Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Ponkan By-Product Samples before Processing
2.3. Proximate Analysis
2.4. Flavonoid Extraction
2.5. Analysis of Phytochemical Content
2.5.1. Total Phenolic Contents (TPC)
2.5.2. Total Flavonoid Content (TFC)
2.5.3. Total Ascorbic Acid Content (TAAC)
2.5.4. Analysis of Flavanone Content by HPLC-PDA
2.6. Measurement of Antioxidant Capacity
2.6.1. ABTS (2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulphonic Acid) Assay
2.6.2. DPPH Assay
2.6.3. FRAP Assay
2.7. Pectin Extract
2.8. Total Dietary Fiber (TDF)
2.9. Statistical Analysis
3. Results
3.1. Drying Results for Ponkan By-Products
3.2. Proximate Analysis of Ponkan By-Products
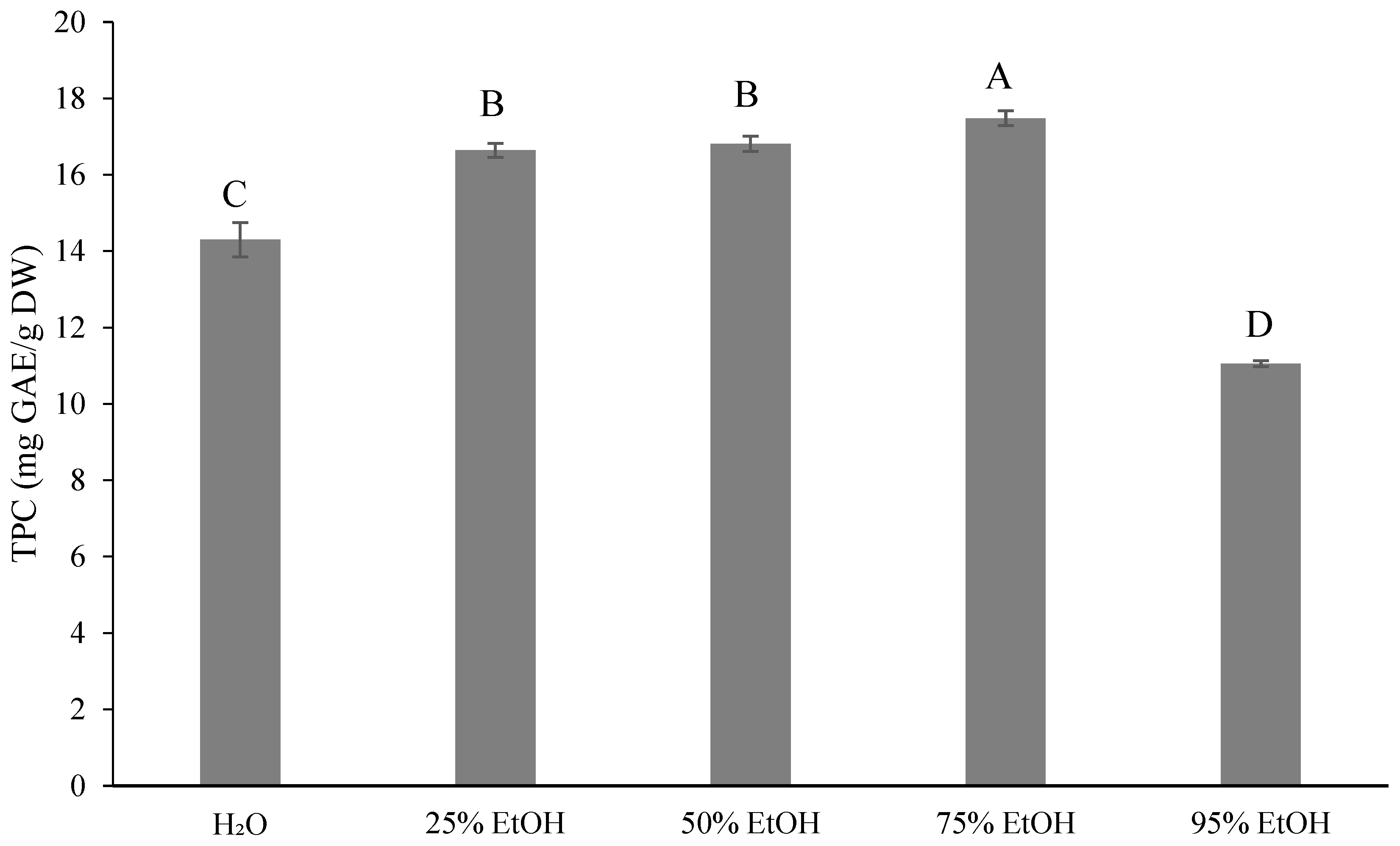
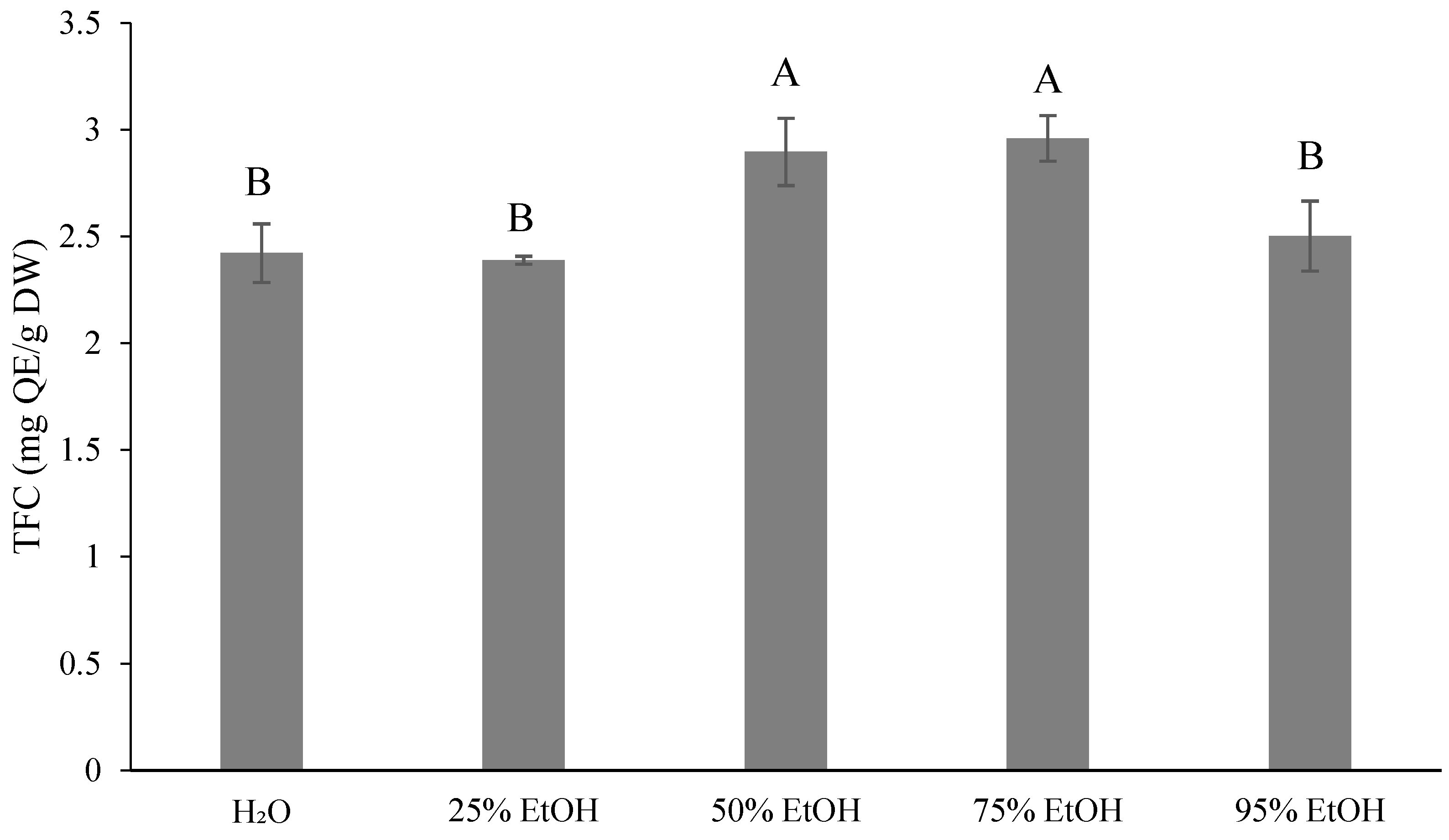
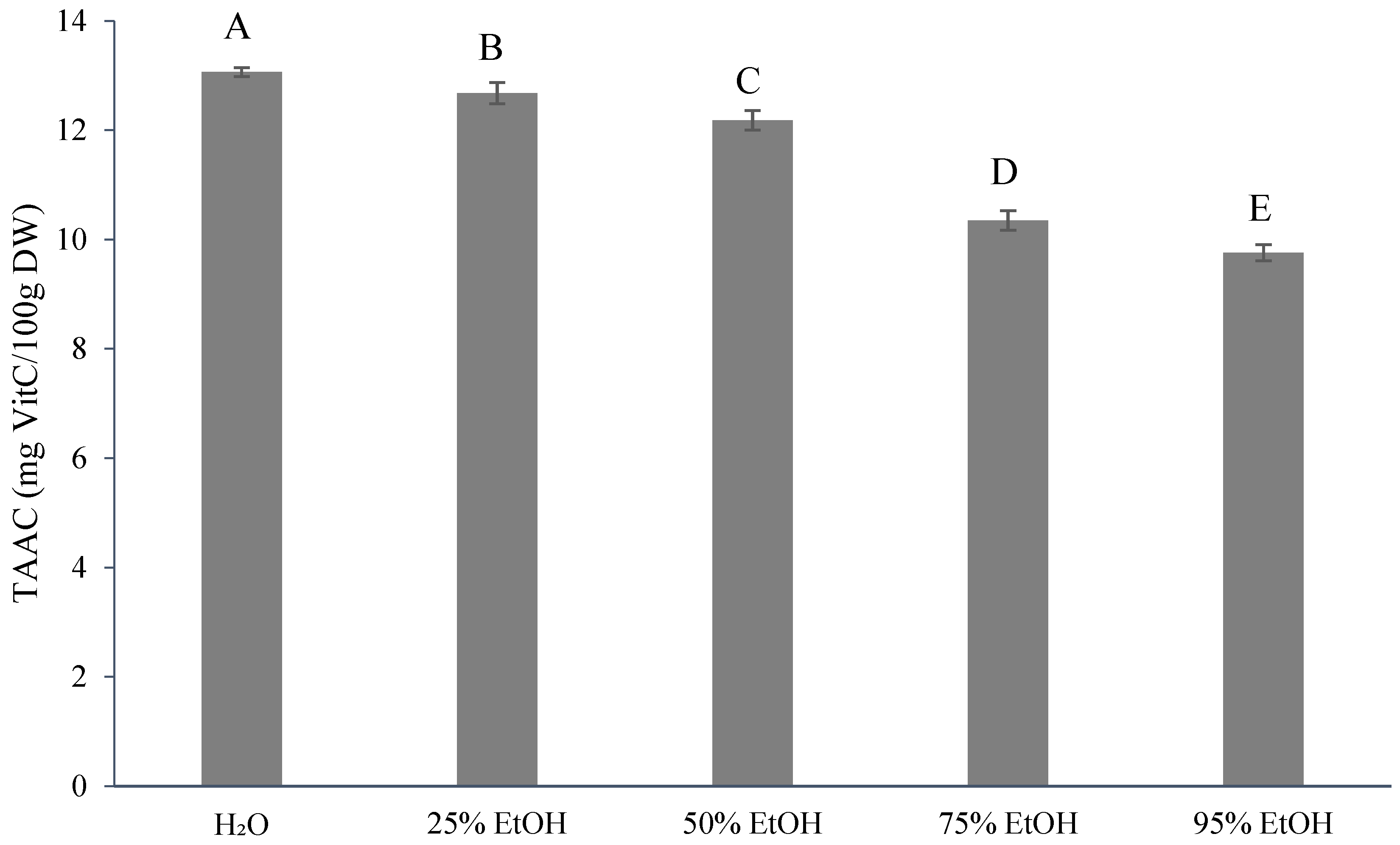
3.3. Results for the Phytochemical Content of Ponkan By-Products by Extractive Solvents
3.4. Analysis of the Flavanone Content of Ponkan by HPLC-PDA
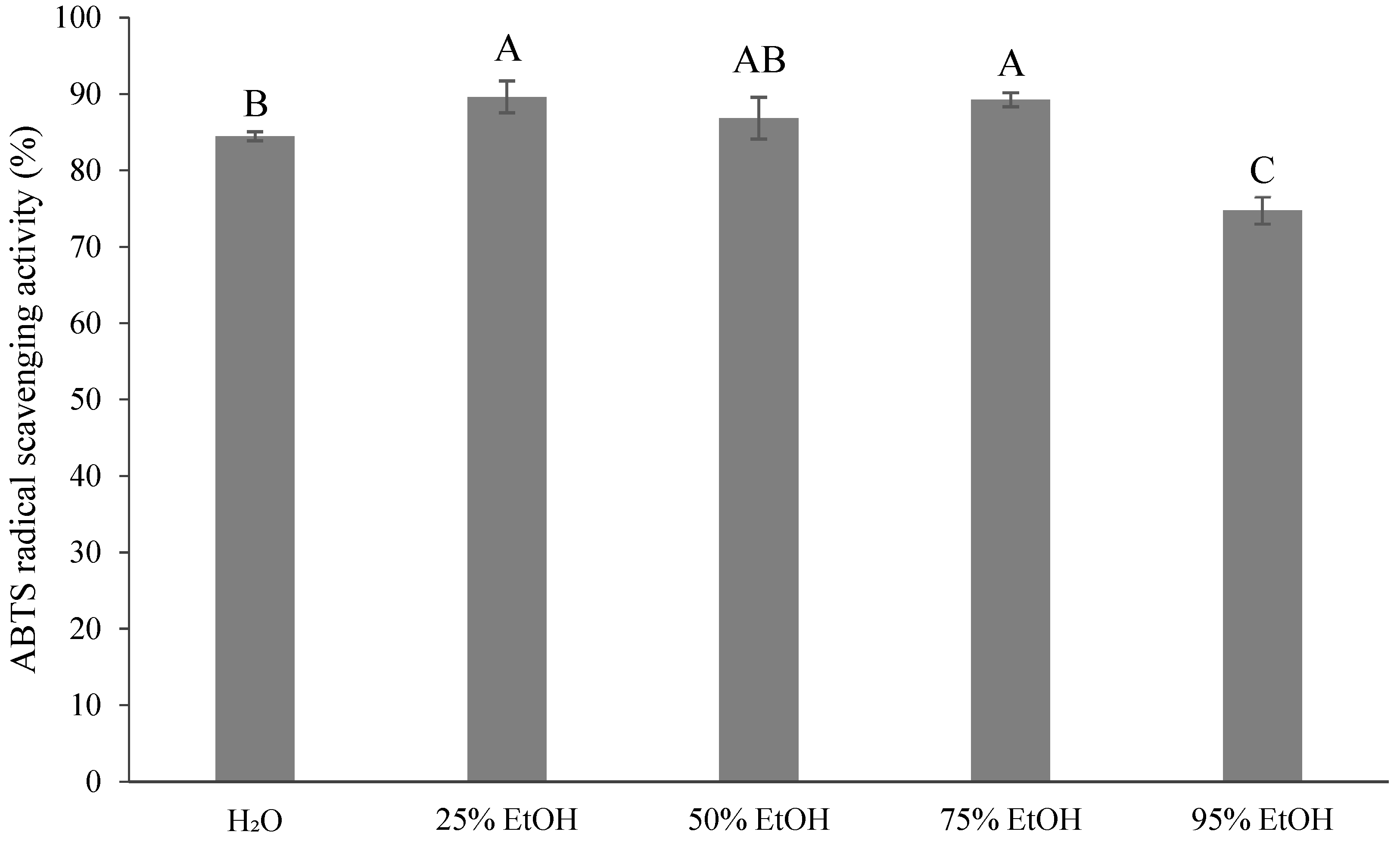
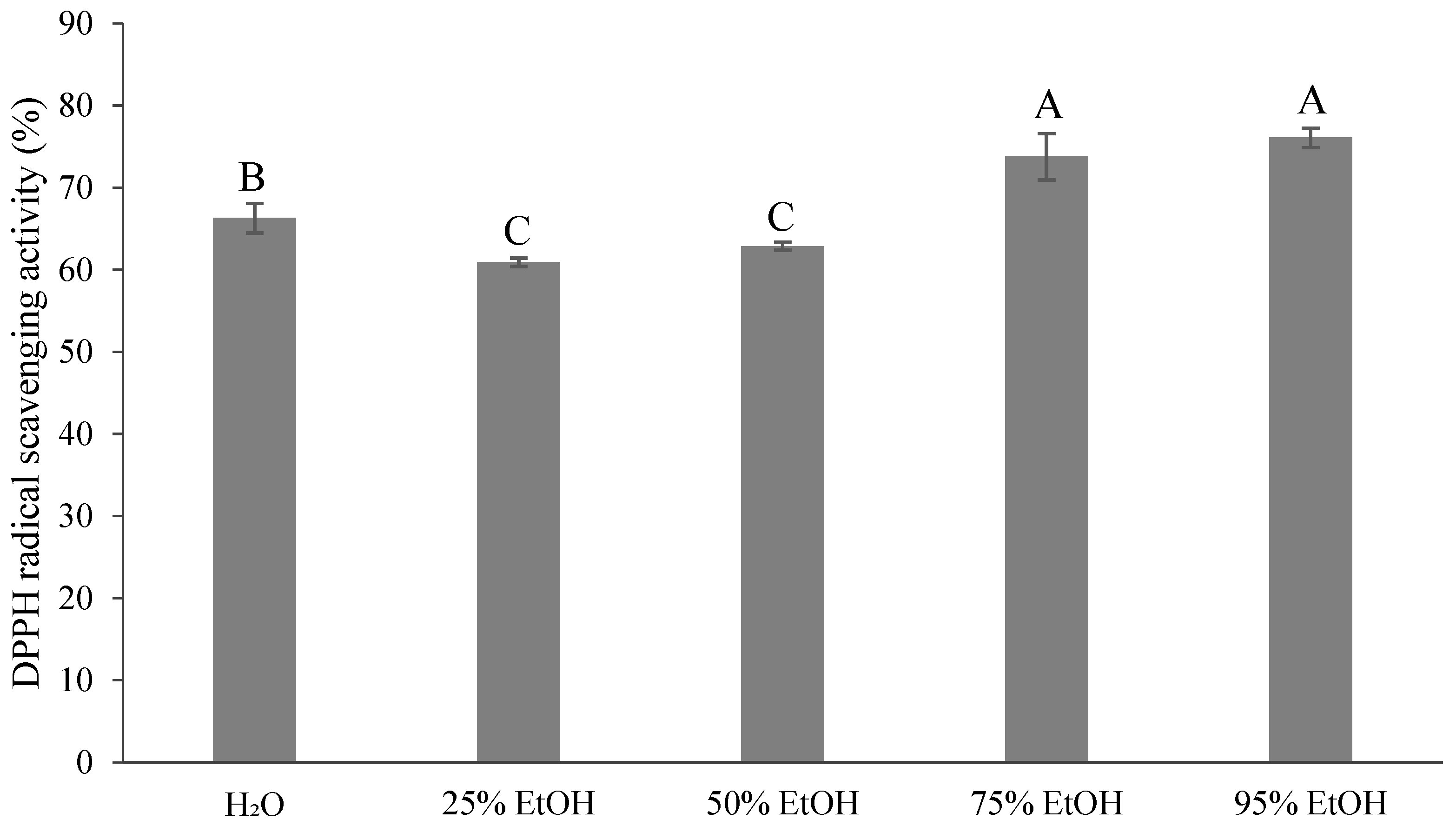
3.5. Antioxidant Activity of the Extracted Solvent on Ponkan By-Products
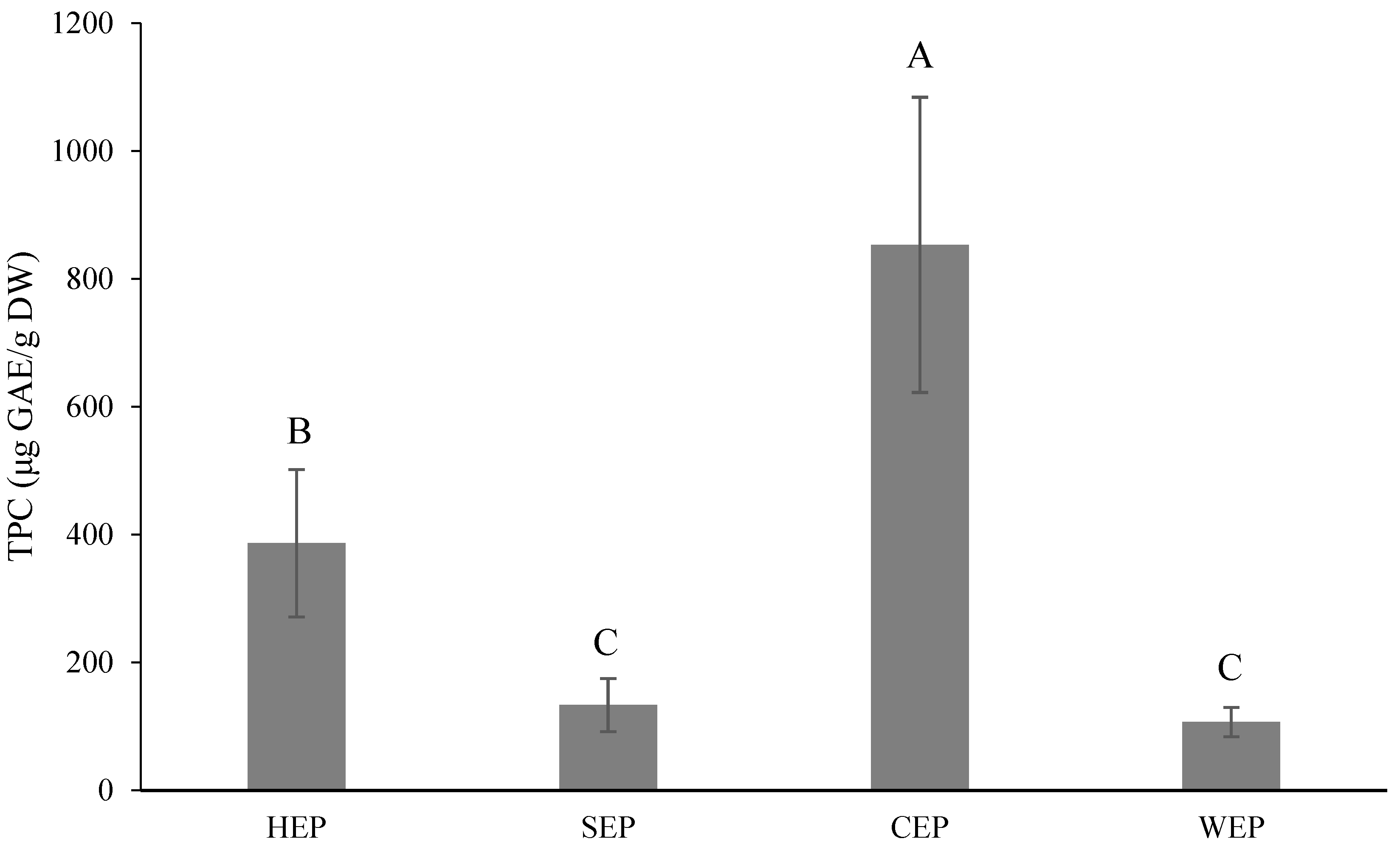
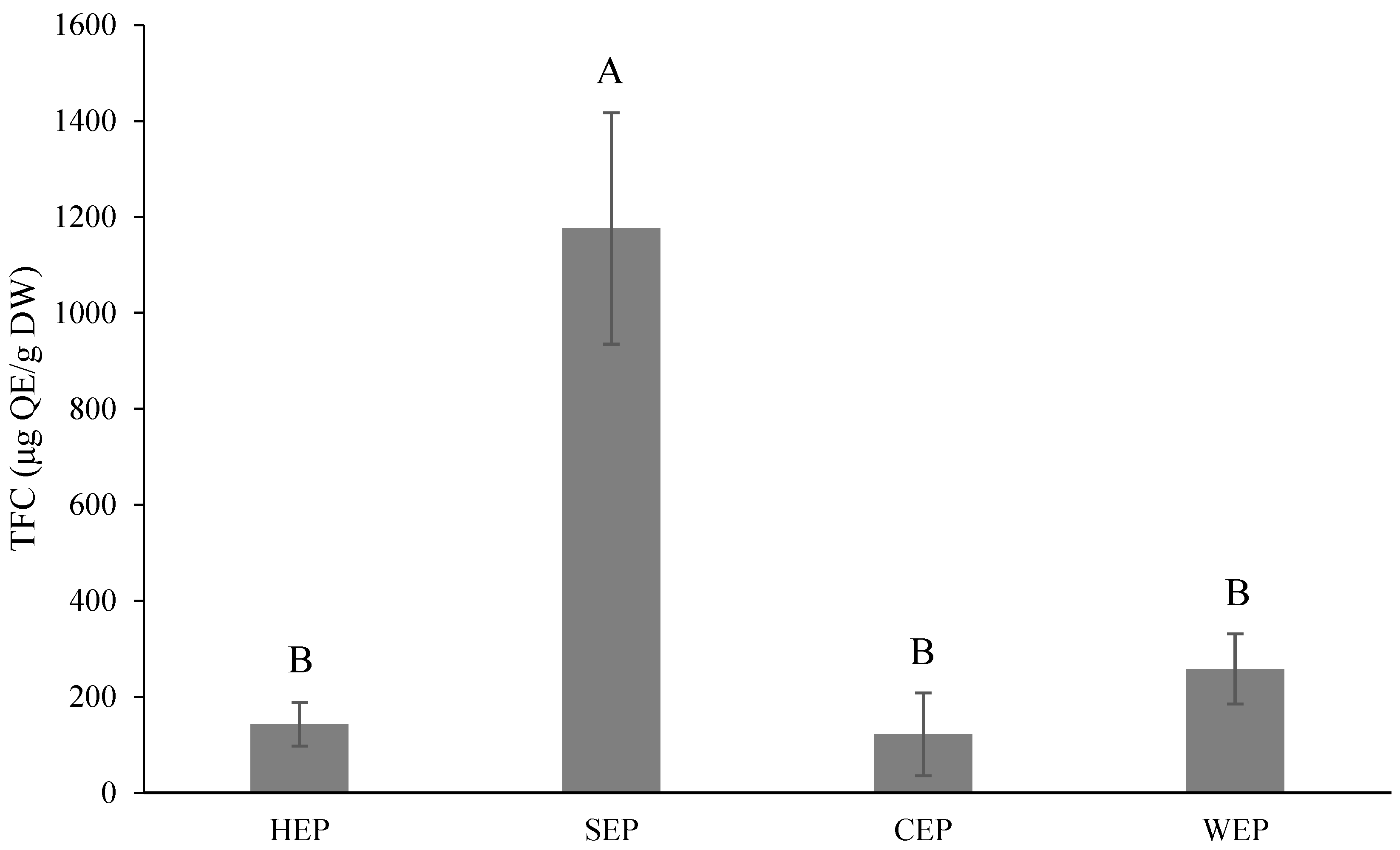
3.6. Effect of Extraction Conditions on the Pectin Yield and Results for the Phytochemical Content of Pectin Using Extraction Solvents
TPC and TFC
3.7. Antioxidant Activity of Pectin Extract Solvents
3.7.1. DPPH
3.7.2. FRAP
3.8. Total Dietary Fiber (TDF) of Ponkan By-Products
4. Discussion
4.1. Drying Results for Ponkan By-Products
4.2. Results for the Phytochemical Content of Ponkan By-Products with Extractive Solvents
4.3. Analysis of the Flavanone Content of Ponkan by HPLC-PDA
4.4. Antioxidant Activity of the Extracted Solvent on Ponkan By-Products
