Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Experimental Design
2.3. Analysis of Fecal Parameters
2.4. Measurement of Intestinal Transit Rate
2.5. Histological Analysis
2.6. Analysis of Serotonin and Substance P Content in Colon Tissue
2.7. Gene Expression Level Analysis by qPCR
2.8. Western Blot Analysis
2.9. Short Chain Fatty Acid (SCFA) Analysis
2.10. Cecal Microbiota Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of Lactitol on Fecal Parameters in SD Rats with Loperamide-Induced Constipation
3.2. Effect of Lactitol on Gastrointestinal Transit Rate in SD Rats with Loperamide-Induced Constipation
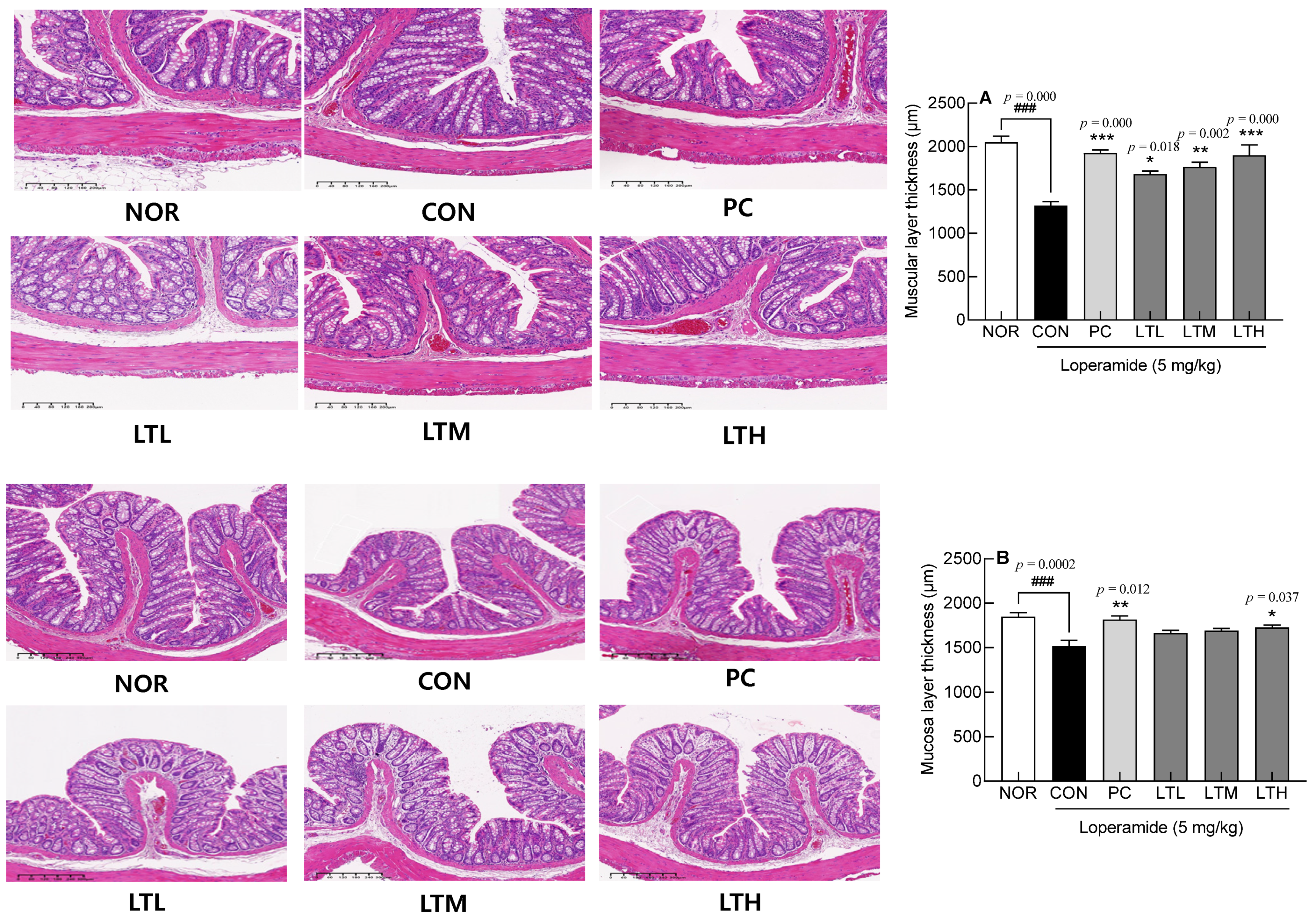
3.3. Intestinal Epithelial Cells through H&E Staining
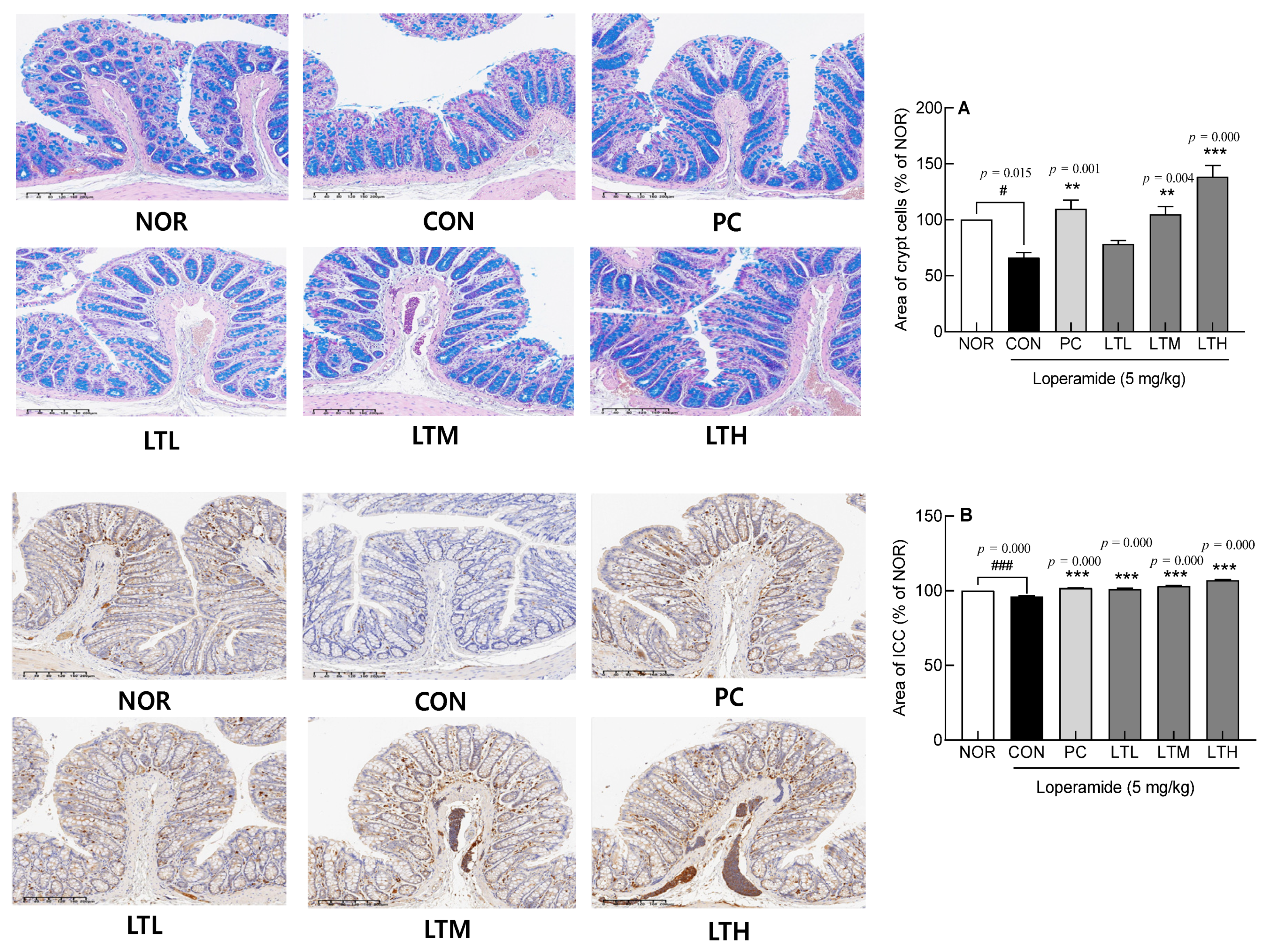
3.4. Effect of Lactitol on Intestinal Crypt Cells and Interstitial ICCs
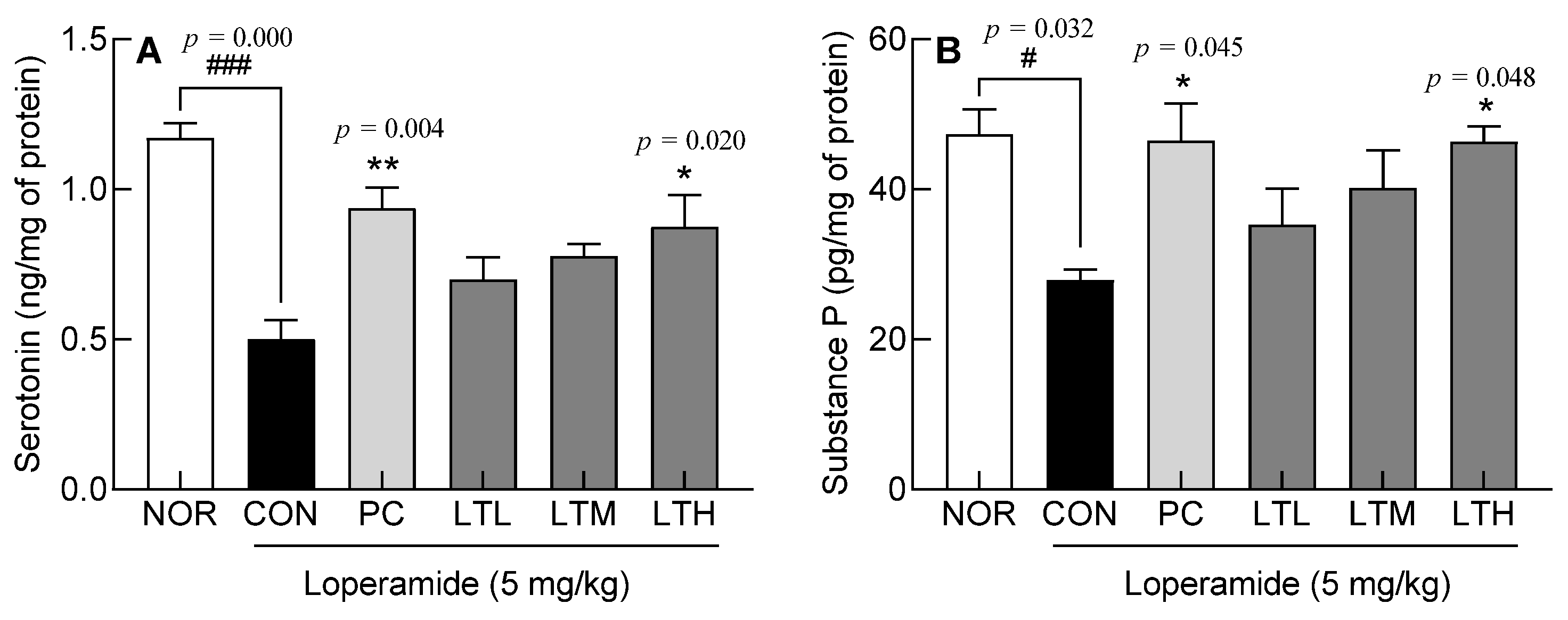
3.5. Effects of Lactitol on Serotonin and Substance P Content in Colon Tissue of SD rats with Loperamide-Induced Constipation
3.6. Effect of Lactitol on Gene Expression Levels of Constipation-Related Genes
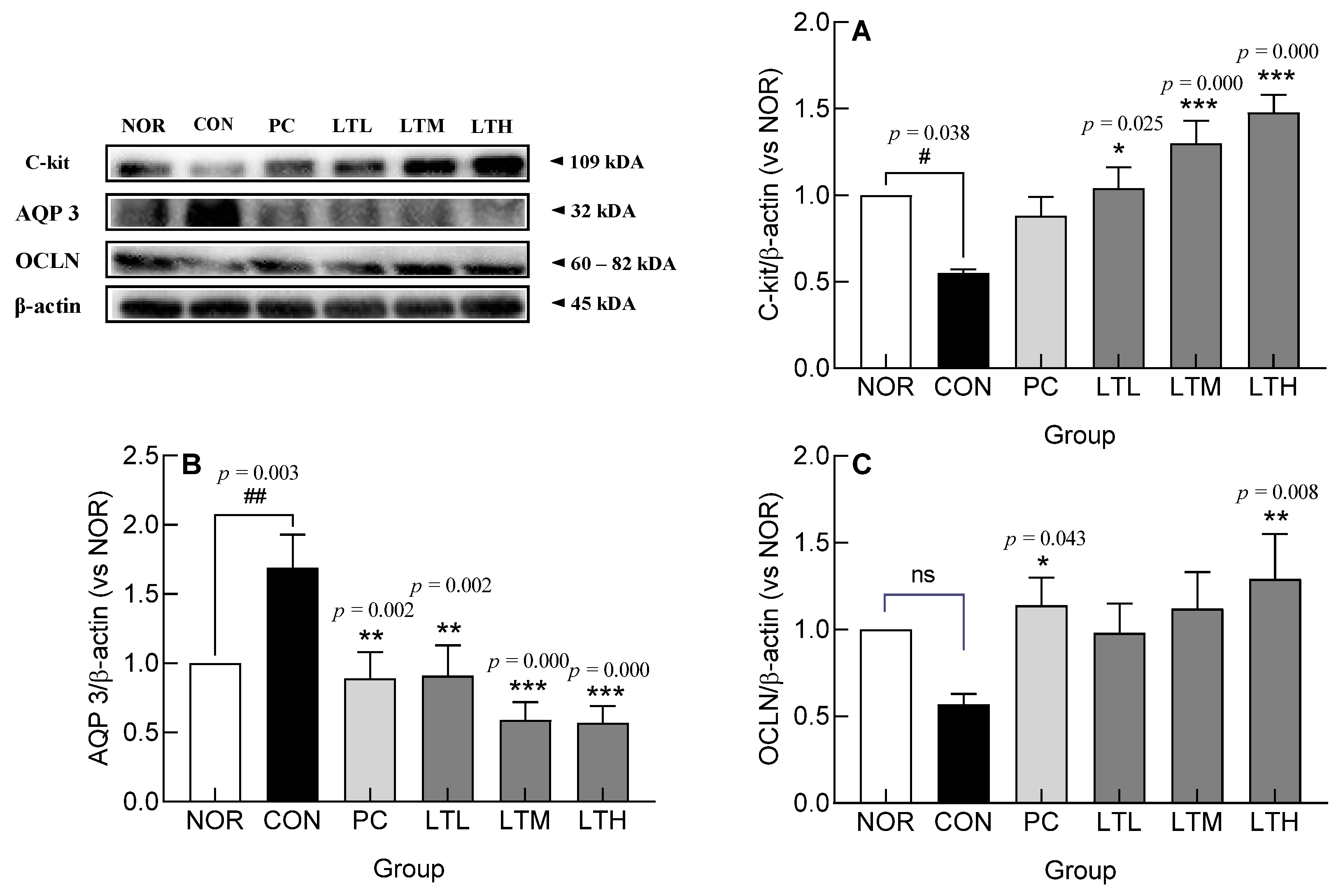
3.7. Effect of Lactitol on c-kit, AQP3, and OCLN Protein Levels in Colonic Tissue of SD Rats with Loperamide-Induced Constipation
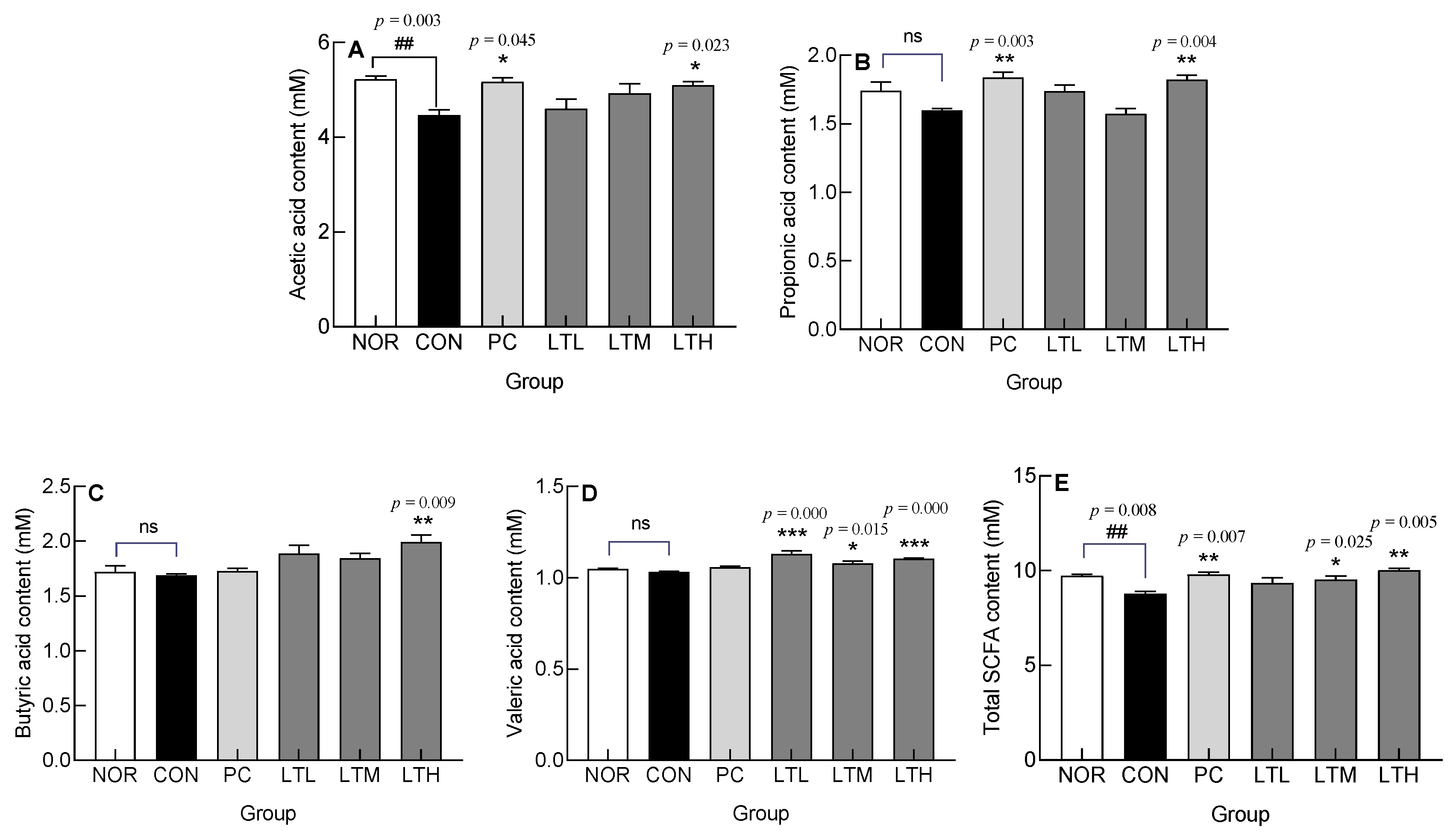
3.8. Effect of Lactitol on Short Chain Fatty Acids (SCFA) Content in Ceca of SD Rats with Loperamide-Induced Constipation
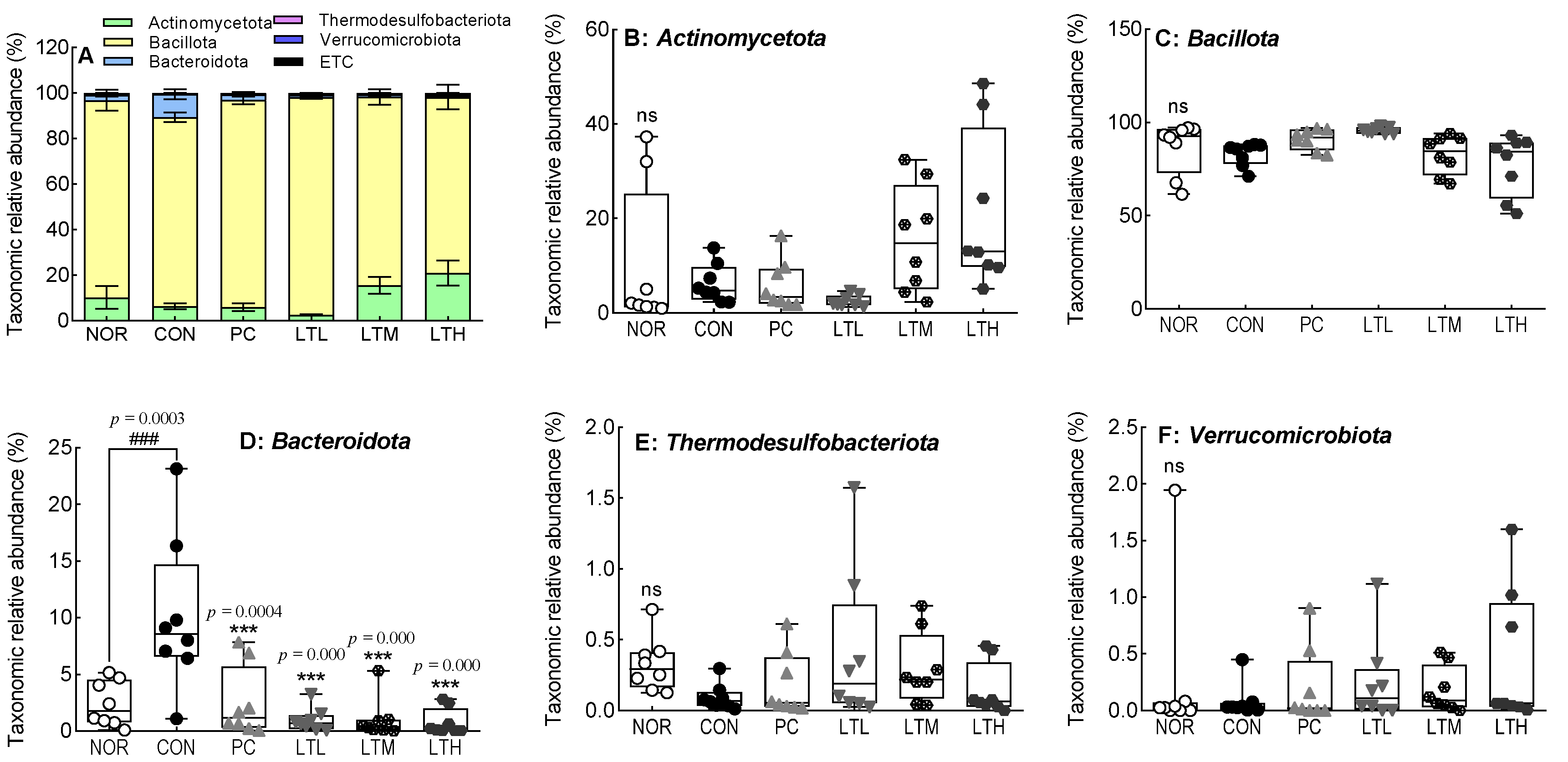
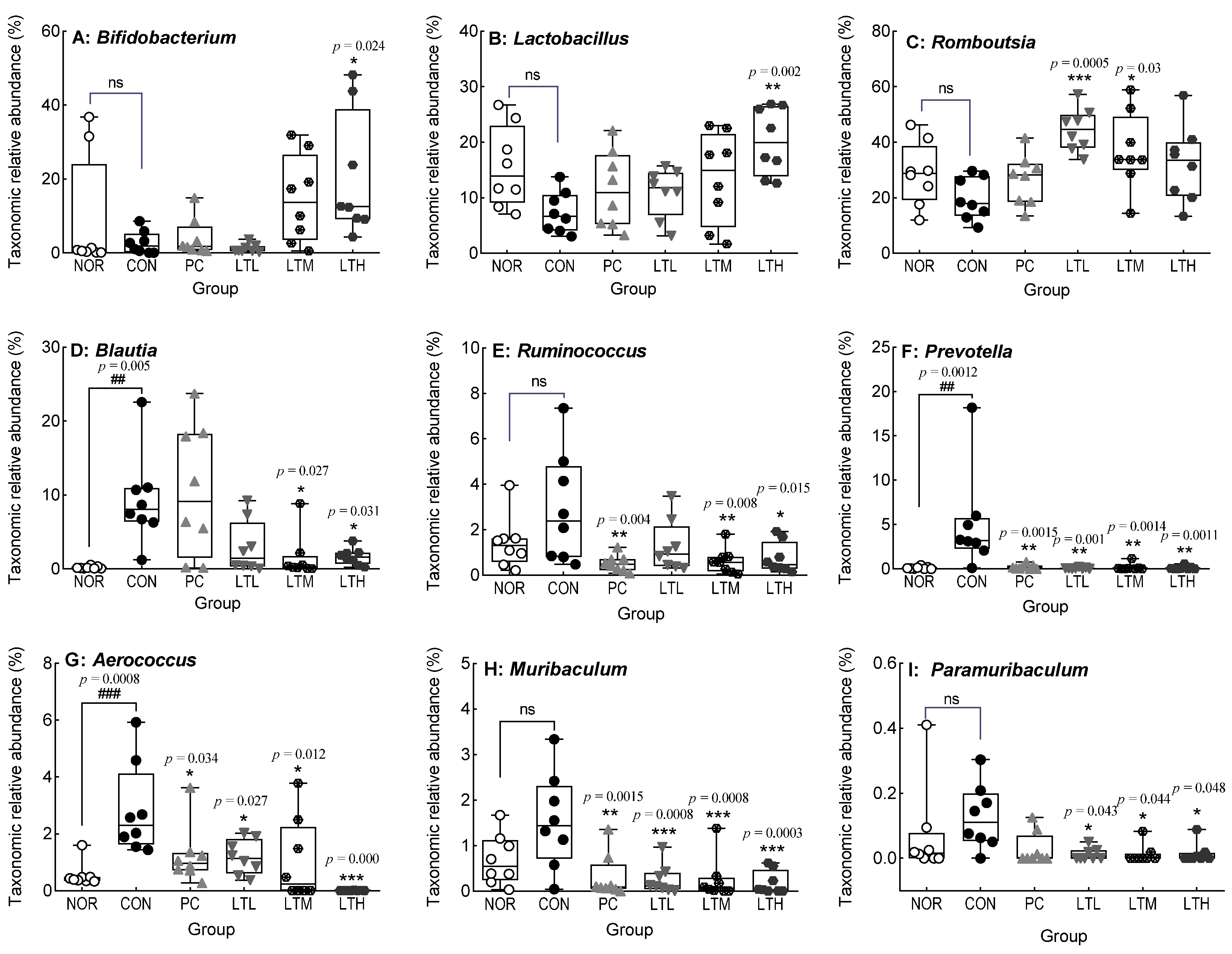
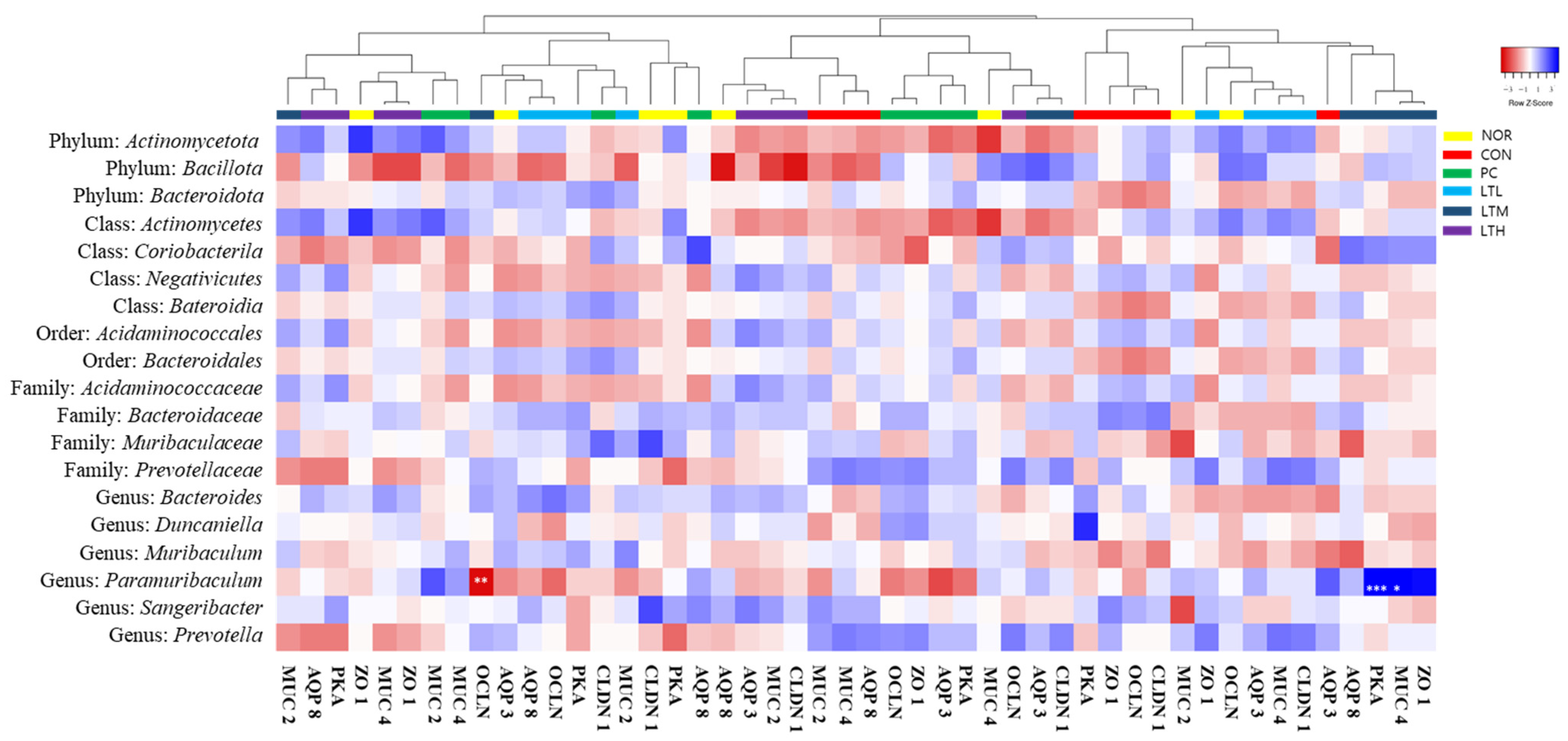
