Valorization of Seafood Waste for Food Packaging Development
Abstract
1. Introduction
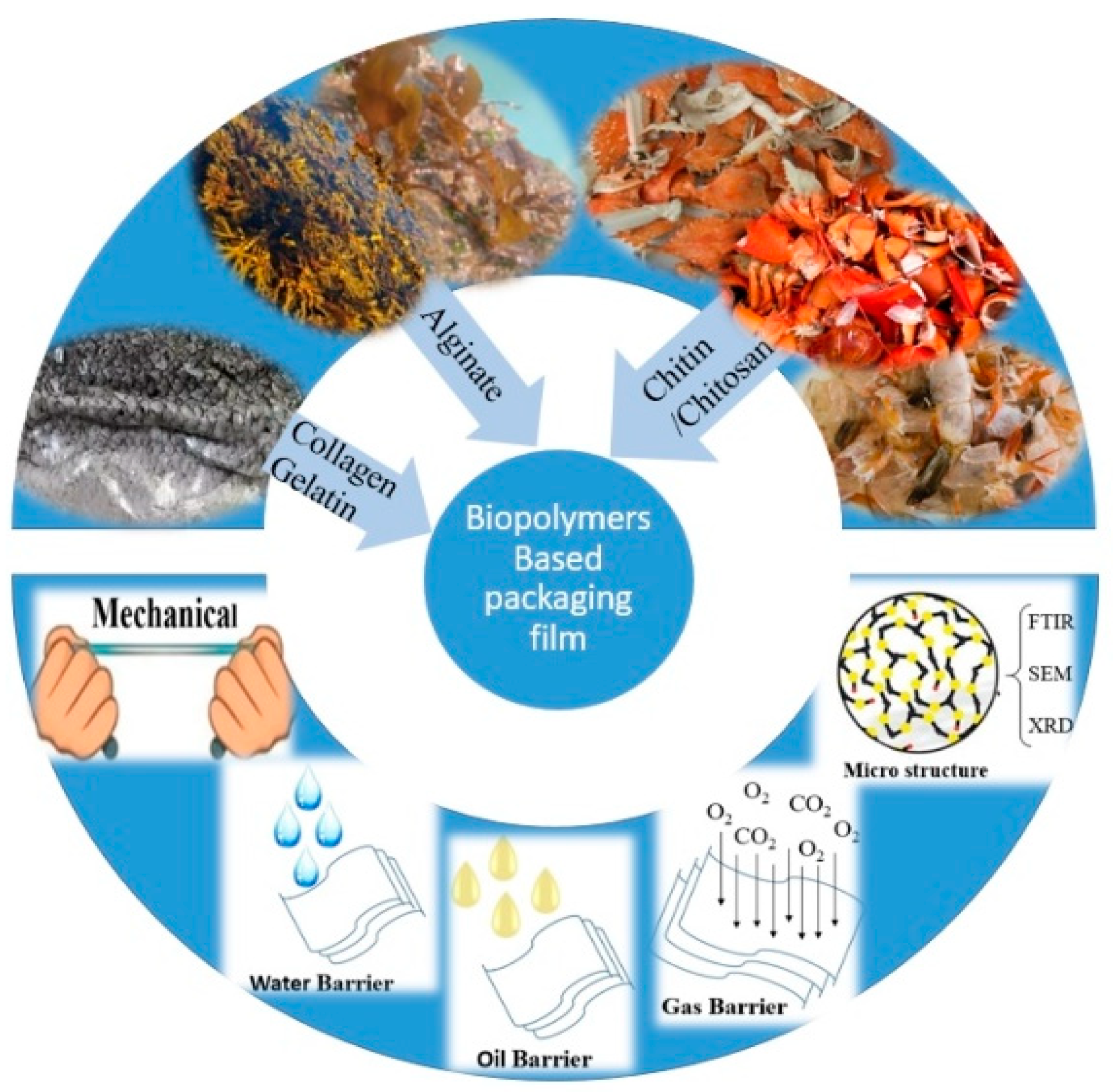
2. Seafood Waste-Derived Biopolymers
2.1. Chitin
2.1.1. Structure and Sources

2.1.2. Extraction Method
2.1.3. Nanochitin
2.2. Chitosan
2.2.1. Structure and Characteristics
2.2.2. Extraction Method
2.2.3. Nanochitosan
2.3. Gelatin
2.3.1. Structure and Characteristics
2.3.2. Extraction Method
2.4. Alginate
2.4.1. Structure and Properties
2.4.2. Extraction Method
3. Seafood Waste-Derived Biopolymer Used as Packaging Materials
3.1. Nanochitin Reinforcement
3.2. Chitosan-Based Packaging
3.3. Chitosan Reinforcement
3.4. Gelatin-Based Film
3.5. Alginate-Based Film
3.6. Antimicrobial Properties of Bio-Based Film
4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3.