4.5. Total Dietary Fiber (TDF) in Ponkan By-Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different Citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Nair, A.; Kurup Sr, R.; Nair, A.S.; Baby, S. Citrus peels prevent cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Kaul, R.; Sofi, S.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Lachowicz, S.; Michalska, A.; Lech, K.; Majerska, J.; Oszmiański, J.; Figiel, A. Comparison of the effect of four drying methods on polyphenols in saskatoon berry. LWT-Food Sci. Technol. 2019, 111, 727–736. [Google Scholar] [CrossRef]
- Feng, H.; Tang, J.; Cavalieri, R.; Plumb, O. Heat and mass transport in microwave drying of porous materials in a spouted bed. AIChE J. 2001, 47, 1499–1512. [Google Scholar] [CrossRef]
- Ute, R. Microwave thawing, drying and baking in the food industry. J. Food Sci. Technol. 1987, 6, 85–94. [Google Scholar]
- Luque-García, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. Trends Analyt. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Chemat, S.; Lagha, A.; AitAmar, H.; Bartels, P.V.; Chemat, F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour Fragr. J. 2004, 19, 188–195. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Londoño-Londoño, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C.; Arango, G.J.; Pineda, J.R.R. Clean recovery of antioxidant flavonoids from Citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onion skin and their use in functional bread production. LWT-Food Sci. Technol. 2020, 117, 108614. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Rockville, MD, USA, 2003. [Google Scholar]
- Xi, W.; Fang, B.; Zhao, Q.; Jiao, B.; Zhou, Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014, 161, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Nielsen, S.S. Vitamin C determination by indophenol method. In Food Analysis Laboratory Manual; Springer International Publishing: Cham, Switzerland, 2017; pp. 143–146. [Google Scholar]
