Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
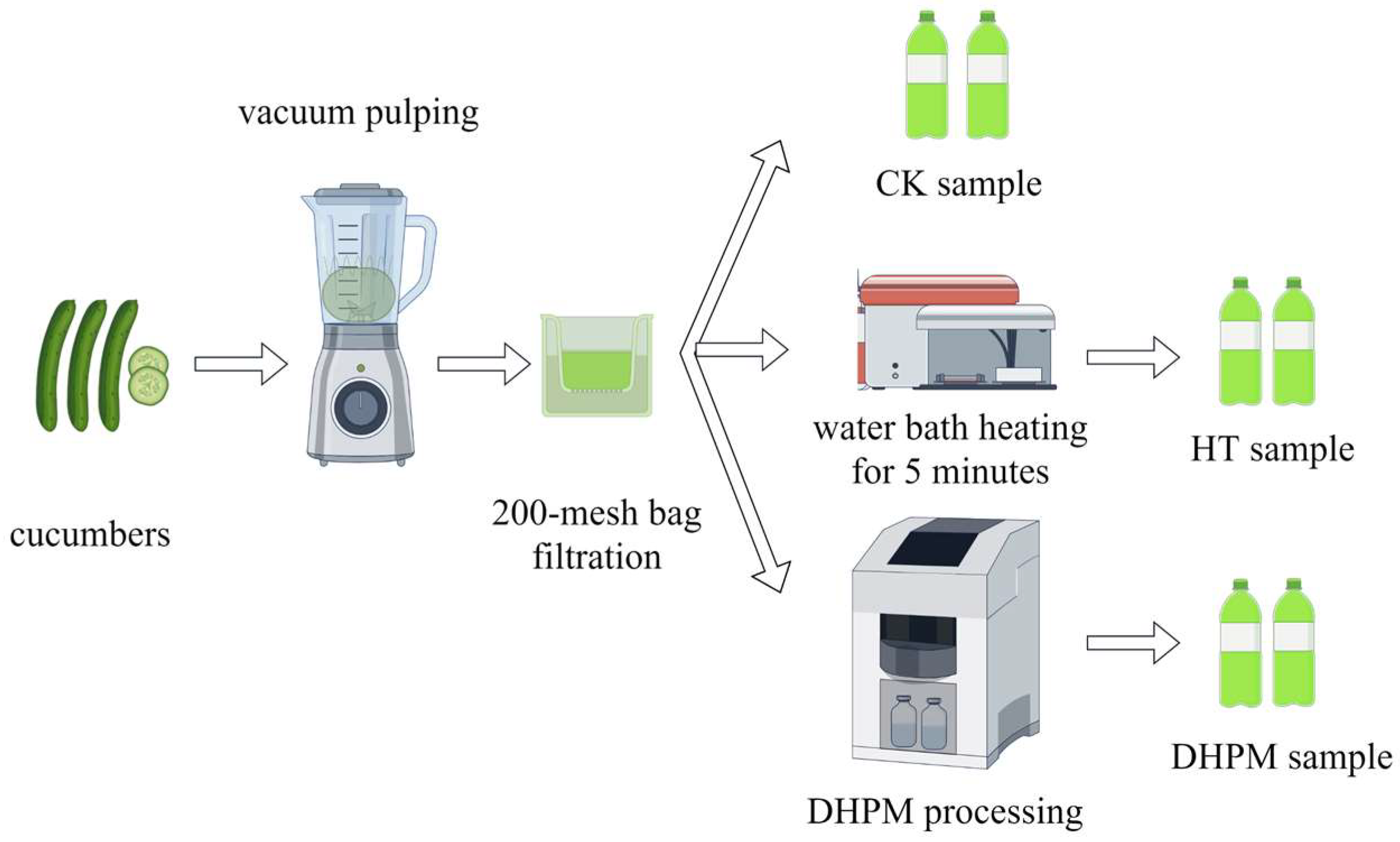
2.2. Preparation of Samples
2.2.1. Preparation of Cucumber Juice
2.2.2. DHPM
2.3. Microbial Analysis
2.4. Determination of Titratable Acidity, pH, and Total Soluble Solids
2.5. Color
2.6. Chlorophyll Content
2.7. Ascorbic Acid
2.8. Determination of Total Phenolic
2.9. Stability Indicators
2.9.1. Turbidity
2.9.2. Particle Size
2.9.3. Rheological Properties
2.10. GC-IMS Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
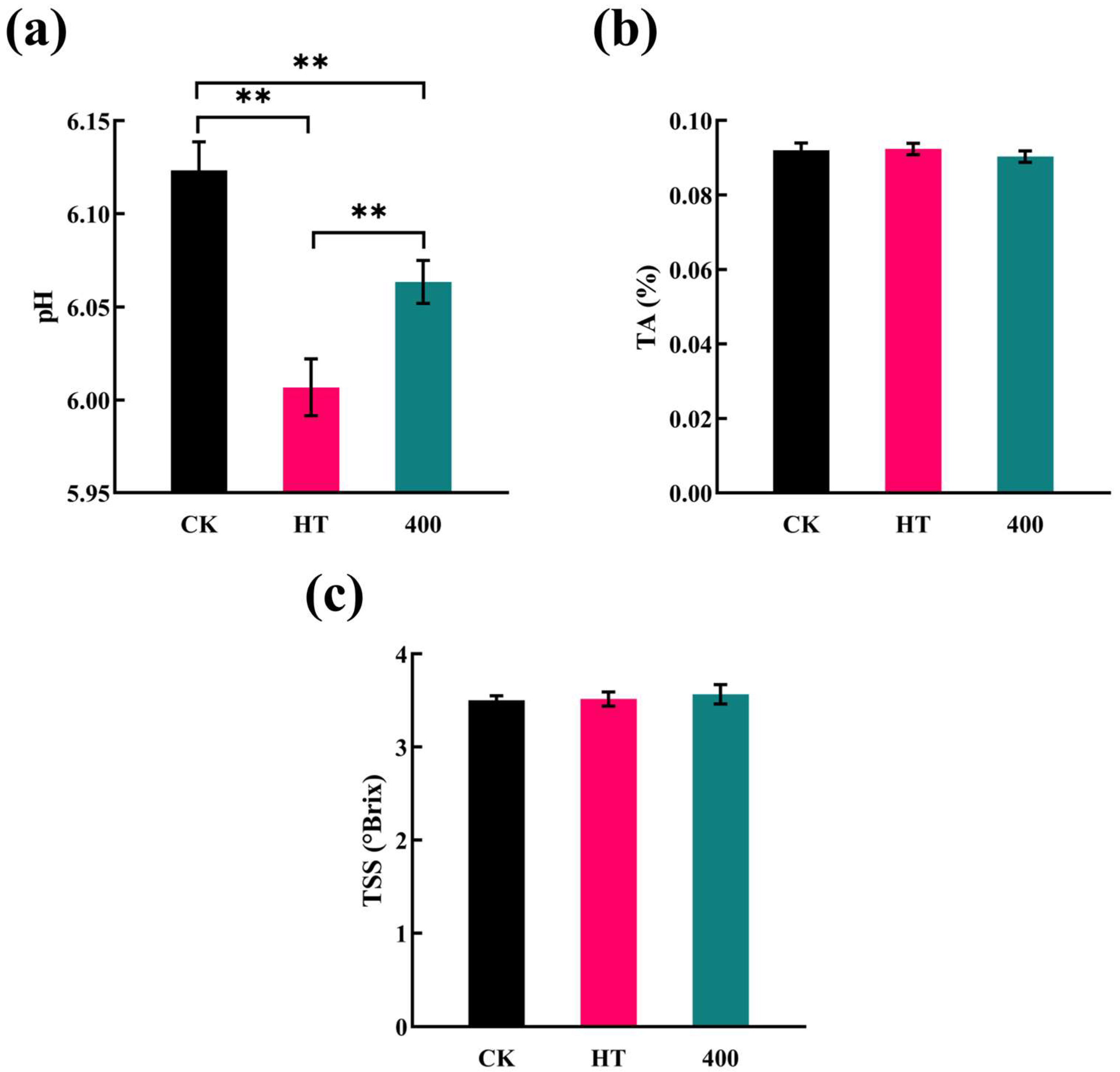
3.2. TA, pH, and TSS
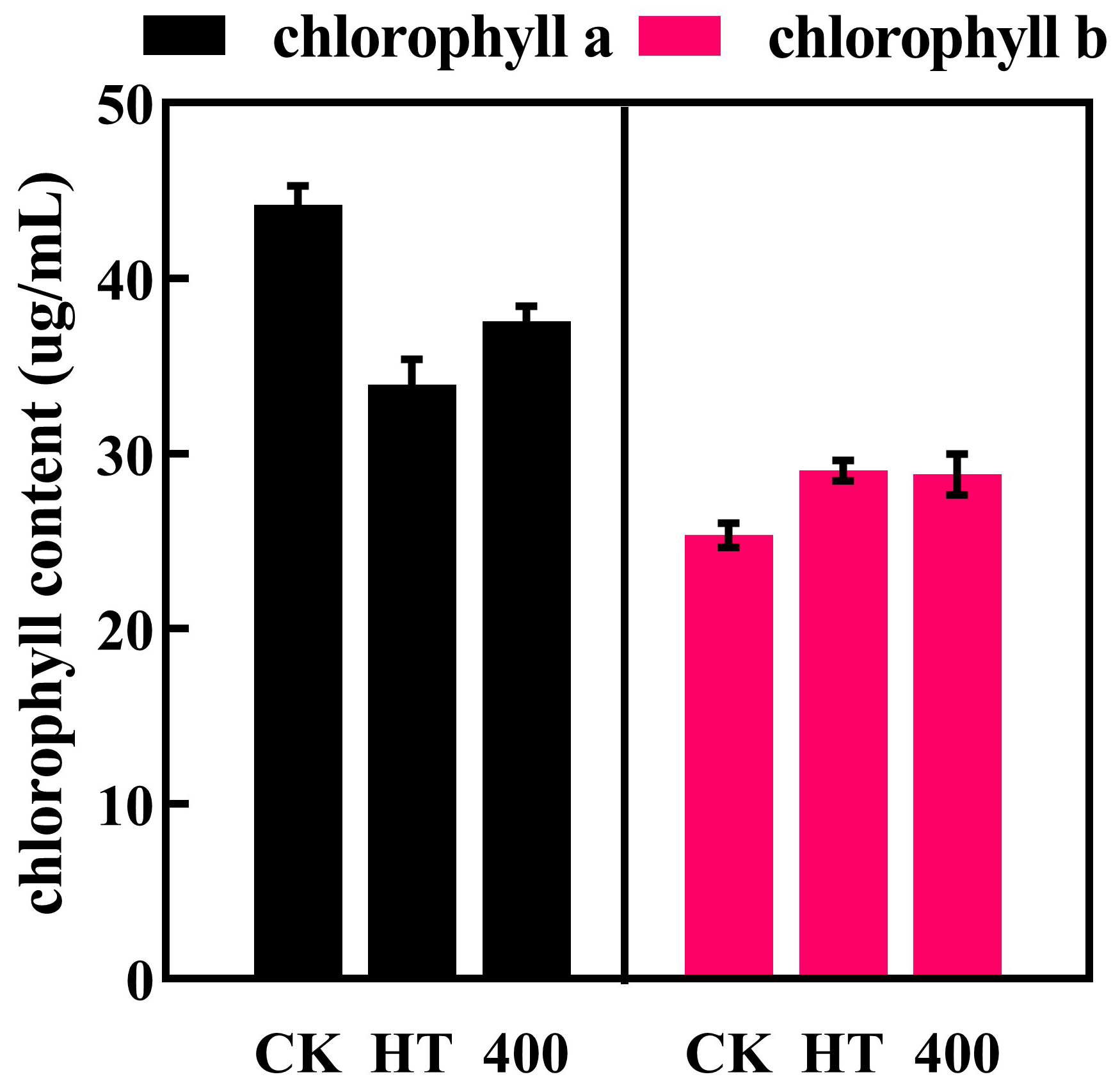
3.3. Color Analysis and Chlorophyll a b
3.4. Ascorbic Acid and Total Phenols
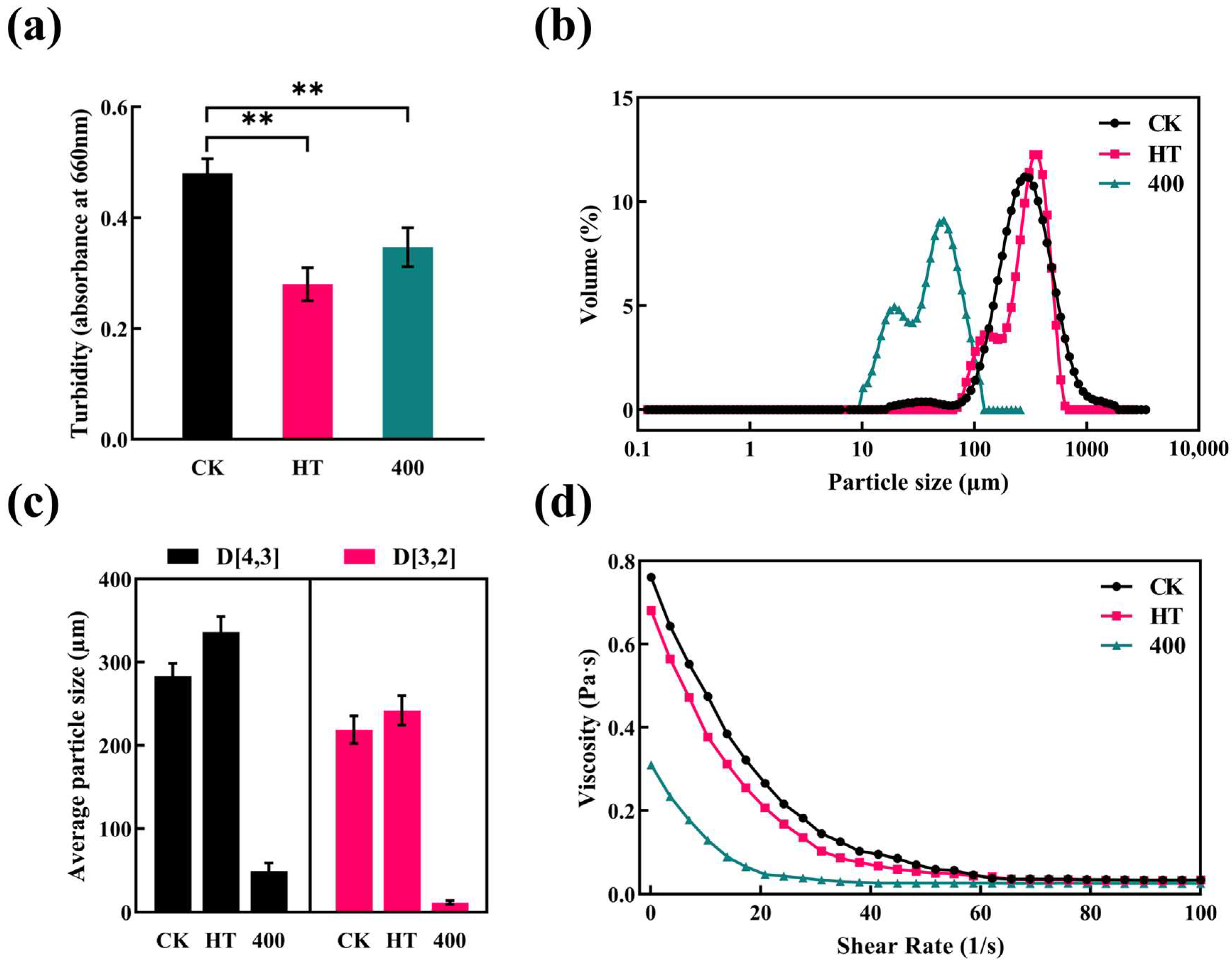
3.5. Turbidity, PSD, and Rheological Properties
3.6. GC-IMS Analysis
3.6.1. Volatile Components
3.6.2. Two-Dimensional Mapping
3.6.3. Analysis of GC-IMS Fingerprints of NFC Cucumber Juice
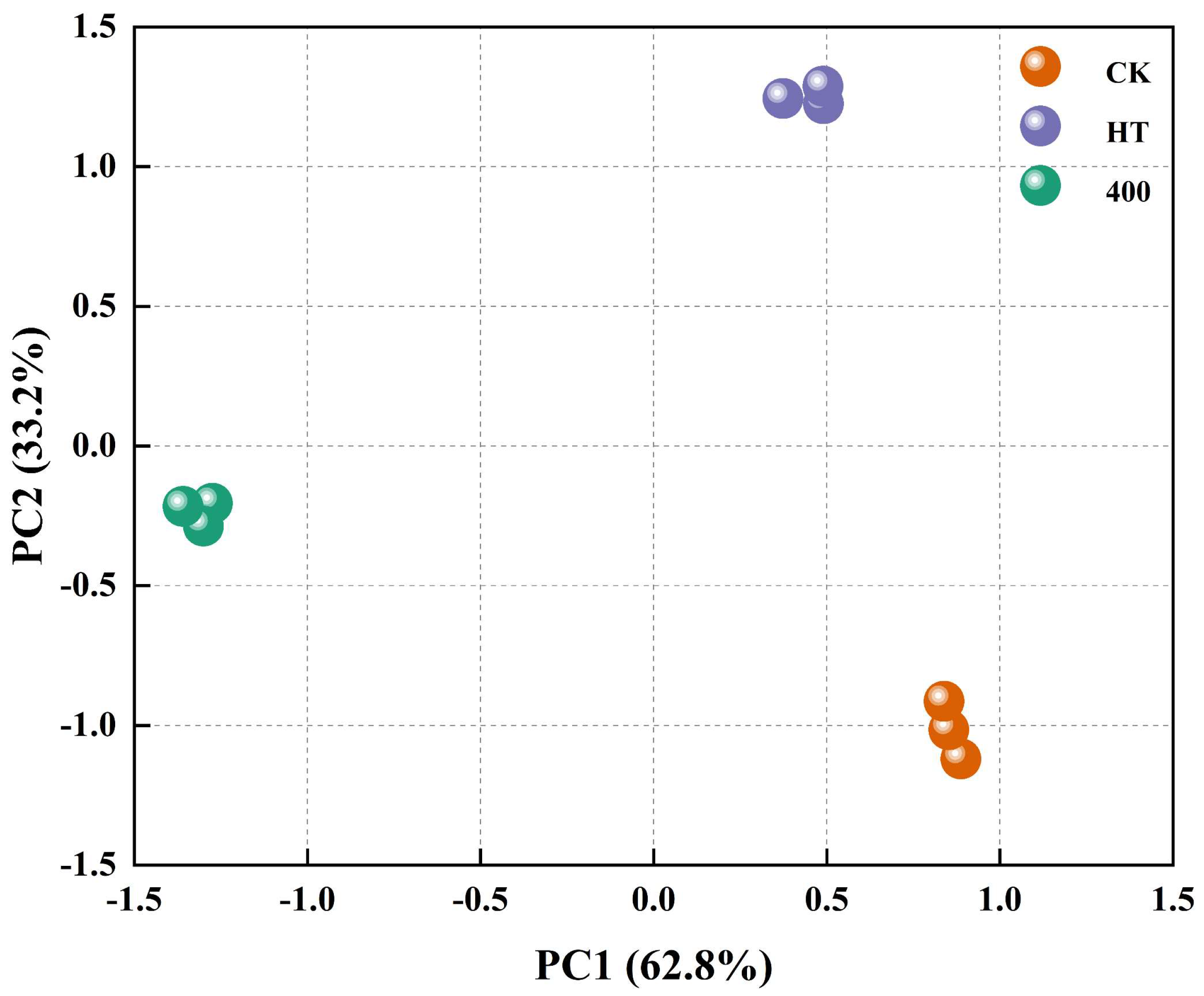
3.6.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Liu, H.; Sun, R.; Zhao, Y.; Xing, R.; Yu, N.; Deng, T.; Ni, X.; Chen, Y. Volatolomics approach for authentication of not-from-concentrate (NFC) orange juice based on characteristic volatile markers using headspace solid phase microextraction (HS-SPME) combined with GC-MS. Food Control 2022, 136, 108856. [Google Scholar] [CrossRef]
- Teixeira, J.C.; Ribeiro, C.; Simoes, R.; Alegria, M.J.; Mateus, N.; de Freitas, V.; Perez-Gregorio, R.; Soares, S. Characterization of the Effect of a Novel Production Technique for ’Not from Concentrate’ Pear and Apple Juices on the Composition of Phenolic Compounds. Plants 2023, 12, 3397. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Chen, L.; Ban, Z.; Zheng, Y.; Jiang, Y.; Jiang, Y.; Li, X. Effects of Fruit Storage Temperature and Time on Cloud Stability of Not from Concentrated Apple Juice. Foods 2022, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Kosiński, J.; Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Jasińska, U.T.; Woźniak, Ł.; Kaniewska, B.; Marszałek, K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Appl. Sci. 2024, 14, 662. [Google Scholar] [CrossRef]
- Gan, X.; Ma, Q.; Wang, L.; Liu, W.; Chen, Z.; Wang, W.; Wang, J.; Mu, J. Physicochemical, sensory characterisation and volatile components of 16 NFC pear juice. J. Food Meas. Charact. 2023, 17, 3534–3547. [Google Scholar] [CrossRef]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT 2021, 148, 111668. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, L.; Wang, Y.; Liao, X. Potential of high-pressure processing and high-temperature/short-time thermal processing on microbial, physicochemical and sensory assurance of clear cucumber juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 51–58. [Google Scholar] [CrossRef]