- The European Green Deal—European Commission. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 18 April 2024).
- Trung, T.S.; Phuong, P.T.D. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam. J. Food Drug Anal. 2012, 20, 64. [Google Scholar] [CrossRef]
- Venugopal, V. (Ed.) Seafood Processing: Adding Value through Quick Freezing, Retortable Packaging and Cook-Chilling; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-429-12118-0. [Google Scholar]
- Xu, Y.; Bajaj, M.; Schneider, R.; Grage, S.L.; Ulrich, A.S.; Winter, J.; Gallert, C. Transformation of the Matrix Structure of Shrimp Shells during Bacterial Deproteination and Demineralization. Microb. Cell Factories 2013, 12, 90. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Ocean Disposal of Fish Wastes. Available online: https://www.epa.gov/ocean-dumping/ocean-disposal-fish-wastes (accessed on 18 April 2024).
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic Digestion of Food Waste—Challenges and Opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-Wealth: Biowaste Valorization into Valuable Bio(Nano)Materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine Organisms as a Source of Natural Matrix for Bone Tissue Engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Kwon, C.W.; Chang, P.-S. Influence of Alkyl Chain Length on the Action of Acetylated Monoglycerides as Plasticizers for Poly (Vinyl Chloride) Food Packaging Film. Food Packag. Shelf Life 2021, 27, 100619. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Development of Polystyrene-Based Films with Temperature Buffering Capacity for Smart Food Packaging. J. Food Eng. 2015, 164, 55–62. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Review of Surface Treatment Methods for Polyamide Films for Potential Application as Smart Packaging Materials: Surface Structure, Antimicrobial and Spectral Properties. Food Packag. Shelf Life 2020, 24, 100475. [Google Scholar] [CrossRef]
- Ge, L.; Li, X.; Zhang, R.; Yang, T.; Ye, X.; Li, D.; Mu, C. Development and Characterization of Dialdehyde Xanthan Gum Crosslinked Gelatin Based Edible Films Incorporated with Amino-Functionalized Montmorillonite. Food Hydrocoll. 2015, 51, 129–135. [Google Scholar] [CrossRef]
- Kunkel, J.G.; Rosa, M.; Bahadur, A.N. 3D-Xray-Tomography of American Lobster Shell-Structure. An Overview. Fish. Res. 2017, 186, 372–382. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and Analysis of Chitosan Nanocomposites Reinforced with Chitin Whiskers and Tannic Acid as a Crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A State-of-the-Art Review on the Elaboration of Fish Gelatin as Bioactive Packaging: Special Emphasis on Nanotechnology-Based Approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage Extraction and Purification of Waste Sargassum Natans to Produce Sodium Alginate: An Optimization Approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of Bio-Nanocomposite Films Based on Fish Gelatin Reinforced with Chitosan Nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W.; Kim, H.-Y. Preparation, Characterization, and Antimicrobial Activity of Chitin Nanofibrils Reinforced Carrageenan Nanocomposite Films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef]
- Nikolov, S.; Fabritius, H.; Petrov, M.; Friák, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and Optimal Use of Design Principles of Arthropod Exoskeletons Studied by Ab Initio-Based Multiscale Simulations. J. Mech. Behav. Biomed. Mater. 2011, 4, 129–145. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and Mechanical Properties of Insect Cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The Structure of α-Chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; pp. 5–18. ISBN 9789811373183. [Google Scholar]
- Lavall, R.; Assis, O.; Campanafilho, S. β-Chitin from the Pens of Loligo sp.: Extraction and Characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Hurlburt, N.K.; Chen, L.-H.; Stergiopoulos, I.; Fisher, A.J. Structure of the Cladosporium fulvum Avr4 Effector in Complex with (GlcNAc)6 Reveals the Ligand-Binding Mechanism and Uncouples Its Intrinsic Function from Recognition by the Cf-4 Resistance Protein. PLoS Pathog. 2018, 14, e1007263. [Google Scholar] [CrossRef]