- Sun, Y.; Wang, J.; Gu, S.; Liu, Z.; Zhang, Y.; Zhang, X. Simultaneous determination of flavonoids in different parts of Citrus reticulata ‘Chachi’fruit by high performance liquid chromatography—Photodiode array detection. Molecules 2010, 15, 5378–5388. [Google Scholar] [CrossRef]
- Chew, K.; Khoo, M.; Ng, S.; Thoo, Y.Y.; Aida, W.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive compounds, antioxidant activity and inhibition of key enzymes relevant to Alzheimer’s disease from sweet pepper (Capsicum annuum) extracts. Prev. Nutr. Food Sci. 2019, 24, 327. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Bu, Z.; Ren, H.; He, Q.; Yu, Y.; Xu, Y.; Wu, J.; Cheng, L.; Li, L. Physicochemical, structural, and functional properties of wampee (Clausena lansium (Lour.) Skeels) fruit peel pectin extracted with different organic acids. Food Chem. 2022, 386, 132834. [Google Scholar] [CrossRef]
- Minkov, S.; Minchev, A.; Paev, K. Modelling of the hydrolysis and extraction of apple pectin. J. Food Eng. 1996, 29, 107–113. [Google Scholar] [CrossRef]
- Kliemann, E.; De Simas, K.N.; Amante, E.R.; Prudêncio, E.S.; Teófilo, R.F.; Ferreira, M.M.; Amboni, R.D. Optimisation of pectin acid extraction from passion fruit peel (Passiflora edulis flavicarpa) using response surface methodology. Int. J. Food Sci. Technol. 2009, 44, 476–483. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef] [PubMed]
- El Fihry, N.; El Mabrouk, K.; Eeckhout, M.; Schols, H.A.; Filali-Zegzouti, Y.; Hajjaj, H. Physicochemical and functional characterization of pectin extracted from Moroccan Citrus peels. LWT-Food Sci. Technol. 2022, 162, 113508. [Google Scholar] [CrossRef]
- AOAC Official Method 2011.25. Insoluble, Soluble, and Total Dietary Fiber in Foods Enzymatic-Gravimetric-Liquid Chromatography, First Action 2011; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. In Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Boluda-Aguilar, M.; García-Vidal, L.; González-Castañeda, F.D.P.; López-Gómez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef]