3.9. Effects of Lactitol on Cecal Microbiota of SD Rats with Loperamide-Induced Constipation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salari, N.; Ghasemianrad, M.; Ammari-Allahyari, M.; Rasoulpoor, S.; Shohaimi, S.; Mohammadi, M. Global prevalence of constipation in older adults: A systematic review and meta-analysis. Wien. Klin. Wochenschr. 2023, 135, 389–398. [Google Scholar] [CrossRef]
- Parkman, H.P.; Sharkey, E.; McCallum, R.W.; Hasler, W.L.; Koch, K.L.; Sarosiek, I.; Abell, T.L.; Kuo, B.; Shulman, R.J.; Grover, M.; et al. Constipation in patients with symptoms of gastroparesis: Analysis of symptoms and gastrointestinal transit. Clin. Gastroenterol. Hepatol. 2022, 20, 546–558. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, Y.J.; Shin, J.E.; Jung, H.K.; Park, S.Y.; Kang, S.J.; Song, K.H.; Kim, J.W.; Lim, H.C.; Park, H.S.; et al. 2022 Seoul consensus on clinical practice guidelines for functional constipation. J. Neurogastroenterol. Motil. 2023, 29, 271–305. [Google Scholar] [CrossRef]
- de Geus, A.; Koppen, I.J.; Flint, R.B.; Benninga, M.A.; Tabbers, M.M. An update of pharmacological management in children with functional constipation. Pediatr. Drugs 2023, 25, 343–358. [Google Scholar] [CrossRef]
- Sari, Y.P.; Candraruna, D.B. The potential of polysaccharides from various plants as constipation treatment. J. Appl. Food Technol. 2023, 10, 48–57. [Google Scholar] [CrossRef]
- Eum, J.B.; Rhee, K.H.; Cho, Y.K.; Byeon, J.S.; Myung, S.J.; Jung, H.Y.; Kim, J.H.; Kim, S.J.; Kim, J.E.; Son, H.J. Therapeutic effects of agio? 18 g per Day in patients with constipation dominant irritable bowel syndrome and chronic constipation. Korean J. Neurogastroenterol. Motil. 2008, 14, 115–121. [Google Scholar]