- Hossin, M.A.; Al Shaqsi, N.H.K.; Al Touby, S.S.J.; Al Sibani, M.A. A Review of Polymeric Chitin Extraction, Characterization, and Applications. Arab. J. Geosci. 2021, 14, 1870. [Google Scholar] [CrossRef]
- Yang, H.; Gözaydın, G.; Nasaruddin, R.R.; Har, J.R.G.; Chen, X.; Wang, X.; Yan, N. Towards the Shell Biorefinery: Processing Crustacean Shell Waste Using Hot Water and Carbonic Acid. ACS Sustain. Chem. Eng. 2019, 7, 5532–5542. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef]
- Morgan, K.; Conway, C.; Faherty, S.; Quigley, C. A Comparative Analysis of Conventional and Deep Eutectic Solvent (DES)-Mediated Strategies for the Extraction of Chitin from Marine Crustacean Shells. Molecules 2021, 26, 7603. [Google Scholar] [CrossRef]
- Xu, Z. Solubility of Polysaccharides; BoD—Books on Demand: Norderstedt, Germany, 2017; ISBN 978-953-51-3649-1. [Google Scholar]
- Wineinger, H.B.; Kelly, A.; Shamshina, J.L.; Rogers, R.D. Farmed Jumbo Shrimp Molts: An Ionic Liquid Strategy to Increase Chitin Yield per Animal While Controlling Molecular Weight. Green Chem. 2020, 22, 6001–6007. [Google Scholar] [CrossRef]
- Shimo, M.; Abe, M.; Ohno, H. Functional Comparison of Polar Ionic Liquids and Onium Hydroxides for Chitin Dissolution and Deacetylation to Chitosan. ACS Sustain. Chem. Eng. 2016, 4, 3722–3727. [Google Scholar] [CrossRef]
- Wang, W.-T.; Zhu, J.; Wang, X.-L.; Huang, Y.; Wang, Y.-Z. Dissolution Behavior of Chitin in Ionic Liquids. J. Macromol. Sci. Part B 2010, 49, 528–541. [Google Scholar] [CrossRef]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J. Facile Production of Chitin from Crab Shells Using Ionic Liquid and Citric Acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-Pot Production of Chitin with High Purity from Lobster Shells Using Choline Chloride–Malonic Acid Deep Eutectic Solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process. Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile Acid Base Sustainable Solvent for Fast Extraction of Various Molecular Weight Chitin from Lobster Shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of Deproteinization and Demineralization Using Natural Deep Eutectic Solvents for Production of Insect Chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Qiao, M.; Luo, Y. Glycerol/Organic Acid-Based Ternary Deep Eutectic Solvents as a Green Approach to Recover Chitin with Different Molecular Weight from Seafood Waste. Int. J. Biol. Macromol. 2024, 257, 128714. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.d.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and Chitosan on the Nanoscale. Nanoscale Horiz. 2021, 6, 505–542. [Google Scholar] [CrossRef]
- Ma, X.; Gözaydın, G.; Yang, H.; Ning, W.; Han, X.; Poon, N.Y.; Liang, H.; Yan, N.; Zhou, K. Upcycling Chitin-Containing Waste into Organonitrogen Chemicals via an Integrated Process. Proc. Natl. Acad. Sci. USA 2020, 117, 7719–7728. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Yao, Y.; Wang, Y.; Fei, X.; Qi, P.; Lin, S.; Kaplan, D.L.; Buehler, M.J.; Ling, S. Biological Material Interfaces as Inspiration for Mechanical and Optical Material Designs. Chem. Rev. 2019, 119, 12279–12336. [Google Scholar] [CrossRef]
- Arwin, H.; Magnusson, R.; Landin, J.; Järrendahl, K. Chirality-Induced Polarization Effects in the Cuticle of Scarab Beetles: 100 Years after Michelson. Philos. Mag. 2012, 92, 1583–1599. [Google Scholar] [CrossRef]
- Arzt, E.; Gorb, S.; Spolenak, R. From Micro to Nano Contacts in Biological Attachment Devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef]
- Rizzo, N.W.; Gardner, K.H.; Walls, D.J.; Keiper-Hrynko, N.M.; Ganzke, T.S.; Hallahan, D.L. Characterization of the Structure and Composition of Gecko Adhesive Setae. J. R. Soc. Interface 2005, 3, 441–451. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Marcovich, N.E.; Pereda, M. Food Biopackaging Based on Chitosan. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–27. ISBN 978-3-319-48281-1. [Google Scholar]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Chen, P.-W.; Kuo, T.-Y.; Lin, C.-M.; Wang, D.-M.; Hsien, T.-Y.; Hsieh, H.-J. Chitosan/Pectin/Gum Arabic Polyelectrolyte Complex: Process-Dependent Appearance, Microstructure Analysis and Its Application. Carbohydr. Polym. 2014, 101, 752–759. [Google Scholar] [CrossRef]