- Kaushal, S.; Kalia, A.; Kaur, V. Proximate, mineral, chemical composition, antioxidant and antimicrobial potential of dropped fruits of Citrus reticulata Blanco. J Food Meas. Charact. 2022, 16, 4303–4317. [Google Scholar]
- Tareen, H.; Mengal, F.; Masood, Z.; Mengal, R.; Ahmed, S.; Bibi, S.; Shoaib, S.; Sami, U.; Mandokhail, F.; Riaz, M. Determination of vitamin C content in Citrus fruits and in non-Citrus fruits by titrimetric method, with special reference to their nutritional importance in human diet. Biol. Forum 2015, 7, 367. [Google Scholar]
- Nweze, C.; Abdulganiyu, M.; Erhabor, O. Comparative analysis of vitamin C in fresh fruits juice of Malus domestica, Citrus sinensi, Ananas comosus and Citrullus lanatus by iodometric titration. Int. J. Environ. Sci. Technol. 2015, 4, 17–22. [Google Scholar]
- Shamloo, M.M.; Sharifani, M.; Daraei Garmakhany, A.; Seifi, E. Alternation of secondary metabolites and quality attributes in Valencia Orange fruit (Citrus sinensis) as influenced by storage period and edible covers. J. Food Sci. Technol. 2015, 52, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT-Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Ho, S.-C.; Lin, C.-C. Investigation of heat treating conditions for enhancing the anti-inflammatory activity of Citrus fruit (Citrus reticulata) peels. J. Agric. Food Chem. 2008, 56, 7976–7982. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.; Monteiro, M. Simultaneous HPLC determination of flavonoids and phenolic acids profile in Pêra-Rio orange juice. Food Res. Int. 2018, 106, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Guo, Z.; Feng, X.; Huang, P.; Du, M.; Zalán, Z.; Kan, J. Distribution and natural variation of free, esterified, glycosylated, and insoluble-bound phenolic compounds in brocade orange (Citrus sinensis L. Osbeck) peel. Food Res. Int. 2022, 153, 110958. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid mandarin peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef]