- Rao, S.S.; Lichtlen, P.; Habibi, S. Effects of lubiprostone, an intestinal secretagogue, on electrolyte homeostasis in chronic idiopathic and opioid-induced constipation. J. Clin. Gastroenterol. 2021, 55, 512–519. [Google Scholar] [CrossRef]
- Lee, B.E.; Lee, T.H.; Kim, S.-E.; Park, K.S.; Park, S.-Y.; Shin, J.E.; Jung, K.W.; Choi, S.C.; Hong, K.S.; Hong, S.N. Conventional laxatives. Korean J. Med. 2015, 88, 1–8. [Google Scholar] [CrossRef]
- Schiller, L. The therapy of constipation. Aliment. Pharmacol. Ther. 2001, 15, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E. Functional Constipation. In Sex/Gender-Specific Medicine in the Gastrointestinal Diseases; Kim, N., Ed.; Springer Nature: Singapore, 2022; pp. 259–272. [Google Scholar]
- Faruqui, A.A.; Joshi, C.; Balakumar, P.; Mahadevan, N.; Kumar, R.; Kumar, M. Lactitol: A review of its use in the treatment of constipation. Int. J. Recent Adv. Pharm. Sci. 2012, 2, 1–5. [Google Scholar]
- Li, X.Q.; Zhang, X.M.; Wu, X.; Lan, Y.; Xu, L.; Meng, X.C.; Li, J.N. Beneficial effects of lactitol on the composition of gut microbiota in constipated patients. J. Dig. Dis. 2020, 21, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Tennilä, J.; Ouwehand, A.C. Efficacy and tolerance of lactitol supplementation for adult constipation: A systematic review and meta-analysis. Clin. Exp. Gastroenterol. 2014, 7, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Maydeo, A. Lactitol or lactulose in the treatment of chronic constipation: Result of a systematic. J. Indian Med. Assoc. 2010, 108, 789–792. [Google Scholar] [PubMed]
