Flavor Variations in Precious Tricholoma matsutake under Different Drying Processes as Detected with HS-SPME-GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Materials and Sample Production
2.2. Headspace Solid-Phase Microextraction (HS-SPME)
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.4. Statistical Analysis
3. Results
3.1. Changes in HS-SPME-GC-MS of T. matsutake with Differing Drying Processes
3.2. Variation in VOCs of T. matsutake
3.2.1. Changes in the Types and Relative Contents of VOCs
3.2.2. Analysis of Major VOCs in Different-Drying T. matsutake
3.2.3. Analysis of the Unique and Common VOCs in Different Drying Processes of T. matsutake
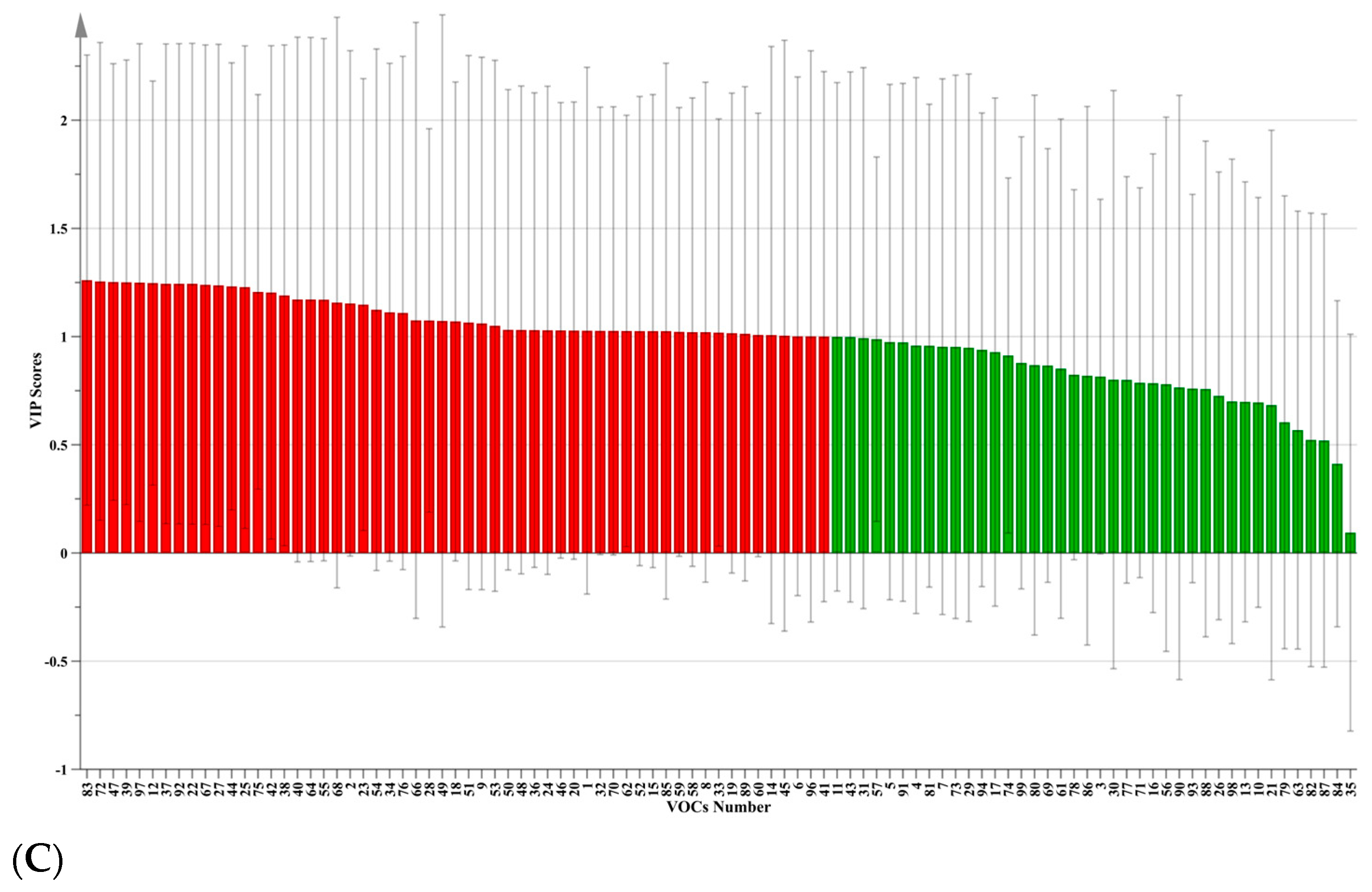
3.3. Characteristic VOCs via PCA and PLS-DA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Adelina, N.; Hu, J.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in four pine-mushrooms using HS-SPME/GC-MS and E-nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Li, M.; Du, H.; Lin, S. Flavor changes of Tricholoma matsutake Singer under different processing conditions by using HS-GC-IMS. Foods 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Pueschel, V.; Schieberle, P. Changes in the key aroma compounds of matsutake mushroom (Tricholoma matsutake Sing.) from Canada during pan-frying elucidated by application of the sensomics approach. Eur. Food Res. Technol. 2021, 247, 51–65. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y. Identification of genetic characterization and volatile compounds of Tricholoma matsutake from different geographical origins. Biochem. Syst. Ecol. 2012, 44, 233–239. [Google Scholar] [CrossRef]
- Cho, I.; Chio, H.; Yim, Y. Difference in the volatile composition of pine mushrooms (Tricholoma matsutake Sing) according to their grades. J. Agric. Food Chem. 2006, 54, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Geng, F.; Xu, Y.; Li, X.; Liu, D.; Liu, Z.; Zhang, L.; Wang, J. Quantitative transcriptomic and metabolomic analyses reveal the changes in Tricholoma matsutake fruiting bodies during cold storage. Food Chem. 2022, 381, 132292. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Namgung, H.; Choi, H.; Kim, Y. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. HS-GC-IMS和HS-SPME-GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Cho, I.; Kim, S.; Choi, H.; Kim, Y. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A. Development of flavor during drying and applications of edible mushrooms: A review. Drying Technol. 2021, 39, 1685–1703. [Google Scholar] [CrossRef]
- Ai, J. Headspace solid phase microextration. Dynamics and quantitative analysis before reaching a partition equilibrium. Anal. Chem. 1997, 69, 3260–3266. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC-MS and electronic nose. Food Sci. Technol. 2006, 72, 343–350. [Google Scholar] [CrossRef]
- Siegmund, B. 7–Biogenesis of aroma compounds: Flavour formation in fruits and vegetables. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 127–149. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT—Food Sci. Technol. 2021, 146, 111402–111412. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef]
- Tain, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Cruz, C.; Noëlsuberville, C.; Montury, M. Fatty acid content and some flavor compound release in two strains of Agaricus bisporus, according to three stages of development. J. Agric. Food Chem. 1997, 45, 64–67. [Google Scholar] [CrossRef]
- Burton, K.; Combet, E.; Henderson, J.; Combet, E. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Qing, Z.; Cheng, J.; Wang, X.; Tang, D.; Liu, X.; Zhu, M. The effects of four edible mushrooms (Volvariella volvacea, Hypsizygus marmoreus, Pleurotus ostreatus and Agaricus bisporus) on physicochemical properties of beef paste. LWT—Food Sci. Technol. 2021, 135, 110063–110071. [Google Scholar] [CrossRef]
- Zhou, Y.; El-Seedi, H.; Xu, B. Insights into health promoting effects and myochemical profiles of pine mushroom. Tricholoma Matsutake 2023, 63, 5698–5723. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xin, G.; Sun, B.; Bao, X.; Wei, Y.; Zhao, X.; Xu, H. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Hou, Z.; Xia, R.; Li, Y.; Xu, H.; Wang, Y.; Feng, Y.; Pan, S.; Wang, Z.; Ren, H.; Qian, G.; et al. Key components, formation pathways, affecting factors, and emerging analytical strategies for edible mushrooms aroma: A review. Food Chem. 2024, 438, 137993. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Claver, I.; Zhu, K.; Peng, W.; Zhou, H. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]