- Singanusong, R.; Nipornram, S.; Tochampa, W.; Rattanatraiwong, P. Low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) and lime (Citrus aurantifolia) peels and the antioxidant. Food Anal. Methods 2015, 8, 1112–1123. [Google Scholar] [CrossRef]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.T.; Mawson, J.; Williams, M.A.K.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Yadav, S.K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, H.; Huang, Q.; An, F.; Song, H. Flash extraction optimization of low-temperature soluble pectin from passion fruit peel (Passiflora edulis f. flavicarpa) and its soft gelation properties. Food Bioprod. Process. 2020, 123, 409–418. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lee, M.-S.; Lin, C.-J.; Chen, M.-L. Dietary fiber content and composition of fruits in Taiwan. Asia Pac. J. Clin. Nutr. 1998, 7, 206–210. [Google Scholar]
- Oduntan, A.O.; Arueya, G.L. Design, formulation, and characterization of a potential ‘whole food’ using fibre rich orange (Citrus sinensis Lin) pomace as base. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100172. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Lech, K.; Tkacz, K.; Nowicka, P. Influence of different drying methods on the quality of Japanese quince fruit. LWT-Food Sci. Technol. 2019, 114, 108416. [Google Scholar] [CrossRef]
- Chen, M.L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Queimada, A.J.; Mota, F.L.; Pinho, S.P.; Macedo, E.A. Solubilities of biologically active phenolic compounds: Measurements and modeling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of drying methods on total phenolic contents and antioxidant capacity of the pomelo (Citrus grandis (L.) Osbeck) peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.-F.; Zhou, Z.-Q. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integ. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- Rafiq, S.; Singh, B.; Gat, Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J. Food Sci. Technol. 2019, 56, 2458–2466. [Google Scholar] [CrossRef]