- Perera, U.M.S.P.; Rajapakse, N. Chitosan Nanoparticles: Preparation, Characterization, and Applications. In Seafood Processing By-Products; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 371–387. ISBN 978-1-4614-9589-5. [Google Scholar]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-Water Emulsions Stabilized by Chitin Nanocrystal Particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid Crystal Systems from Fibrillar Polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Revol, J.-F.; Marchessault, R.H. In Vitro Chiral Nematic Ordering of Chitin Crystallites. Int. J. Biol. Macromol. 1993, 15, 329–335. [Google Scholar] [CrossRef]
- Paillet, M.; Dufresne, A. Chitin Whisker Reinforced Thermoplastic Nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation of α-Chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Hernández-Ramos, F.; Salaberria, A.M.; Andrés, M.Á.; Labidi, J.; Fernandes, S.C.M. Eco-Friendly Isolation and Characterization of Nanochitin from Different Origins by Microwave Irradiation: Optimization Using Response Surface Methodology. Int. J. Biol. Macromol. 2021, 186, 218–226. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different Routes to Turn Chitin into Stunning Nano-Objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable Applications of Chitin and Chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Gómez-Guillén, M.C.; Pérez-Mateos, M.; Montero, P. A Chitosan–Gelatin Blend as a Coating for Fish Patties. Food Hydrocoll. 2005, 19, 303–311. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Reporting Degree of Deacetylation Values of Chitosan: The Influence of Analytical Methods. J. Pharm. Pharm. Sci. 2002, 5, 205–212. [Google Scholar]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Srinivasa Gopal, T.K. Effect of Chitosan Edible Coating on the Quality of Double Filleted Indian Oil Sardine (Sardinella longiceps) during Chilled Storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Pavinatto, A.; Pavinatto, F.J.; Delezuk, J.A.D.M.; Nobre, T.M.; Souza, A.L.; Campana-Filho, S.P.; Oliveira, O.N. Low Molecular-Weight Chitosans Are Stronger Biomembrane Model Perturbants. Colloids Surf. B Biointerfaces 2013, 104, 48–53. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Mariniello, L.; Di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase Crosslinked Pectin- and Chitosan-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial Action of Chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.-E.; Wang, N.; Liu, X.; An, Q.; Ye, X.-M.; Zhao, Z.-T.; Zhao, M.; Han, Y.; et al. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxidative Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Gaur, P. A Simple and Effective Method for Preparation of Chitosan from Chitin. Int. J. Life. Sci. Sci. Res. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Alagui, A.; Vottero, P. Investigation of Different Natural Sources of Chitin: Influence of the Source and Deacetylation Process on the Physicochemical Characteristics of Chitosan. Polym. Int. 2000, 49, 337–344. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, H.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhang, H.; Fan, D. The Physicochemical Properties of Chitosan Prepared by Microwave Heating. Food Sci. Nutr. 2020, 8, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- El Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and Efficient Extraction of Chitin and Chitosan for Scale-up Production: Effect of Process Parameters on Deacetylation Degree and Molecular Weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef]
- Apriyanti, D.T.; Susanto, H.; Rokhati, N. Influence of Microwave Irradiation on Extraction of Chitosan from Shrimp Shell Waste. Reaktor 2018, 18, 45–50. [Google Scholar] [CrossRef]
- Mahardika, R.G.; Jumnahdi, M.; Widyaningrum, Y. Chitin Deacetylation Shells of Portunus pelagicus L. Using Microwave Irradiation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 353, p. 012037. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, X.; Bi, X.; Han, Q.; Tu, L.; Yang, Y.; Shen, Y.; Wang, M. Dissolution and Deacetylation of Chitin in Ionic Liquid Tetrabutylammonium Hydroxide and Its Cascade Reaction in Enzyme Treatment for Chitin Recycling. Carbohydr. Polym. 2020, 230, 115605. [Google Scholar] [CrossRef]