| No. | CAS | Compound | Formula | MW | RT [min] | Relative Amount % | ||
|---|---|---|---|---|---|---|---|---|
| VFD | HAD | FRESH | ||||||
| Acids | ||||||||
| 1 | 503-74-2 | 3-Methyl-butanoic acid | C5H10O2 | 102.1 | 5.52 | 0.23 | nd | nd |
| 2 | 107-92-6 | Butanoic acid | C4H8O2 | 88.1 | 9.40 | nd | 0.18 | nd |
| Aldehydes | ||||||||
| 3 | 100-52-7 | Benzaldehyde | C7H6O | 106.1 | 7.72 | 0.87 | 1.85 | 1.16 |
| 4 | 122-78-1 | Benzeneacetaldehyde | C8H8O | 120.1 | 10.79 | 0.12 | 0.86 | 0.57 |
| 5 | 2548-87-0 | (E)-2-Octenal | C8H14O | 126.2 | 11.45 | 0.74 | 0.16 | 2.80 |
| 6 | 4432-63-7 | Atropaldehyde | C9H8O | 132.1 | 14.94 | nd | nd | 0.06 |
| 7 | 18829-56-6 | trans-2-Nonenal | C9H16O | 140.2 | 15.16 | nd | nd | 0.05 |
| 8 | 112-31-2 | Decanal | C10H20O | 156.2 | 17.16 | 0.02 | nd | 0.05 |
| 9 | 5910-87-2 | (E,E)-2,4-Nonadienal | C9H14O | 138.2 | 17.49 | nd | nd | 0.12 |
| 10 | 4411-89-6 | 2-Phenylbut-2-enal | C10H10O | 146.1 | 19.33 | nd | 0.19 | nd |
| 11 | 25152-84-5 | (E,E)-2,4-Decadienal | C10H16O | 152.2 | 20.82 | nd | 0.21 | 0.09 |
| 12 | 13019-16-4 | 2-Butyl-2-octenal | C12H22O | 152.2 | 22.09 | 0.65 | nd | |
| Alcohols | ||||||||
| 13 | 111-27-3 | 1-Hexanol | C6H14O | 102.1 | 4.74 | nd | nd | 0.15 |
| 14 | 3391-86-4 | 1-Octen-3-ol | C8H16O | 128.2 | 8.79 | 31.41 | 0.77 | 68.67 |
| 15 | 589-98-0 | 3-Octanol | C8H18O | 130.2 | 9.48 | 0.06 | nd | 16.38 |
| 16 | 18185-81-4 | 3-Octen-1-ol | C8H16O | 128.2 | 11.77 | nd | 0.59 | nd |
| 17 | 18409-17-1 | (E)-2-Octen-1-ol | C8H16O | 128.2 | 11.97 | 0.28 | 0.44 | 3.33 |
| 18 | 111-87-5 | 1-Octanol | C8H18O | 130.2 | 12.05 | 0.02 | 0.75 | 0.94 |
| 19 | 34995-77-2 | trans-Furan linalool oxide | C10H18O2 | 170.2 | 12.45 | 0.25 | 0.73 | nd |
| 20 | 818-81-5 | 2-Methyl-1-octanol | C9H20O | 144.2 | 12.65 | 0.29 | 0.50 | nd |
| 21 | 3913-02-8 | 2-Butyl-1-octanol | C12H26O | 186.3 | 20.35 | nd | 0.07 | nd |
| 22 | 40716-66-3 | Nerolidol | C15H26O | 222.3 | 25.61 | 0.07 | 0.28 | nd |
| Esters | ||||||||
| 23 | 106-70-7 | Methyl hexanoate | C7H14O2 | 130.1 | 6.43 | 0.11 | nd | 0.01 |