- Han, S.H.; Park, K.; Kim, E.Y.; Ahn, S.H.; Lee, H.S.; Suh, H.J. Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats. BMC Complement. Altern. Med. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, E.J.; Park, C.; Lee, J.S.; Kim, W.J.; Yu, K.W.; Suh, H.J.; Ahn, Y.; Moon, S.K. Modulation of gut microbiota ecosystem by a glucan-rich snail mucin heteropolysaccharide attenuates loperamide-induced constipation. Int. J. Biol. Macromol. 2023, 253, 126560. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef] [PubMed]
- Key, M. Immunohistochemistry staining methods. In Education Guide Immunohistochemical Staining Methods Fourth Edition; Dako Denmark A/S: Glostrup, Denmark, 2006; p. 57. [Google Scholar]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Singh, V.; Muthuramalingam, K.; Kim, Y.M.; Park, S.; Kim, S.H.; Lee, J.; Hyun, C.; Unno, T.; Cho, M. Synbiotic supplementation with prebiotic derived β-(1,3/1,6)-glucan and probiotic concoction benefits gut microbiota and its associated metabolic activities. Appl. Biol. Chem. 2021, 64, 7. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.D. Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases. J. Transl. Intern. Med. 2023, 11, 115–127. [Google Scholar] [CrossRef]
- Hibberd, T.J.; Ramsay, S.; Spencer-Merris, P.; Dinning, P.G.; Zagorodnyuk, V.P.; Spencer, N.J. Circadian rhythms in colonic function. Front. Physiol. 2023, 14, 1239278. [Google Scholar] [CrossRef]
