish Oil and Protein Hydrolysates. Food Bioprocess Technol. 2022, 15, 2267–2281. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Jacobsen, C.; Rustad, T.; Nielsen, N.S.; Falch, E.; Jansson, S.; Storrø, I. Processing of marine lipids and factors affecting their quality when used for functional foods. In Marine Functional Food; Luten, J.B., Ed.; Wageningen Academic Publisher: Amsterdam, The Netherlands, 2009; Volume 2, pp. 89–114. [Google Scholar]
- Paluchowski, L.A.; Misimi, E.; Grimsmo, L.; Randeberg, L.L. Towards automated sorting of Atlantic cod (Gadus morhua) roe, milt, and liver–Spectral characterization and classification using visible and near-infrared hyperspectral imaging. Food Control 2016, 62, 337–345. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Espe, M.; Raa, J.; Njaa, L.R. Nutritional value of stored fish silage as a protein source for young rats. J. Sci. Food Agric. 1989, 49, 259–270. [Google Scholar] [CrossRef]
- Haaland, H.; Njaa, L.R. Total volatile nitrogen—A quality criterion for fish silage? Aquaculture 1989, 79, 311–316. [Google Scholar] [CrossRef]
- Dapkevičius, M.d.L.E.; Batista, I.; Nout, M.R.; Rombouts, F.M.; Houben, J.H. Lipid and protein changes during the ensilage of blue whiting (Micromesistius poutassou Risso) by acid and biological methods. Food Chem. 1998, 1, 97–102. [Google Scholar]
- van’t Land, M.; Vanderperren, E.; Raes, K. The effect of raw material combination on the nutritional composition and stability of four types of autolyzed fish silage. Anim. Feed. Sci. Technol. 2017, 234, 284–294. [Google Scholar] [CrossRef]
- Sajib, M.; Albers, E.; Langeland, M.; Undeland, I. Understanding the effect of temperature and time on protein degree of hydrolysis and lipid oxidation during ensilaging of herring (Clupea harengus) filleting co-products. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Raeesi, R.; Shabanpour, B.; Pourashouri, P. Quality evaluation of produced silage and extracted oil from rainbow trout (Oncorhynchus mykiss) wastes using acidic and fermentation methods. Waste Biomass Valorization 2021, 12, 4931–4942. [Google Scholar] [CrossRef]
- Sajib, M.; Langeland, M.; Undeland, I. Effect of antioxidants on lipid oxidation in herring (Clupea harengus) co-product silage during its production, heat-treatment and storage. Sci. Rep. 2022, 12, 1–13. [Google Scholar]
- Arason, S. Production of fish silage. In Fisheries Processing; Springer: Berlin/Heidelberg, Germany, 1994; pp. 244–272. [Google Scholar]
- AOAC Official Methods of Analysis; Association of Official Analytic Chemists: Washington, DC, USA, 1990.