- Chan, S.; Lee, C.; Yap, C.; Wan Aida, W.; Ho, C. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int. Food Res. J. 2009, 16, 203–213. [Google Scholar]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Naggy, S. Vitamin C contents of citrus fruit and their products. J. Agric. Food Chem. 1980, 28, 8–15. [Google Scholar] [CrossRef]
- Murcia, M.A.; López-Ayerra, B.; Martinez-Tomé, M.; Vera, A.; García-Carmona, F. Evolution of ascorbic acid and peroxidase during industrial processing of broccoli. J. Sci. Food Agric. 2000, 80, 1882–1886. [Google Scholar] [CrossRef]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus flavonoids, aaringin and aaringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.; Bahna, S.L. Hypersensitivity reactions to food additives. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, P.; Umamaheswari, M.; Asokkumar, K. In vitro antioxidant activities of leaves, fruits and peel extracts of Citrus. Int. J. Phytopharm. 2012, 2, 13–20. [Google Scholar]
- Kumaran, A.; Joel Karunakaran, R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Tomar, M.; Punia, S.; Singh, S.; Patil, S.; Singh, S.; Ilakiya, T.; Kaur, C.; Kennedy, J.F. Jackfruit seed slimy sheath, a novel source of pectin: Studies on antioxidant activity, functional group, and structural morphology. Carbohydr. Polym. Technol. Appl. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.-M.; Cheng, X.-C.; Wang, X.-D. Effect of drying pretreatment methods on structure and properties of pectins extracted from Chinese quince fruit. Int. J. Biol. Macromol. 2019, 137, 801–808. [Google Scholar] [CrossRef]