- Mohd Rosli, N.N.H.; Harun, N.H.; Abdul Rahman, R.; Ngadi, N.; Samsuri, S.; Amran, N.A.; Safiei, N.Z.; Ab Hamid, F.H.; Zakaria, Z.Y.; Jusoh, M. Preservation of total phenolic content (TPC) in cucumber juice concentrate using non-thermal Progressive Freeze Concentration: Quantitative design characteristics and process optimization. J. Clean. Prod. 2022, 330, 129705. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, N.; Huang, S.; Kan, J.; Zhang, F. In vitro digestion and structural properties of rice starch modified by high methoxyl pectin and dynamic high-pressure microfluidization. Carbohydr. Polym. 2021, 274, 118649. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Meng, Y.; Liu, S.; Ding, Y.; Zhou, X.; Ding, Y. Synergistic effect of microfluidization and transglutaminase cross-linking on the structural and oil-water interface functional properties of whey protein concentrate for improving the thermal stability of nanoemulsions. Food Chem. 2023, 408, 135147. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Xing, K.; Huang, Z.; Elfalleh, W.; Zhang, H.; Yu, D. Microfluidization of soybean protein isolate-tannic acid complex stabilized emulsions: Characterization of emulsion properties, stability and in vitro digestion properties. Food Chem. 2024, 430, 137065. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng. Rev. 2020, 13, 490–508. [Google Scholar] [CrossRef]
- Morata, A.; Guamis, B. Use of UHPH to Obtain Juices With Better Nutritional Quality and Healthier Wines With Low Levels of SO2. Front. Nutr. 2020, 7, 598286. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Huang, L.; Wang, H. Effects of dynamic high pressure microfluidization on the physical and chemical properties of carrot (Daucus carota L.). Bangladesh J. Bot. 2024, 53, 123–129. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.; Li, J.; Zhang, L.; Zhang, J.; Zong, W.; Mo, W. Dynamic high pressure microfluidization (DHPM): Physicochemical properties, nutritional constituents and microorganisms of yam juice. Czech J. Food Sci. 2021, 39, 217–225. [Google Scholar] [CrossRef]
- Abliz, A.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Effect of dynamic high pressure microfluidization treatment on physical stability, microstructure and carotenoids release of sea buckthorn juice. LWT 2021, 135, 110277. [Google Scholar] [CrossRef]
- GB 4789.2-2022; Food Microbiological Examination: Determination of Aerobic Plate Count. National Standards of the People’s Republic of China: Beijing, China, 2022.
- GB 4789.15-2016; Food Microbiological Examination: Enumeration of Moulds and Yeasts. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
- Tighiceanu, C.; Bulai, E.R.; Iatcu, O.C.; Dulucheanu, C.; Nemtoi, A. Effect of Vegetable Juices on Properties of Two Resin Composites Used for Dental Caries Management. Medicina 2023, 59, 774. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, X.; Han, W.; Wu, X.; Wang, Y.; Hu, X.; Ling, J.; Liao, X. Novel application of CO2-assisted high pressure processing in cucumber juice and apple juice. LWT 2018, 96, 491–498. [Google Scholar] [CrossRef]
- GB 5009.86-2016; Determination of Ascorbic Acid in Foods. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Rizvi, N.B.; Fatima, A.; Busquets, R.; Khan, M.R.; Ashraf, S.; Khan, M.S.; Oz, F. Effect of the Media in the Folin-Ciocalteu Assay for the Analysis of the Total Phenolic Content of Olive Products. Food Anal. Methods 2023, 16, 1627–1634. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Jiang, Y.; Hu, X.; Yi, J. A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing. Foods 2023, 12, 581. [Google Scholar] [CrossRef]