| 24 | 108-29-2 | γ-Valerolactone | C5H8O2 | 100.1 | 7.34 | 0.24 | 0.25 | nd |
| 25 | 637-65-0 | Tetrahydrofurfuryl propionate | C8H14O3 | 158.2 | 10.85 | 0.10 | 0.16 | 0.07 |
| 26 | 695-06-7 | 5-Ethyloxolan-2-one | C6H10O2 | 114.1 | 11.08 | 0.10 | nd | nd |
| 27 | 111-11-5 | Caprylic acid methyl ester | C9H18O2 | 158.2 | 13.85 | nd | nd | 0.17 |
| 28 | 7367-81-9 | Methyl oct-2-enoate | C9H16O2 | 156.2 | 15.59 | 0.32 | 0.27 | 0.74 |
| 29 | 959067-41-5 | 2-Ethylhexyl hexyl sulfite | C14H30O3S | 278.4 | 20.24 | nd | 0.10 | nd |
| 30 | 1191-02-2 | Methyl dec-4-enoate | C11H20O2 | 184.2 | 20.51 | 0.16 | nd | 0.54 |
| 31 | 103-26-4 | Methyl cinnamate | C10H10O2 | 162.2 | 22.45 | 19.93 | 52.76 | 2.07 |
| 32 | 4493-42-9 | Methyl 2E,4Z-decadienoate | C11H18O2 | 182.2 | 22.62 | nd | 0.43 | 0.06 |
| 33 | 79837-88-0 | Methyl (Z)-dodec-5-enoate | C13H24O2 | 212.3 | 24.80 | nd | 0.54 | 0.18 |
| 34 | 84-69-5 | Diisobutyl phthalate | C16H22O4 | 278.3 | 30.10 | 0.02 | nd | 0.01 |
| 35 | 84-74-2 | Dibutyl phthalate | C16H22O4 | 278.3 | 31.67 | 0.03 | 0.09 | nd |
| Ketones | ||||||||
| 36 | 497-23-4 | 2(5H)-Furanone | C4H4O2 | 84.0 | 5.97 | 0.75 | 6.38 | nd |
| 37 | 4312-99-6 | 1-Octen-3-one | C8H14O | 126.1 | 8.36 | 1.95 | 0.72 | nd |
| 38 | 2918-13-0 | 1-Hepten-3-one | C7H12O | 112.1 | 8.54 | nd | 0.14 | 0.24 |
| 39 | 106-68-3 | 3-Octanone | C8H16O | 128.2 | 8.66 | nd | 0.84 | nd |
| 40 | 14705-50-1 | 2,2,5-Trimethyl-3-hexanone | C9H18O | 142.2 | 9.20 | nd | 0.35 | nd |
| 41 | 1073-11-6 | Lavender lactone | C7H10O2 | 126.1 | 10.46 | 1.08 | 0.66 | nd |
| 42 | 18402-82-9 | 3-Octen-2-one | C8H14O | 126.2 | 10.64 | 0.46 | 0.19 | nd |
| 43 | 98-86-2 | Acetophenone | C8H8O | 120.1 | 11.67 | nd | nd | 0.02 |
| 44 | 3508-78-9 | 3-Allylpentane-2,4-dione | C8H12O2 | 140.1 | 11.74 | nd | 0.38 | 0.05 |
| 45 | 693-54-9 | 2-Decanone | C10H20O | 156.2 | 16.45 | 0.29 | 0.26 | 0.01 |
| 46 | 927-49-1 | 6-Undecanone | C11H22O | 170.2 | 19.47 | 0.08 | 0.44 | nd |
| 47 | 112-12-9 | 2-Undecanone | C11H22O | 170.3 | 20.13 | 0.26 | 0.53 | 0.08 |
| Alkanes | ||||||||
| 48 | 5750-02-7 | 2-Cyclopropyl-butane | C7H14 | 98.2 | 4.14 | nd | nd | 0.01 |