- Pichaiyongvongdee, S.; Haruenkit, R. Comparative studies of limonin and naringin distribution in different parts of pummelo (Citrus grandis (L.) Osbeck) cultivars grown in Thailand. Agric. Nat. Resour. 2009, 43, 28–36. [Google Scholar]
- Zhang, H.; Li, J.; Xia, J.; Lin, S. Antioxidant activity and physicochemical properties of an acidic polysaccharide from Morinda officinalis. Int. J. Biol. Macromol. 2013, 58, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Kumquat (Fortunella japonica Swingle) juice: Flavonoid distribution and antioxidant properties. Food Res. Int. 2011, 44, 2190–2197. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Chitrakar, B.; Jiang, H. Effect of combined drying method on phytochemical components, antioxidant capacity and hygroscopicity of Huyou (Citrus changshanensis) fruit. LWT-Food Sci. Technol. 2020, 123, 109102. [Google Scholar] [CrossRef]
- Jang, H.-D.; Chang, K.-S.; Chang, T.-C.; Hsu, C.-L. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010, 118, 554–558. [Google Scholar] [CrossRef]
- Rosamond, W.D. Dietary fiber and prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2002, 39, 57–59. [Google Scholar] [CrossRef]
- Dong, T.; Xia, R.; Wang, M.; Xiao, Z.; Liu, P. Changes in dietary fibre, polygalacturonase, cellulase of navel orange (Citrus sinensis (L.) Osbeck ‘Cara Cara’) fruits under different storage conditions. Sci. Hortic. 2008, 116, 414–420. [Google Scholar] [CrossRef]
- Hapdang, A.; Maruddin, F.; Malaka, R. Physical characteristics of ice cream with the addition of tangerines (Citrus reticulata). IOP Conf. Ser. Earth Environ. Sci. 2021, 788, 012105. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.A.L.; Estévez, A.M.A.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and Citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
| Solvents | Naringin | Hesperidin | Neohesperidin | Naringenin |
|---|---|---|---|---|