- Han, S.H.; Zhu, J.K.; Shao, L.; Yue, C.H.; Li, P.Y.; Bai, Z.Y.; Luo, D.L. Effects of Ultrasonic Treatment on Physical Stability of Lily Juice: Rheological Behavior, Particle Size, and Microstructure. Foods 2024, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWT 2023, 187, 115322. [Google Scholar] [CrossRef]
- Xi, B.-N.; Zhang, J.-J.; Xu, X.; Li, C.; Shu, Y.; Zhang, Y.; Shi, X.; Shen, Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2024, 435, 137547. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Y.; Zou, B.; Yu, Y.; Yang, J.; Xu, Y.; Chen, X.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Xie, J.; Yuan, H.; Wang, L.; Liu, F.; Deng, Y.; Jiang, Y.; Yang, Y. Characterization of volatile metabolites in Pu-erh teas with different storage years by combining GC-E-Nose, GC–MS, and GC-IMS. Food Chem. X 2023, 18, 100693. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Xi, J. Dynamic high pressure microfluidization-assisted extraction of plant active ingredients: A novel approach. Crit. Rev. Food Sci. Nutr. 2023, 63, 12413–12421. [Google Scholar] [CrossRef]
- Reverter-Carrión, L.; Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Inactivation study of Bacillus subtilis, Geobacillus stearothermophilus, Alicyclobacillus acidoterrestris and Aspergillus niger spores under Ultra-High Pressure Homogenization, UV-C light and their combination. Innov. Food Sci. Emerg. Technol. 2018, 48, 258–264. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, M.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Effect of ultra-high pressure homogenization on microorganism and quality of composite pear juice. Food Sci. Nutr. 2022, 10, 3072–3084. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F.; et al. (Ultra) High Pressure Homogenization Potential on the Shelf-Life and Functionality of Kiwifruit Juice. Front. Microbiol. 2019, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G. Application of ultrasound and high-pressure homogenization against high temperature-short time in peach juice. J. Food Process Eng. 2019, 42, e12997. [Google Scholar] [CrossRef]
- Arakawa, G.Y.; Yokoi, K.J. Application of multiple ultra-high-pressure homogenization to the pasteurization process of Japanese rice wine, sake. J. Biosci. Bioeng. 2023, 136, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M.; Marszałek, K.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 102279. [Google Scholar] [CrossRef]
- Ravichandran, C.; Jayachandran, L.E.; Kothakota, A.; Pandiselvam, R.; Balasubramaniam, V.M. Influence of high pressure pasteurization on nutritional, functional and rheological characteristics of fruit and vegetable juices and purees-an updated review. Food Control 2023, 146, 109516. [Google Scholar] [CrossRef]