- Barret, A. Lysosomal enzymes. In Lysosomes—A Laboratory Handbook, 2nd ed.; Dingle, J.T., Ed.; North Holland Publishing Co.: Amsterdam, The Netherland, 1972. [Google Scholar]
- Bergvik, M.; Overrein, I.; Bantle, M.; Evjemo, J.O.; Rustad, T. Properties of Calanus finmarchicus biomass during frozen storage after heat inactivation of autolytic enzymes. Food Chem. 2012, 132, 209–215. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D. Laboratory Techniques in Food Analysis (Laboratory Techniques Series); Butterworths: London, UK, 1973. [Google Scholar]
- Taylor, W. Formol titration: An evaluation of its various modifications. Analyst 1957, 82, 488–498. [Google Scholar] [CrossRef]
- Kvangarsnes, K.; Kendler, S.; Rustad, T.; Aas, G.H. Induced oxidation and addition of antioxidant before enzymatic hydrolysis of heads of rainbow trout (Oncorhynchus mykiss)—Effect on the resulting oil and protein fraction. Heliyon 2021, 7, e06816. [Google Scholar] [CrossRef]
- Osnes, K.K.; Mohr, V. Peptide hydrolases of Antartic krill, Euphausia superba. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 82, 599–606. [Google Scholar] [CrossRef]
- Sovik, S.L.; Rustad, T. Seasonal changes in trypsin and chymotrypsin activity in viscera from cod species. J. Aquat. Food Prod. Technol. 2004, 13, 13–30. [Google Scholar] [CrossRef]
- Søvik, S.L.; Rustad, T. Effect of season and fishing ground on the activity of lipases in byproducts from cod (Gadus morhua). LWT-Food Sci. Technol. 2005, 38, 867–876. [Google Scholar] [CrossRef]
- Slizyte, R.; Daukšas, E.; Falch, E.; Storrø, I.; Rustad, T. Yield and composition of different fractions obtained after enzymatic hydrolysis of cod (Gadus morhua) by-products. Process Biochem. 2005, 40, 1415–1424. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Balcázar, J.L.; Daniels, C.; Zhou, Z.; Carnevali, O.; Sun, Y.Z.; Hoseinifar, S.H.; Ringø, E. Indigenous lactic acid bacteria in fish and crustaceans. Aquac. Nutr. Gut Health Probiotics Prebiotics 2014, 128–168. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. (Eds.) Fennema’s Food Chemistry, 5th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Søvik, S.L.; Rustad, T. Proteolytic activity in byproducts from cod species caught at three different fishing grounds. J. Agric. Food Chem. 2005, 53, 452–458. [Google Scholar] [CrossRef]
- Meidell, L.S.; Slizyte, R.; Mozuraityte, R.; Carvajal, A.K.; Rustad, T.; Standal, I.B.; Kopczyk, M.; Falch, E. Silage for upcycling oil from saithe (Pollachius virens) viscera–Effect of raw material freshness on the oil quality. Heliyon 2023, 9, E16972. [Google Scholar] [CrossRef]
- Bower, C.; Malemute, C.; Bechtel, P. Endogenous protease activity in by-products of pink salmon (Oncorhynchus gorbuscha). J. Food Biochem. 2011, 35, 628–637. [Google Scholar] [CrossRef]
- Stoknes, I.; Rustad, T.; Mohr, V. Comparative studies of the proteolytic activity of tissue extracts from cod (Gadus morhua) and herring (Clupea harengus). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 613–619. [Google Scholar] [CrossRef]
- Mutilangi, W.; Panyam, D.; Kilara, A. Hydrolysates from proteolysis of heat-denatured whey proteins. J. Food Sci. 1995, 60, 1104–1109. [Google Scholar] [CrossRef]
- Gildberg, A.; Olsen, R.L.; Bjarnason, J.B. Characteristics and composition of pepsins from Atlantic cod. In Structure and Function of the Aspartic Proteinases: Genetics, Structures, and Mechanisms; Springer: Berlin/Heidelberg, Germany, 1991; pp. 107–110. [Google Scholar]
- Sikorski, Z.E.; Kolakowska, A.; Burt, J.R. Postharvest biochemical and microbial changes. In Seafood: Resources, Nutritional Composition and Preservation; CRC Press: Boca Raton, FL, USA, 1990; pp. 55–75. [Google Scholar]
- ICMSF. Microorganisms in Foods 2. Sampling for Microbiological Analysis: Principles and Specific Applications; University of Toronto Press: Toronto, ON, Canada, 1986. [Google Scholar]
- Haaland, H.; Espe, M.; Njaa, L.R.; Myklestad, H. Chemical composition and variation in some parameters during storage of 8 formic acid silages prepared from capelin. Fisk. Dir. Skr. Ser. Ermring. 1990, III, 59–74. [Google Scholar]
- Bekhit, A.E.-D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Haaland, H.; Njaa, L.R. Ammonia (NH3) and total volatile nitrogen (TVN) in preserved and unpreserved stored, whole fish. J. Sci. Food Agric. 1988, 44, 335–342. [Google Scholar] [CrossRef]
- Haaland, H.; Njaa, L.R. Fish silages prepared from raw material of varying quality: Chemical analysis related to balance experiments in rats. Fisk. Dir. Skr. Ser. Ernering. 1990, III, 27–35. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/627 of 15 March 2019 Laying Down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls (Text with EEA Relevance); European Parliament and the Council of the European Union: Washington, DC, USA, 2019.