- Gilet, M.; Eutamene, H.; Han, H.; Kim, H.W.; Bueno, L. Influence of a new 5-HT4 receptor partial agonist, YKP10811, on visceral hypersensitivity in rats triggered by stress and inflammation. Neurogastroenterol. Motil. 2014, 26, 1761–1770. [Google Scholar] [CrossRef]
- Bisht, P.; Dagar, N.; Kumar, N.; Velayutham, R.; Arumugam, S. Potential targets in constipation research: A review. Curr. Drug Targets 2023, 24, 247–260. [Google Scholar]
- Camilleri, M. Serotonergic modulation of visceral sensation: Lower gut. Gut 2002, 51 (Suppl. S1), i81–i86. [Google Scholar] [CrossRef]
- Silberstein, C.; Kierbel, A.; Amodeo, G.; Zotta, E.; Bigi, F.; Berkowski, D.; Ibarra, C. Functional characterization and localization of AQP3 in the human colon. Braz. J. Med. Biol. Res. 1999, 32, 1303–1313. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Aquaporin: Targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr. 2022, 106, 167–180. [Google Scholar] [CrossRef]
- Leforestier, G.; Blais, A.; Blachier, F.; Marsset-Baglieri, A.; Davila-Gay, A.M.; Perrin, E.; Tome, D. Effects of galacto-oligosaccharide ingestion on the mucosa-associated mucins and sucrase activity in the small intestine of mice. Eur. J. Nutr. 2009, 48, 457–464. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Ma, X.; Yu, Y.; Yu, X.; Chen, X.; Suo, H. Efficacy of Bacillus coagulans BC01 on loperamide hydrochloride-induced constipation model in Kunming mice. Front. Nutr. 2022, 9, 964257. [Google Scholar] [CrossRef]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Fang, F.F.; Liu, Y.; Xiong, Y.L.; Li, X.Y.; Li, G.P.; Jiang, Y.D.; Hou, X.H.; Song, J. Slowed intestinal transit induced by less mucus in intestinal goblet cell piezo1-deficient mice through impaired epithelial homeostasis. Int. J. Mol. Sci. 2023, 24, 14377. [Google Scholar] [CrossRef]