- Rolandelli, G.; Favre, L.C.; Mshicileli, N.; Vhangani, L.N.; Farroni, A.E.; van Wyk, J.; Buera, M.d.P. The complex dependence of non-enzymatic browning development on processing conditions in maize snacks. LWT 2021, 147, 111636. [Google Scholar] [CrossRef]
- Sajib, M.; Undeland, I. Towards valorization of herring filleting by-products to silage 2.0: Effect of temperature and time on lipid oxidation and non-enzymatic browning reactions. LWT 2020, 127, 109441. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wu, S.H.; Zong, W. Comparison of the Influence of Dynamic High-Pressure Microfluidization and Conventional Homogenization on the Quality of Kiwi Fruit Juice. Appl. Eng. Agric. 2018, 34, 1039–1045. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef] [PubMed]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. Ananassa) juice. LWT Food Sci. Technol. 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, Y.; Bi, J.; Liu, X.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Change of the rheological properties of mango juice by high pressure homogenization. LWT Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Combined effects of ultra-high pressure homogenization and short-wave ultraviolet radiation on the properties of cloudy apple juice. LWT 2021, 136, 110286. [Google Scholar] [CrossRef]
- Rossi, S.; Burgess, P.; Jespersen, D.; Huang, B. Heat-Induced Leaf Senescence Associated with Chlorophyll Metabolism in Bentgrass Lines Differing in Heat Tolerance. Crop Sci. 2017, 57, S-169–S-178. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Zheng, Q.; Hu, X.; Zhang, Y.; Liao, X. Effects of high hydrostatic pressure on color of spinach purée and related properties. J. Sci. Food Agric. 2012, 92, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, J.; He, M.; Tian, H.; Qi, X.; Xu, Q.; Chen, X. Transcriptome profiling reveals key genes related to astringency during cucumber fruit development. 3 Biotech 2019, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Gan, Z.; Nie, J.; Liu, H.; Wang, Z.; Sui, X. Comprehensive Characterization of Fruit Volatiles and Nutritional Quality of Three Cucumber (Cucumis sativus L.) Genotypes from Different Geographic Groups after Bagging Treatment. Foods 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jacobo, Á.; Saldo, J.; Rüfer, C.E.; Guamis, B.; Roig-Sagués, A.X.; Gervilla, R. Aseptically packaged UHPH-treated apple juice: Safety and quality parameters during storage. J. Food Eng. 2012, 109, 291–300. [Google Scholar] [CrossRef]
- He, X.-H.; Luo, S.-J.; Chen, M.-S.; Xia, W.; Chen, J.; Liu, C.-M. Effect of industry-scale microfluidization on structural and physicochemical properties of potato starch. Innov. Food Sci. Emerg. Technol. 2020, 60, 102278. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, W.; Ge, Z.; Zhang, L.; Li, C.; Zhang, B.; Zong, W. Comparison of the effects of dynamic high-pressure microfluidization and conventional homogenization on the quality of peach juice. J. Sci. Food Agric. 2019, 99, 5994–6000. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.S.; Augusto, P.E.D.; Cristianini, M. Structural and Rheological Properties of Frozen Concentrated Orange Juice (FCOJ) by Multi-Pass High-Pressure Homogenisation (MP-HPH). Int. J. Food Prop. 2017, 20, 2107–2117. [Google Scholar] [CrossRef]