- FAO. The Production of Fishmeal and Oil; FAO, Fishery Industries Division: Rome, Italy, 1986. [Google Scholar]
- Slizyte, R.; Rustad, T.; Storrø, I. Enzymatic hydrolysis of cod (Gadus morhua) by-products: Optimization of yield and properties of lipid and protein fractions. Process Biochem. 2005, 40, 3680–3692. [Google Scholar] [CrossRef]
- Kristinsson, H. Aquatic food protein hydrolysates. In Maximising the Value of Marine by-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 229–248. [Google Scholar]
- Hilmarsdottir, G.S.; Ogmundarson, Ó.; Arason, S.; Gudjónsdóttir, M. Efficiency of fishmeal and fish oil processing of different pelagic fish species: Identification of processing steps for potential optimization toward protein production for human consumption. J. Food Process. Preserv. 2021, 45, e15294. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, A.; Gomes, R.M.; Gregersen, S.; Sloth, J.J.; Jacobsen, C.; Sørensen, A.-D.M. Characterization of cod (Gadus morhua) frame composition and its valorization by enzymatic hydrolysis. J. Food Compos. Anal. 2020, 89, 103469. [Google Scholar] [CrossRef]
- Farvin, K.S.; Andersen, L.L.; Otte, J.; Nielsen, H.H.; Jessen, F.; Jacobsen, C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: Fractionation and characterisation of peptide fractions. Food Chem. 2016, 204, 409–419. [Google Scholar] [CrossRef]
- Ween, O.; Stangeland, J.K.; Fylling, T.S.; Aas, G.H. Nutritional and functional properties of fishmeal produced from fresh by-products of cod (Gadus morhua L.) and saithe (Pollachius virens). Heliyon 2017, 3, E00343. [Google Scholar] [CrossRef]
- Slizyte, R.; Daukšas, E.; Falch, E.; Storrø, I.; Rustad, T. Characteristics of protein fractions generated from hydrolysed cod (Gadus morhua) by-products. Process Biochem. 2005, 40, 2021–2033. [Google Scholar] [CrossRef]
- Opheim, M.; Šližytė, R.; Sterten, H.; Provan, F.; Larssen, E.; Kjos, N.P. Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials—Effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochem. 2015, 50, 1247–1257. [Google Scholar] [CrossRef]
- Espe, M.; Lied, E. Fish silage prepared from different cooked and uncooked raw materials: Chemical changes during storage at different temperatures. J. Sci. Food Agric. 1999, 79, 327–332. [Google Scholar] [CrossRef]
- Bhaskar, N.; Mahendrakar, N. Chemical and microbiological changes in acid ensiled visceral waste of Indian major carp Catla catla (Hamilton) with emphasis on proteases. Indian J. Fish. 2007, 54, 217–225. [Google Scholar]
- Nørgaard, J.V.; Petersen, J.K.; Tørring, D.B.; Jørgensen, H.; Lærke, H.N. Chemical composition and standardized ileal digestibility of protein and amino acids from blue mussel, starfish, and fish silage in pigs. Anim. Feed. Sci. Technol. 2015, 205, 90–97. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Inhibition of proteolysis in oxidized lipid-damaged proteins. J. Agric. Food Chem. 2001, 49, 6006–6011. [Google Scholar] [CrossRef]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef]