| 49 | 2415-72-7 | Propyl-cyclopropane | C6H12 | 84.1 | 4.65 | 0.23 | nd | nd |
| 50 | 5911-04-6 | 3-Methyl-nonane | C10H22 | 142.2 | 8.12 | 0.04 | 0.09 | nd |
| 51 | 107-83-5 | 2-Methyl-pentane | C6H14 | 86.1 | 9.61 | nd | 0.04 | nd |
| 52 | 17301-32-5 | 4,7-Dimethyl-undecane | C13H28 | 184.3 | 11.25 | nd | 0.45 | nd |
| 53 | 16747-50-5 | 1-Ethyl-1-methyl-cyclopentane | C8H16 | 112.2 | 11.36 | nd | 0.21 | nd |
| 54 | 1120-21-4 | Undecane | C11H24 | 156.3 | 13.02 | 3.75 | nd | nd |
| 55 | 4292-92-6 | Pentyl-cyclohexane | C11H22 | 154.2 | 14.21 | 0.05 | nd | nd |
| 56 | 71138-64-2 | 3-Methyl-undecane | C12H26 | 168.3 | 14.52 | nd | 0.14 | nd |
| 57 | 62238-12-4 | 2,3,6-Trimethyl-decane | C13H28 | 184.3 | 15.36 | 0.03 | nd | nd |
| 58 | 1502-38-1 | Methyl-cyclooctane | C9H18 | 126.2 | 16.46 | 0.25 | nd | nd |
| 59 | 112-40-3 | Dodecane | C12H26 | 170.3 | 16.88 | 4.14 | 3.80 | nd |
| 60 | 560-21-4 | 2,3,3-Trimethyl-pentane | C8H18 | 114.2 | 19.21 | 0.01 | 0.05 | nd |
| 61 | 5881-17-4 | 3-Ethyl-octane | C10H22 | 142.2 | 19.58 | 0.02 | 0.63 | nd |
| 62 | 111-65-9 | Octane | C8H18 | 114.2 | 20.39 | 1.45 | 1.07 | 0.01 |
| 63 | 17301-33-6 | 4,8-Dimethyl-undecane | C13H28 | 184.3 | 21.37 | nd | 0.28 | nd |
| 64 | 17312-57-1 | 3-Methyl-dodecane | C8H18 | 184.3 | 21.83 | 0.04 | 0.07 | nd |
| 65 | 1072-16-8 | 2,7-Dimethyl-octane | C10H22 | 142.2 | 22.11 | 0.24 | 0.08 | 0.01 |
| 66 | 3891-98-3 | 2,6,10-Trimethyl-dodecane | C15H32 | 212.4 | 22.24 | 0.09 | nd | nd |
| 67 | 629-59-4 | Tetradecane | C14H30 | 198.3 | 22.79 | 0.96 | 0.32 | 0.11 |
| 68 | 14905-56-7 | 2,6,10-Trimethyltetradecane | C17H36 | 240.4 | 23.97 | 0.25 | nd | nd |
| 69 | 629-62-9 | Pentadecane | C15H32 | 212.4 | 24.71 | 0.17 | nd | 0.01 |
| 70 | 17302-01-1 | 3-Ethyl-3-methylheptane | C10H22 | 142.2 | 25.27 | nd | 0.05 | 0.01 |
| 71 | 563-16-6 | 3,3-Dimethyl-hexane | C8H18 | 114.2 | 26.38 | 0.09 | 0.07 | 0.01 |
| Olefins | ||||||||
| 72 | 1002-33-1 | 1,3-Octadiene | C8H14 | 110.1 | 3.29 | 0.63 | nd | 0.03 |
| 73 | 19549-87-2 | 2,4-Dimethyl-1-heptene | C9H18 | 126.2 | 3.46 | nd | 0.39 | nd |
| 74 | 694-87-1 | Benzocyclobutene | C8H8 | 104.1 | 5.21 | 0.18 | nd | 0.48 |
| 75 | 16746-86-4 | 2,3-Dimethyl-1-hexene | C8H16 | 112.2 | 3.63 | nd | 0.08 | 0.04 |