- Yu, W.; Cui, J.; Zhao, S.; Feng, L.; Wang, Y.; Liu, J.; Zheng, J. Effects of High-Pressure Homogenization on Pectin Structure and Cloud Stability of Not-From-Concentrate Orange Juice. Front. Nutr. 2021, 8, 647748. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Jiang, S.-W.; Cao, X.-M.; Jiang, S.-T.; Pan, L.-J. Effect of high pressure homogenization (HPH) on the physical properties of taro ( Colocasia esculenta (L). Schott) pulp. J. Food Eng. 2016, 177, 1–8. [Google Scholar] [CrossRef]
- Peng, X.-y.; Mu, T.-h.; Zhang, M.; Sun, H.-n.; Chen, J.-w.; Yu, M. Effects of pH and high hydrostatic pressure on the structural and rheological properties of sugar beet pectin. Food Hydrocoll. 2016, 60, 161–169. [Google Scholar] [CrossRef]
- Du, X.; Routray, J.; Williams, C.; Weng, Y. Association of Refreshing Perception with Volatile Aroma Compounds, Organic Acids, and Soluble Solids in Freshly Consumed Cucumber Fruit. ACS Food Sci. Technol. 2022, 2, 1495–1506. [Google Scholar] [CrossRef]
- Xuan, X.; Sun, R.; Zhang, X.; Cui, Y.; Lin, X.; Sun, Y.; Deng, W.; Liao, X.; Ling, J. Novel application of HS-GC-IMS with PCA for characteristic fingerprints and flavor compound variations in NFC Chinese bayberry (Myrica rubra) juice during storage. Lwt 2022, 167, 113882. [Google Scholar] [CrossRef]
- Telloli, C.; Tagliavini, S.; Passarini, F.; Salvi, S.; Rizzo, A. ICP-MS triple quadrupole as analytical technique to define trace and ultra-trace fingerprint of extra virgin olive oil. Food Chem. 2023, 402, 134247. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Huang, L.; Tan, X.; Wu, H.; Li, G. Association between flavor composition and sensory profile in thermally processed mandarin juices by multidimensional gas chromatography and multivariate statistical analysis. Food Chem 2023, 419, 136026. [Google Scholar] [CrossRef]
- Luo, D.; Pan, X.; Zhang, W.; Bi, S.; Wu, J. Effect of glucose oxidase treatment on the aroma qualities and release of cooked off-odor components from heat-treated Hami melon juice. Food Chem. 2022, 371, 131166. [Google Scholar] [CrossRef]
- Castura, J.C.; Varela, P.; Næs, T. Investigating paired comparisons after principal component analysis. Food Qual. Prefer. 2023, 106, 104814. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Wu, L.; Xue, X.; Zhao, J.; Li, Y.; Ye, Z.; Lin, G. Classification of Chinese Honeys According to Their Floral Origins Using Elemental and Stable Isotopic Compositions. J. Agric. Food Chem. 2015, 63, 5388–5394. [Google Scholar] [CrossRef] [PubMed]

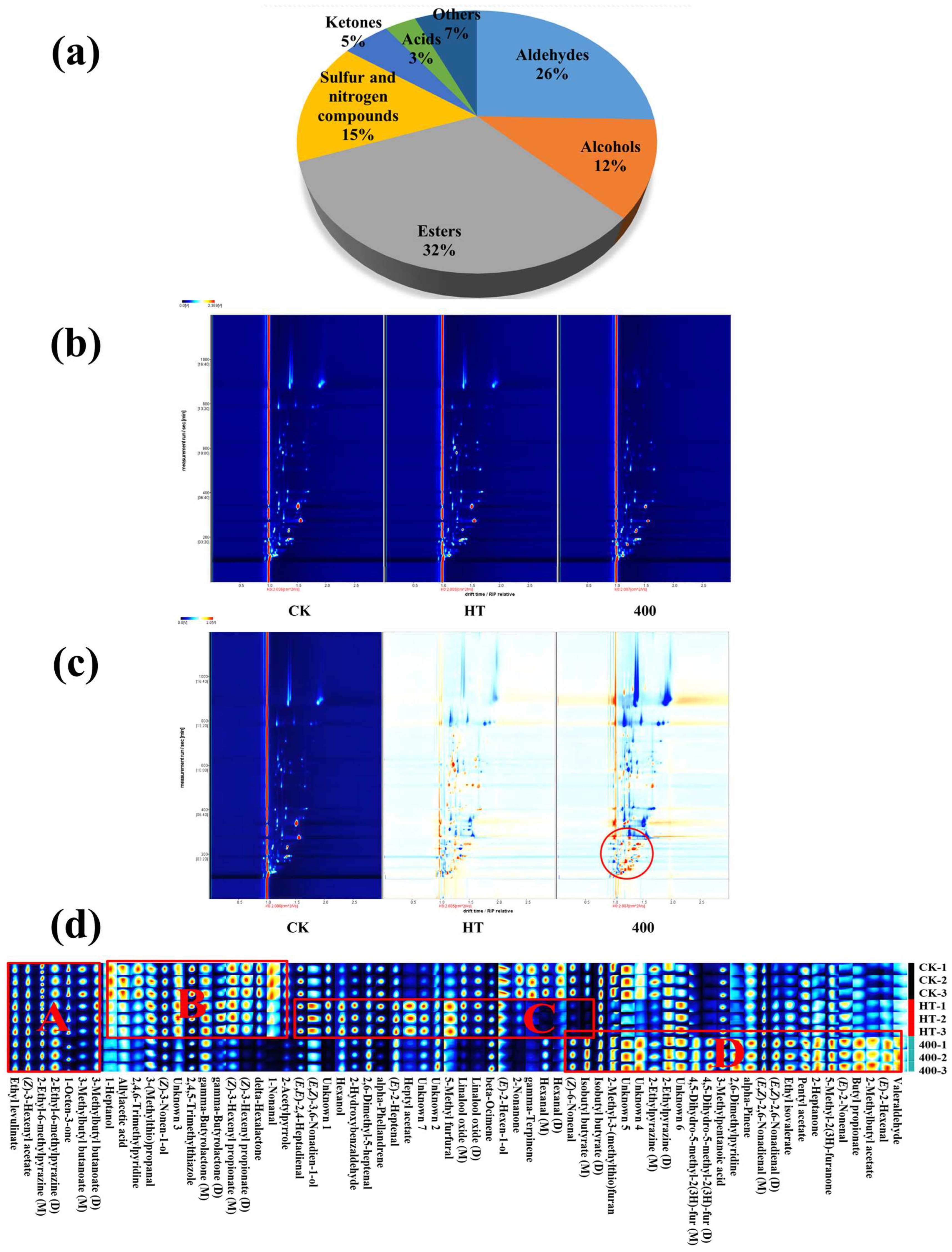
| Microbial Species | CK Sample | HT Sample | 200 MPa DHPM-Treated Sample | 300 MPa DHPM-Treated Sample | 400 MPa DHPM-Treated Sample |
|---|---|---|---|---|---|
| Total aerobic bacteria | 4.56 ± 0.13 a | ND | 2.49 ± 0.12 b | 1.66 ± 0.08 c | ND |
| Yeast and molds | 3.37 ± 0.08 a | ND | ND | ND | ND |
| Treatment Condition | L* | a* | b* | ∆E |
|---|---|---|---|---|
| CK | 27.73 ± 0.22 a | −2.66 ± 0.12 a | 3.54 ± 0.08 c | Control |