- Takaki, M. Gut pacemaker cells: The interstitial cells of Cajal (ICC). J. Smooth Muscle Res. 2003, 39, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front. Immunol. 2021, 12, 4783. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Muller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Probert, H.M.; Apajalahti, J.H.; Rautonen, N.; Stowell, J.; Gibson, G.R. Polydextrose, lactitol, and fructo-oligosaccharide fermentation by colonic bacteria in a three-stage continuous culture system. Appl. Environ. Microbiol. 2004, 70, 4505–4511. [Google Scholar] [CrossRef]
- Scheppach, W.; Weiler, F. The butyrate story: Old wine in new bottles? Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 563–567. [Google Scholar] [CrossRef]
- Piva, A.; Panciroli, A.; Meola, E.; Formigoni, A. Lactitol enhances short-chain fatty acid and gas production by swine cecal microflora to a greater extent when fermenting low rather than high fiber diets. J. Nutr. 1996, 126, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Yang, F.; Liu, W.; Bai, H.; Ma, J.; Ma, X.; Zhang, J.; Fang, Y.; Miao, Y.; et al. The composition of intestinal microbiota and its association with functional constipation of the elderly patients. Future Microbiol. 2020, 15, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Klewicki, R.; Klewicka, E. Antagonistic activity of lactic acid bacteria as probiotics against selected bacteria of the Enterobaceriacae family in the presence of polyols and their galactosyl derivatives. Biotechnol. Lett. 2004, 26, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, L.; Wu, Z.; Chen, H.; Fu, S. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J. Infect. 2007, 54, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101742. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Fang, B.; Pang, G.F.; Zhang, M.; Ren, F.Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, T.; Wu, X.; Chen, X.; Wang, J.; Ba, Y. Polygalae radix oligosaccharide esters may relieve depressive-like behavior in rats with chronic unpredictable mild stress via modulation of gut microbiota. Int. J. Mol. Sci. 2023, 24, 13877. [Google Scholar] [CrossRef]
- Zhang, M.M.; Gong, Z.C.; Zhao, Q.; Xu, D.Q.; Fu, R.J.; Tang, Y.P.; Chen, Y.Y. Time-dependent laxative effect of sennoside A, the core functional component of rhubarb, is attributed to gut microbiota and aquaporins. J. Ethnopharmacol. 2023, 311, 116431. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Peng, X.; Yi, X.; Deng, N.; Liu, J.; Tan, Z.; Cai, Y. Zhishi Daozhi decoction alleviates constipation induced by a high-fat and high-protein diet via regulating intestinal mucosal microbiota and oxidative stress. Front. Microbiol. 2023, 14, 1214577. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; He, C.; Hua, R.; Liang, C.; Wang, B.; Du, Y.; Xin, S.; Guo, Y.; Gao, L.; Zhang, L.; et al. Underlying beneficial effects of Rhubarb on constipation-induced inflammation, disorder of gut microbiome and metabolism. Front. Pharmacol. 2022, 13, 1048134. [Google Scholar] [CrossRef]