| 76 | 80-56-8 | α-Pinene | C10H16 | 136.2 | 6.64 | 1.15 | 0.21 | 0.03 |
| 77 | 690-92-6 | (3Z)-2,2-Dimethyl-3-hexene | C8H16 | 112.2 | 7.60 | 0.10 | 0.33 | 0.12 |
| 78 | 13269-52-8 | (E)-3-Hexene | C6H12 | 84.1 | 9.31 | 6.65 | 0.16 | nd |
| 79 | 61142-36-7 | 3-Ethyl-2-methyl-1,3-hexadiene | C9H16 | 124.2 | 10.38 | nd | 0.69 | 0.18 |
| 80 | 13877-91-3 | Ocimene | C10H16 | 136.2 | 13.00 | 0.23 | 5.43 | nd |
| 81 | 56728-10-0 | 3,4,5-Trimethyl-1-hexene | C9H18 | 126.2 | 13.46 | 0.18 | 0.66 | 0.01 |
| 82 | 71138-64-2 | 3-Methylene-undecane | C12H24 | 168.3 | 16.15 | 0.12 | 0.08 | nd |
| 83 | 3650-28-0 | (+)-Sativen | C15H24 | 204.3 | 23.05 | 0.75 | nd | nd |
| 84 | 39863-73-5 | (±)-β-Barbatene | C15H24 | 204.3 | 23.77 | 15.02 | 7.64 | 0.21 |
| 85 | 30364-38-6 | Dehydro-ar-ionene | C15H22O | 172.2 | 27.04 | nd | 0.64 | nd |
| Aromatic hydrocarbons | ||||||||
| 86 | 100-41-4 | Ethylbenzene | C8H10 | 106.1 | 4.26 | 0.04 | nd | nd |
| 87 | 95-47-6 | o-Xylene | C8H10 | 106.1 | 4.54 | 0.24 | nd | 0.07 |
| 88 | 99-87-6 | p-Cymene | C10H14 | 134.2 | 13.57 | 0.10 | nd | nd |
| 89 | 91-20-3 | Naphthalene | C10H8 | 128.1 | 15.96 | 0.23 | nd | nd |
| 90 | 90-12-0 | 1-Methyl-naphthalene | C11H10 | 142.1 | 20.09 | 0.34 | 0.16 | nd |
| 91 | 575-43-9 | 1,6-Dimethyl-naphthalene | C12H12 | 156.2 | 23.12 | 0.06 | nd | nd |
| 92 | 96-76-4 | 2,4-Di-tert-butylphenol | C14H22O | 206.3 | 24.82 | nd | 0.22 | nd |
| Heterocycle compounds | ||||||||
| 93 | 109-08-0 | Methyl-pyrazine | C5H6N2 | 94.1 | 4.39 | 0.03 | nd | nd |
| 94 | 123-32-0 | 2,5-Dimethyl-pyrazine | C6H8N2 | 108.1 | 5.99 | 0.11 | 2.63 | nd |
| 95 | 3777-69-3 | 2-Pentyl-furan | C9H14O | 138.2 | 8.84 | 0.36 | nd | nd |
| 95 | 100-84-5 | 3-Methylanisole | C8H10O | 122.1 | 9.97 | 0.78 | nd | 0.04 |
| 97 | 1003-46-9 | 1,1-Dioxide-2-methylthiolane | C5H10O2S | 134.1 | 18.48 | nd | 0.07 | nd |
| 98 | 111150-30-2 | 3,5-Dimethyl-2-(3-methylbutyl)pyrazine | C11H18N2 | 178.2 | 20.64 | nd | 0.16 | nd |
| 99 | 113604-56-1 | 1,2,3-Trimethyldiaziridine | C4H10N2 | 86.1 | 25.90 | nd | 0.02 | nd |
| Unique VOCs | Common VOCs | ||
|---|---|---|---|
| VFD | HAD | FRESH | VFD/HAD/FRESH |
| Ethylbenzene | 2,4-Dimethyl-1-heptene | 2-Cyclopropyl-butane | α-Pinene |