| HT | 24.39 ± 0.28 d | −2.17 ± 0.18 c | 5.49 ± 0.19 a | 3.90 ± 0.47 a |
| 400-MPa DHPM | 25.57 ± 0.32 c | −2.20 ± 0.2 c | 4.57 ± 0.11 b | 2.44 ± 0.14 b |
| No. | Compound | CAS# | Formula | MW | RI | Rt [s] | Dt [a.u.] | Peak Intensity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CK | HT | 400 | ||||||||
| 1 | (E)-2-Nonenal | C18829566 | C9H16O | 114.2 | 863.9 | 118.9 | 1.12 | 2906.36 ± 33.57 c | 4270.49 ± 9.61 b | 4948.25 ± 233.29 a |
| 2 | (E)-2-Hexen-1-ol | C928950 | C6H12O | 100.2 | 866.1 | 120.83 | 1.16 | 1537.16 ± 50.02 a | 1150.46 ± 57.8 b | 787.65 ± 58.49 c |
| 3 | 5-Methyl-2(3H)-furanone | C591128 | C5H6O2 | 98.1 | 878.1 | 131.74 | 1.12 | 1849.13 ± 28.89 b | 1881.91 ± 56.72 b | 2478.6 ± 132.42 a |
| 4 | (E)-2-Hexenal | C6728263 | C6H10O | 98.1 | 881.1 | 134.66 | 1.2 | 126.52 ± 5.84 c | 217.85 ± 8.16 b | 244.43 ± 2.78 a |
| 5 | 2-Methylbutyl acetate | C624419 | C7H14O2 | 130.2 | 881.6 | 135.17 | 1.29 | 39.6 ± 4.81 c | 50.49 ± 1.26 b | 96.44 ± 10.67 a |
| 6 | 2-Heptanone | C110430 | C7H14O | 114.2 | 888.4 | 141.96 | 1.25 | 540.44 ± 9.62 c | 742.99 ± 24.23 b | 957.91 ± 9.95 a |
| 7 | Allylacetic acid | C591800 | C5H8O2 | 100.1 | 890.3 | 143.89 | 1.14 | 704.46 ± 26.45 a | 548.87 ± 12.77 b | 483.8 ± 17.64 c |
| 8 | Butyl propionate | C590012 | C7H14O2 | 130.2 | 891.9 | 145.63 | 1.29 | 92.97 ± 9.1 b | 90.09 ± 5.41 b | 161.55 ± 1.15 a |
| 9 | 2,6-Dimethylpyridine | C108485 | C7H9N | 107.2 | 895.6 | 149.95 | 1.1 | 286.16 ± 10.88 b | 249.31 ± 42.89 b | 514.65 ± 13.87 a |
| 10 | Pentyl acetate | C628637 | C7H14O2 | 130.2 | 911.4 | 170.63 | 1.31 | 708.04 ± 16.12 b | 636.12 ± 3.6 c | 944.58 ± 37.47 a |
| 11 | 3-(Methylthio)propanal | C3268493 | C4H8OS | 104.2 | 912.5 | 172.16 | 1.4 | 753.05 ± 26.95 b | 806.07 ± 34.32 a | 578.76 ± 22.06 c |
| 12 | Valeraldehyde | C110623 | C5H10O | 122.2 | 919.5 | 182.19 | 1.26 | 137.59 ± 5.36 a | 119.37 ± 7.6 b | 146.99 ± 5.78 a |
| 13 | gamma-Butyrolactone (M) | C96480 | C4H6O2 | 86.1 | 919.7 | 182.5 | 1.08 | 555.05 ± 9.68 b | 646.08 ± 10.05 a | 530.52 ± 14.66 c |
| 14 | gamma-Butyrolactone (D) | C96480 | C4H6O2 | 86.1 | 921.5 | 185.18 | 1.31 | 1781.82 ± 17.37 b | 3325.87 ± 43.26 a | 1251.36 ± 38.44 c |
| 15 | 2-Ethylpyrazine (D) | C13925003 | C6H8N2 | 108.1 | 923.5 | 188.25 | 1.2 | 452.83 ± 2.39 b | 379.99 ± 8.91 c | 549.71 ± 26.53 a |
| 16 | (E,Z)-3,6-Nonadien-1-ol | C56805233 | C9H16O | 96.1 | 926 | 192.08 | 1.39 | 1913.27 ± 51.67 b | 2595.77 ± 54.64 a | 1974.83 ± 37.44 b |
| 17 | alpha-Pinene | C80568 | C10H16 | 136.2 | 928.4 | 195.86 | 1.2 | 351.74 ± 3.53 b | 349.81 ± 10.54 b | 415.6 ± 9.38 a |
| 18 | 2-Ethylpyrazine (M) | C13925003 | C6H8N2 | 108.1 | 929.6 | 197.79 | 1.11 | 408.22 ± 29.24 a | 216.29 ± 8.12 c | 320.28 ± 2.04 b |
| 19 | Ethyl isovalerate | C108645 | C7H14O2 | 130.2 | 940.6 | 216.37 | 1.25 | 220.72 ± 4.64 c | 346.16 ± 15.85 b | 445.73 ± 8.65 a |
| 20 | Isobutyl butyrate (M) | C539902 | C8H16O2 | 144.2 | 944.1 | 222.7 | 1.34 | 2994.96 ± 127.9 b | 676.63 ± 15.92 c | 6409.55 ± 255.32 a |
| 21 | 2-Methyl-3-(methylthio)furan | C63012975 | C6H8OS | 128.2 | 945.1 | 224.53 | 1.09 | 2130.94 ± 34.55 a | 1362.25 ± 32.6 c | 1970.48 ± 45.89 b |