| H2O | 16.74 ± 4.88 e | 102.95 ± 12.54 e | 4.57 ± 1.83 d | 0.72 ± 0.16 c |
| 25% EtOH | 35.98 ± 1.83 d | 329.67 ± 21.23 d | 6.89 ± 2.31 c | 1.14 ± 0.71 c |
| 50% EtOH | 43.20 ± 2.36 c | 377.64 ± 14.29 c | 7.37 ± 1.96 c | 2.89 ± 0.59 b |
| 75% EtOH | 56.17 ± 3.32 b | 496.73 ± 9.83 b | 12.61 ± 2.21 b | 2.76 ± 0.32 b |
| 95% EtOH | 61.83 ± 0.79 a | 622.57 ± 34.50 a | 19.55 ± 3.85 a | 6.69 ± 1.08 a |
| Solvents/Treatment | ABTS (μmol TE/g DW) | DPPH (μmol TE/g DW) | FRAP (μmol TE/g DW) |
|---|---|---|---|
| H2O | 122.16 ± 1.48 b | 22.65 ± 0.27 b | 48.93 ± 4.24 b |
| 25% EtOH | 135.09 ± 5.24 a | 21.76 ± 0.08 c | 56.56 ± 2.19 a |
| 50% EtOH | 128.09 ± 6.84 ab | 22.36 ± 0.07 b | 58.47 ± 3.25 a |
| 75% EtOH | 134.14 ± 2.29 a | 23.43 ± 0.46 a | 58.69 ± 3.84 a |
| 95% EtOH | 97.84 ± 4.45 c | 23.73 ± 0.20 a | 26.54 ± 2.23 c |
| Extraction Solvents/Treatment | DPPH (μmol TE/g DW) | FRAP (μmol TE/g DW) |
|---|---|---|
| HEP | 0.039 ± 0.029 b | 13.03 ± 1.61 a |
| SEP | 0.004 ± 0.005 b | 9.79 ± 1.59 b |
| CEP | 0.043 ± 0.037 b | 6.44 ± 0.15 c |
| WEP | 0.219 ± 0.022 a | 15.04 ± 2.31 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-W.; Chen, Y.-L.; Park, S.H.; Yap, E.E.S.; Sung, W.-C. Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction. Foods 2024, 13, 2129. https://doi.org/10.3390/foods13132129
Chang Y-W, Chen Y-L, Park SH, Yap EES, Sung W-C. Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction. Foods. 2024; 13(13):2129. https://doi.org/10.3390/foods13132129
Chicago/Turabian StyleChang, Yu-Wei, Yen-Ling Chen, Sung Hoon Park, Encarnacion Emilia S. Yap, and Wen-Chieh Sung. 2024. "Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction" Foods 13, no. 13: 2129. https://doi.org/10.3390/foods13132129
APA StyleChang, Y.-W., Chen, Y.-L., Park, S. H., Yap, E. E. S., & Sung, W.-C. (2024). Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction. Foods, 13(13), 2129. https://doi.org/10.3390/foods13132129