- Strøm, T.; Eggum, B.O. Nutritional value of fish viscera silage. J. Sci. Food Agric. 1981, 32, 115–120. [Google Scholar] [CrossRef]
- Olley, J.; Ford, J.E.; Williams, A. Nutritional value of fish visceral meals. J. Sci. Food Agric. 1968, 19, 282–285. [Google Scholar] [CrossRef]
- Søvik, S.L. Characterisation of Enzymatic Activities in by-Products from Cod Species: Effect of Species, Season and Fishing Ground; NTNU: Trondheim, Norway, 2005. [Google Scholar]
- Rustad, T. Physical and chemical properties of protein seafood by-products. In Maximising the Value of Marine by-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–21. [Google Scholar]
- Nguyen, H.T.; Hilmarsdóttir, G.S.; Tómasson, T.; Arason, S.; Gudjónsdóttir, M. Changes in protein and non-protein nitrogen compounds during fishmeal processing—Identification of unoptimized processing steps. Processes 2022, 10, 621. [Google Scholar] [CrossRef]
- Opstvedt, J.; Mundheim, H.; Nygård, E.; Aase, H.; Pike, I.H. Reduced growth and feed consumption of Atlantic salmon (Salmo salar L.) fed fish meal made from stale fish is not due to increased content of biogenic amines. Aquaculture 2000, 188, 323–337. [Google Scholar] [CrossRef]
| Experimental Data | Viscera with Liver (VL) | Viscera without Liver (V) |
|---|---|---|
| Number of fish | 27 | 41 |
| Fish length, cm | 66 ± 5 | 67 ± 5 |
| Round weight, g | 3388 ± 809 | 3521 ± 709 |
| Viscera with liver, g | 593 ± 257 | 612 ± 209 |
| Viscera without liver, g | 445 ± 245 | 485 ± 173 |
| Liver, g | 123 ± 45 | 127 ± 56 |
| Viscera with Liver (VL) | ||||
|---|---|---|---|---|
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| pH | 6.8 ± 0.1 Aa | 6.9 ± 0.0 Aab | 7.0 ± 0.2 Ab | 6.5 ± 0.1 Ac |
| WS protein, % | 4.9 ± 0.3 Aa | 5.1 ± 0.0 Aa | 6.9 ± 0.2 Ab | 6.9 ± 0.0 Ab |
| Viscera (V) | ||||
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| pH | 6.8 ± 0.1 Aa | 6.8 ± 0.1 Ba | 7.2 ± 0.1 Bb | 6.8 ± 0.1 Ba |
| WS protein, % | 9.5 ± 0.2 Ba | 10.6 ± 0.1 Bb | 12.3 ± 0.3 Bc | 8.5 ± 0.3 Bd |
| Viscera with Liver (VL) | ||||
|---|---|---|---|---|
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Bacterial count (Log CFU/g) | 5.7 ± 0.1 Aa | 2.4 ± 0.1 Ab | 3.2 ± 0.3 Ab | 3.3 ± 0.8 Ab |
| TVB-N (mg/100 g) | 60.6 ± 1.5 Aa | 49.2 ± 2.2 Ab | 62.1 ± 1.7 Aa | 83.2 ± 1.2 Ac |
| Viscera (V) | ||||
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Bacterial count (Log CFU/g) | 2.6 ± 0.1 Ba | 2.4 ± 0.3 Aa | 2.6 ± 0.3 Aa | 3.2 ± 0.5 Aa |
| TVB-N (mg/100 g) | 66.2 ± 2.1 Bac | 54.7 ± 1.2 Bb | 61.5 ± 2.2 Aa | 71.7 ± 0.2 Bc |
| VL-S (Viscera with Liver Silage) | ||||
|---|---|---|---|---|