| 22 | Isobutyl butyrate (D) | C539902 | C8H16O2 | 144.2 | 950.3 | 234.14 | 1.36 | 4492.39 ± 55.91 a | 4012.62 ± 21.27 c | 4295.78 ± 95.29 b |
| 23 | (E)-2-Heptenal | C18829555 | C7H12O | 112.2 | 955.9 | 245.18 | 1.26 | 700.37 ± 13.48 c | 1198.95 ± 2.67 a | 1118.65 ± 24.51 b |
| 24 | 4,5-Dihydro-5-methyl-2(3H)-fur (D) | C108292 | C5H8O2 | 100.1 | 956.4 | 246.11 | 1.41 | 367.28 ± 15.71 c | 560.91 ± 16.68 b | 1624.85 ± 73.36 a |
| 25 | 4,5-Dihydro-5-methyl-2(3H)-fur (M) | C108292 | C5H8O2 | 100.1 | 956.5 | 246.28 | 1.13 | 311.4 ± 17.14 b | 286.42 ± 5.25 c | 880.27 ± 19.52 a |
| 26 | 5-Methyl furfural | C620020 | C6H6O2 | 110.1 | 957.2 | 247.74 | 1.48 | 134.44 ± 4.67 b | 251.77 ± 2.88 a | 120.24 ± 9.85 b |
| 27 | Hexanal (D) | C66251 | C6H12O | 106.1 | 961.6 | 256.66 | 1.16 | 263.53 ± 18.16 a | 127.15 ± 8.73 c | 152.61 ± 3.65 b |
| 28 | 3-Methylpentanoic acid | C105431 | C6H12O2 | 116.2 | 967.2 | 268.76 | 1.27 | 679.57 ± 1.51 b | 718.23 ± 12.11 b | 997.25 ± 49.08 a |
| 29 | 1-Heptanol | C111706 | C7H16O | 116.2 | 969.4 | 273.46 | 1.41 | 232.33 ± 15.25 a | 182.83 ± 12.6 b | 255.01 ± 30.35 a |
| 30 | 1-Octen-3-one | C4312996 | C8H14O | 126.2 | 974 | 284.01 | 1.27 | 4131.04 ± 24.61 a | 3498.12 ± 68.05 b | 2462.42 ± 45.47 c |
| 31 | Hexanal (M) | C66251 | C6H12O | 106.1 | 984.8 | 309.98 | 1.5 | 536.49 ± 19.08 a | 102.68 ± 7.51 d | 354.53 ± 25.87 c |
| 32 | (Z)-6-Nonenal | C2277192 | C9H16O | 128.2 | 984.9 | 310.27 | 1.16 | 1070.97 ± 11.52 a | 327.59 ± 5.56 c | 990.24 ± 44 b |
| 33 | 2-Ethyl-6-methylpyrazine (M) | C13925036 | C7H10N2 | 122.2 | 996.5 | 341.19 | 1.18 | 6215.5 ± 52.26 a | 6112.79 ± 18.09 a | 5493.32 ± 56.56 b |
| 34 | 2,4,5-Trimethylthiazole | C13623115 | C6H9NS | 127.2 | 996.8 | 342.04 | 1.52 | 20,699.43 ± 255.71 a | 15,497.9 ± 236.43 b | 9065.59 ± 226.34 c |
| 35 | (E,Z)-2,6-Nonadienal (D) | C557482 | C9H14O | 120.2 | 998.4 | 346.86 | 1.24 | 287.87 ± 6.45 c | 459.01 ± 14.39 b | 751.34 ± 6.74 a |
| 36 | 2,4,6-Trimethylpyridine | C108758 | C8H11N | 121.2 | 1003.2 | 362.23 | 1.58 | 768.36 ± 43.26 a | 468.52 ± 28.72 b | 325.72 ± 21.12 c |
| 37 | 2-Ethyl-6-methylpyrazine (D) | C13925036 | C7H10N2 | 122.2 | 1003.3 | 362.68 | 1.64 | 1160.68 ± 76.02 a | 1231.34 ± 24.95 a | 1215.92 ± 26.31 a |
| 38 | (Z)-3-Hexenyl acetate | C3681718 | C8H14O2 | 142.2 | 1004 | 364.97 | 1.33 | 3285.69 ± 81.98 b | 3500.57 ± 33.28 a | 3602.44 ± 50.5 a |
| 39 | (E,E)-2,4-Heptadienal | C4313035 | C7H10O | 110.2 | 1014.3 | 400.34 | 1.15 | 1902.11 ± 26.94 a | 1783.25 ± 27.64 b | 960.3 ± 30.2 c |
| 40 | alpha-Phellandrene | C99832 | C10H16 | 136.2 | 1015.3 | 403.86 | 1.69 | 1890.17 ± 84.69 b | 2275.26 ± 246.12 a | 526.29 ± 26.74 c |
| 41 | (Z)-3-Nonen-1-ol | C10340235 | C9H18O | 144.2 | 1015.6 | 405.17 | 1.34 | 2539.04 ± 45.5 a | 2595.74 ± 87.74 a | 1679.86 ± 46.29 b |
| 42 | Hexanol | C111273 | C6H14O | 122.2 | 1019.8 | 420.63 | 1.12 | 334.69 ± 17.84 c | 593.83 ± 4.48 a | 492.45 ± 22.03 b |
| 43 | beta-Ocimene | C13877913 | C10H16 | 136.2 | 1040.2 | 505.3 | 1.67 | 1748.84 ± 139.53 c | 2426.53 ± 95.07 b | 3162.82 ± 462.14 a |