- Liu, D.; Li, T.; Zheng, H.; Yin, X.; Chen, M.; Liao, Z.; Hu, M.; Luo, Z.; Lu, B.; Li, Y. Study on alterations of physiological functions in aged constipation rats with fluid-deficiency based on metabonomic and microbiology analysis. RSC Adv. 2017, 7, 48136–48150. [Google Scholar] [CrossRef]
- Liu, T.X.; Zhang, M.T.; Asif, I.M.; Wu, Y.L.; Li, B.; Wang, L. The regulatory effects of fucoidan and laminarin on functional dyspepsia mice induced by loperamide. Food Funct. 2023, 14, 6513–6525. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Hou, Y.; Zhao, Y. Alleviating effects of gut micro-ecologically regulatory treatments on mice with constipation. Front. Microbiol. 2022, 13, 956438. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.H.; Kim, S.M.; Suh, H.J.; Gim, M.; Shin, H.; Jang, H.; Choi, H.-S.; Han, S.H.; Chang, Y.B. Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota. Foods 2024, 13, 2128. https://doi.org/10.3390/foods13132128
Jang JH, Kim SM, Suh HJ, Gim M, Shin H, Jang H, Choi H-S, Han SH, Chang YB. Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota. Foods. 2024; 13(13):2128. https://doi.org/10.3390/foods13132128
Chicago/Turabian StyleJang, Joo Hyun, Sang Min Kim, Hyung Joo Suh, Minchul Gim, Hoyeon Shin, Hyunsook Jang, Hyeon-Son Choi, Sung Hee Han, and Yeok Boo Chang. 2024. "Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota" Foods 13, no. 13: 2128. https://doi.org/10.3390/foods13132128
APA StyleJang, J. H., Kim, S. M., Suh, H. J., Gim, M., Shin, H., Jang, H., Choi, H.-S., Han, S. H., & Chang, Y. B. (2024). Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota. Foods, 13(13), 2128. https://doi.org/10.3390/foods13132128