| 2-Methylpyrazine | 3-Octanone | 1-Hexanol | (3Z)-2,2-Dimethyl-3-hexene |
| Propyl cyclopropane | 2,2,5-Trimethyl-3-hexanone | Acetophenone | Benzaldehyde |
| 3-Methyl-butanoic acid | Butanoic acid | Caprylic acid methyl ester | Benzeneacetaldehyde |
| 2-Pentyl-furan | 2-Methyl-pentane | Atropaldehyde | Tetrahydrofurfuryl propionate |
| Lavender lactone | 4,7-Dimethyl-undecane | trans-2-Nonenal | (E)-2-Octenal |
| Undecane | 1-Ethyl-1-methyl-cyclopentane | (E,E)-2,4-Nonadienal | (E)-2-Octen-1-ol |
| p-Cymene | 3-Octen-1-ol | 1-Octanol | |
| Pentyl-cyclohexane | 3-Methyl-undecane | 3,4,5-Trimethylhexene | |
| 2,3,6-Trimethyl-decade | 1,1-Dioxide-2-methylthiolane | (E)-2-Octenoic acid, methyl ester | |
| Naphthalene | 2-Phenylbut-2-enal | 2-Decanone | |
| Methyl-cyclooctane | 2-Ethylhexyl hexyl sulfite | 2-Undecanone | |
| 2-Butyl-2-octenal | 2-Butyl-1-octanol | Octane | |
| 2,6,10-Trimethyl-dodecane | 3,5-Dimethyl-2-(3-methylbutyl)pyrazine | 2,7-Dimethyl-octane | |
| (+)-Sativen | 4,8-Dimethyl-undecane | Methyl cinnamate | |
| 1,6-Dimethyl-naphthalene | 2,4-Di-tert-butylphenol | Tetradecane | |
| 2,6,10-Trimethyltetradecane | 1,2,3-Trimethyldiaziridine | (±)-β-Barbatene | |
| Dehydro-ar-ionene | 3,3-Dimethyl-Hexane | ||
| 1-Octen-3-ol | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Lu, B.; He, X.; Yu, F. Flavor Variations in Precious Tricholoma matsutake under Different Drying Processes as Detected with HS-SPME-GC-MS. Foods 2024, 13, 2123. https://doi.org/10.3390/foods13132123
Zhang F, Lu B, He X, Yu F. Flavor Variations in Precious Tricholoma matsutake under Different Drying Processes as Detected with HS-SPME-GC-MS. Foods. 2024; 13(13):2123. https://doi.org/10.3390/foods13132123
Chicago/Turabian StyleZhang, Fengming, Bin Lu, Xinhua He, and Fuqiang Yu. 2024. "Flavor Variations in Precious Tricholoma matsutake under Different Drying Processes as Detected with HS-SPME-GC-MS" Foods 13, no. 13: 2123. https://doi.org/10.3390/foods13132123
APA StyleZhang, F., Lu, B., He, X., & Yu, F. (2024). Flavor Variations in Precious Tricholoma matsutake under Different Drying Processes as Detected with HS-SPME-GC-MS. Foods, 13(13), 2123. https://doi.org/10.3390/foods13132123