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Crude protein, % | 79.8 ± 0.5 Aa | 79.7 ± 0.3 Aa | 79.9 ± 0.5 Aa | 74.7 ± 0.6 Ab |
| TVB-N, mg/100 g | 131.1 ± 0.5 Aa | 134.3 ± 1.2 Aa | 152.2 ± 0.8 Ab | 178.6 ± 0.4 Ac |
| DH, % | 46.4 ± 0.7 Aa | 45.5 ± 0.3 Aa | 47.8 ± 0.5 Ab | 57.0 ± 0.2 Ac |
| FAA, mg/g | 137.5 ± 2.9 Aa | 125.6 ± 4.2 Ab | 146.0 ± 4.0 ABc | 177.7 ± 2.4 Ad |
| VL-S-A (Viscera with Liver Silage with Antioxidants) | ||||
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Crude protein, % | 80.1 ± 0.8 Aa | 79.1 ± 0.3 Bab | 77.8 ± 1.0 Bb | 75.8 ± 0.2 ABc |
| TVB-N, mg/100 g | 137.0 ± 0.6 Ba | 132.6 ± 0.7 Ab | 146.0 ± 0.9 Bc | 179.7 ± 0.7 Ad |
| DH, % | 45.5 ± 0.6 Aa | 44.7 ± 0.7 Aa | 47.6 ± 0.7 Ab | 55.5 ± 0.4 Bc |
| FAA, mg/g | 136.7 ± 6.0 Aa | 126.4 ± 2.2 Aa | 136.7 ± 7.2 Aa | 179.4 ± 13.4 Ab |
| V-S (Viscera Silage) | ||||
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Crude protein, % | 80.6 ± 0.2 Aa | 79.3 ± 0.3 ABb | 79.0 ± 0.1 ABb | 76.8 ± 0.8 BCc |
| TVB-N, mg/100 g | 142.3 ± 0.9 Ca | 126.1 ± 0.6 Bb | 134.4 ± 0.4 Cc | 177.1 ± 1.4 ABd |
| DH, % | 45.2 ± 0.4 Aa | 44.8 ± 0.3 Aa | 46.1 ± 0.1 Ba | 52.7 ± 0.9 Cc |
| FAA, mg/g | 141.7 ± 4.0 Aa | 142.9 ± 3.7 Ba | 148.0 ± 5.6 ABa | 171.0 ± 14.4 ABb |
| V-S-A (Viscera Silage with Antioxidants) | ||||
| Parameter | Day 0 | Day 1 | Day 2 | Day 3 |
| Crude protein, % | 80.3 ± 0.3 Aa | 79.0 ± 0.2 Bb | 78.6 ± 0.2 ABb | 77.3 ± 0.5 Cc |
| TVB-N, mg/100 g | 136.9 ± 0.4 Ba | 138.7 ± 1.2 Ca | 139.0 ± 2.2 Ca | 174.9 ± 0.5 Bb |
| DH, % | 44.4 ± 0.3 Aa | 44.7 ± 0.4 Aa | 48.6 ± 0.3 Ab | 52.4 ± 0.6 Cc |
| FAA, mg/g | 145.3 ± 5.4 Aab | 139.6 ± 7.5 Ba | 157.1 ± 4.8 Bb | 152.9 ± 6.6 Bab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meidell, L.S.; Slizyte, R.; Mozuraityte, R.; Carvajal, A.K.; Rustad, T.; Falch, E. Valorization of Saithe (Pollachius virens) Residuals into Protein Hydrolysates—Silaging as Preservation Technology. Foods 2024, 13, 2133. https://doi.org/10.3390/foods13132133
Meidell LS, Slizyte R, Mozuraityte R, Carvajal AK, Rustad T, Falch E. Valorization of Saithe (Pollachius virens) Residuals into Protein Hydrolysates—Silaging as Preservation Technology. Foods. 2024; 13(13):2133. https://doi.org/10.3390/foods13132133
Chicago/Turabian StyleMeidell, Line Skontorp, Rasa Slizyte, Revilija Mozuraityte, Ana Karina Carvajal, Turid Rustad, and Eva Falch. 2024. "Valorization of Saithe (Pollachius virens) Residuals into Protein Hydrolysates—Silaging as Preservation Technology" Foods 13, no. 13: 2133. https://doi.org/10.3390/foods13132133
APA StyleMeidell, L. S., Slizyte, R., Mozuraityte, R., Carvajal, A. K., Rustad, T., & Falch, E. (2024). Valorization of Saithe (Pollachius virens) Residuals into Protein Hydrolysates—Silaging as Preservation Technology. Foods, 13(13), 2133. https://doi.org/10.3390/foods13132133