| 44 | (E,Z)-2,6-Nonadienal (M) | C557482 | C9H14O | 120.2 | 1041 | 508.84 | 1.25 | 4023.9 ± 158.91 b | 4471.85 ± 81.32 b | 5143.62 ± 336.08 a |
| 45 | Heptyl acetate | C112061 | C9H18O2 | 158.2 | 1041.6 | 511.95 | 1.46 | 45.64 ± 3.57 c | 177.78 ± 3.25 a | 57.62 ± 6.87 b |
| 46 | 2-Hydroxybenzaldehyde | C90028 | C7H6O2 | 122.1 | 1041.8 | 512.63 | 1.15 | 586.28 ± 9.54 b | 1121.83 ± 9.1 a | 574.95 ± 14.45 b |
| 47 | 2,6-Dimethyl-5-heptenal | C106729 | C9H16O | 140.2 | 1051.6 | 559.89 | 1.16 | 857.25 ± 32.4 c | 1136.07 ± 9.92 b | 1357.04 ± 56.73 a |
| 48 | 3-Methylbutyl butanoate (D) | C106274 | C9H18O2 | 158.2 | 1052.8 | 566.31 | 1.42 | 1032.34 ± 82.74 b | 1240.77 ± 27.47 a | 872.28 ± 15.93 c |
| 49 | gamma-Terpinene | C99854 | C10H16 | 136.2 | 1053.6 | 570.25 | 1.71 | 249.48 ± 14.52 a | 90.45 ± 15.08 b | 71.59 ± 4.97 b |
| 50 | 3-Methylbutyl butanoate (M) | C106274 | C9H18O2 | 158.2 | 1061 | 609.24 | 1.41 | 1162.15 ± 79.23 a | 781.36 ± 37.9 b | 588.25 ± 27.25 c |
| 51 | Ethyl levulinate | C539888 | C7H12O3 | 144.2 | 1064.4 | 628.55 | 1.2 | 3356.38 ± 99.27 b | 3845.04 ± 85.59 a | 3120.62 ± 56.96 c |
| 52 | Linalool oxide (M) | C60047178 | C10H18O2 | 170.3 | 1070 | 660.85 | 1.27 | 858.81 ± 18.33 b | 967.21 ± 45.01 a | 269.25 ± 6.48 c |
| 53 | Linalool oxide (D) | C60047178 | C10H18O2 | 170.3 | 1077.9 | 709.35 | 1.82 | 378.45 ± 19.03 b | 455.12 ± 16.11 a | 163.47 ± 8.73 c |
| 54 | delta-Hexalactone | C823223 | C6H10O2 | 114.1 | 1089.2 | 785.37 | 1.17 | 4067.24 ± 123.03 a | 2546.55 ± 192.38 b | 807.94 ± 25.67 c |
| 55 | 2-Nonanone | C821556 | C9H18O | 142.2 | 1090 | 791.25 | 1.86 | 899.15 ± 34.19 a | 338.31 ± 40.32 b | 123.37 ± 12.47 c |
| 56 | 2-Acetylpyrrole | C1072839 | C6H7NO | 109.1 | 1090.7 | 796.16 | 1.48 | 2335.48 ± 116.51 a | 1504.98 ± 121.37 b | 713.05 ± 15.95 c |
| 57 | (Z)-3-Hexenyl propionate (M) | C33467742 | C9H16O2 | 156.2 | 1102.2 | 882.58 | 1.37 | 5901.68 ± 32.4 a | 5888.42 ± 54.88 a | 2329.99 ± 16.85 b |
| 58 | (Z)-3-Hexenyl propionate (D) | C33467742 | C9H16O2 | 156.2 | 1102.3 | 883.47 | 1.9 | 5384.49 ± 81.49 a | 4386.57 ± 294.21 b | 499.12 ± 2.71 c |
| 59 | 1-Nonanal | C124196 | C9H18O | 142.2 | 1103.8 | 895.33 | 1.93 | 2881.48 ± 66.19 a | 1917.83 ± 197.68 b | 165.17 ± 9.81 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, M.; Gao, Z.; Cheng, Y.; Yang, X.; Mu, S.; Qu, K. Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice. Foods 2024, 13, 2125. https://doi.org/10.3390/foods13132125
Zhang Z, Zhang M, Gao Z, Cheng Y, Yang X, Mu S, Qu K. Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice. Foods. 2024; 13(13):2125. https://doi.org/10.3390/foods13132125
Chicago/Turabian StyleZhang, Zhiwei, Meiyue Zhang, Zhenhong Gao, Yuying Cheng, Xinyi Yang, Shuaixue Mu, and Kunsheng Qu. 2024. "Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice" Foods 13, no. 13: 2125. https://doi.org/10.3390/foods13132125
APA StyleZhang, Z., Zhang, M., Gao, Z., Cheng, Y., Yang, X., Mu, S., & Qu, K. (2024). Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice. Foods, 13(13), 2125. https://doi.org/10.3390/foods13132125