- Aspras, I.; Kamińska, M.; Karzyński, K.; Kawka, M.; Jaworska, M.M. The Influence of Selected Ionic Liquids on Activity of Chitin Deacetylase. Chem. Process Eng. 2016, 37, 77–82. [Google Scholar] [CrossRef]
- Ishii, D.; Ohashi, C.; Hayashi, H. Facile Enhancement of the Deacetylation Degree of Chitosan by Hydrothermal Treatment in an Imidazolium-Based Ionic Liquid. Green Chem. 2014, 16, 1764–1767. [Google Scholar] [CrossRef]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and Antitumor Activities of Chitosan from Shiitake Stipes, Compared to Commercial Chitosan from Crab Shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent Advances in Extraction, Modification, and Application of Chitosan in Packaging Industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Qin, Y.; Li, L.; Yuan, M. A pH Indicator Film Based on Chitosan and Butterfly Pudding Extract for Monitoring Fish Freshness. Int. J. Biol. Macromol. 2021, 177, 328–336. [Google Scholar] [CrossRef]
- Esmaeili, M.; Ariaii, P.; Nasiraie, L.R.; Pour, M.Y. Comparison of Coating and Nano-Coating of Chitosan- Lepidium Sativum Seed Gum Composites on Quality and Shelf Life of Beef. Food Meas. 2021, 15, 341–352. [Google Scholar] [CrossRef]
- Homayonpour, P.; Jalali, H.; Shariatifar, N.; Amanlou, M. Effects of Nano-Chitosan Coatings Incorporating with Free/Nano-Encapsulated Cumin (Cuminum cyminum L.) Essential Oil on Quality Characteristics of Sardine Fillet. Int. J. Food Microbiol. 2021, 341, 109047. [Google Scholar] [CrossRef]
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2023, 35, 2203325. [Google Scholar] [CrossRef] [PubMed]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan Based-Nanoparticles and Nanocapsules: Overview, Physicochemical Features, Applications of a Nanofibrous Scaffold, and Bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- Huang, K.-S.; Sheu, Y.-R.; Chao, I.-C. Preparation and Properties of Nanochitosan. Polym.-Plast. Technol. Eng. 2009, 48, 1239–1243. [Google Scholar] [CrossRef]
- Ohya, Y.; Shiratani, M.; Kobayashi, H.; Ouchi, T. Release Behavior of 5-Fluorouracil from Chitosan-Gel Nanospheres Immobilizing 5-Fluorouracil Coated with Polysaccharides and Their Cell Specific Cytotoxicity. J. Macromol. Sci. Part A 1994, 31, 629–642. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Wu, Y. Advances in Chitosan-Based Drug Delivery Vehicles. Nanoscale 2013, 5, 3103. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-X.; Groves, M.J. Formulation and Biological Activity of Antineoplastic Proteoglycans Derived from Mycobacterium Vaccae in Chitosan Nanoparticles. J. Pharm. Pharmacol. 1999, 51, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Chen, H.; Paeratakul, O. A Novel Approach to the Oral Delivery of Micro- or Nanoparticles. Pharm. Res. 1989, 6, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.M.; Higuera-Ciapara, I.; Argüelles-Monal, W. Preparation of Chitosan Nanoparticles by Nanoprecipitation and Their Ability as a Drug Nanocarrier. RSC Adv. 2016, 6, 59250–59256. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.-L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of Chitosan Nanoparticles by Spray Drying, and Their Antibacterial Activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Kulvanich, P.; Sinsuebpol, C.; Chatchawalsaisin, J. Preparation and In Vivo Absorption Evaluation of Spray Dried Powders Containing Salmon Calcitonin Loaded Chitosan Nanoparticles for Pulmonary Delivery. Drug Des. Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef]
- Kumosa, L.S.; Zetterberg, V.; Schouenborg, J. Gelatin Promotes Rapid Restoration of the Blood Brain Barrier after Acute Brain Injury. Acta Biomater. 2018, 65, 137–149. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Jakhar, J.; Kumar, A.; Vardia, H.K. Extraction of Gelatin from Skin and Scale of Indian Major Carps. Environ. Ecol. 2019, 34, 1513–1518. [Google Scholar]
- Feng, X.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Liu, T.; Zhang, Y. Food-Grade Gelatin Nanoparticles: Preparation, Characterization, and Preliminary Application for Stabilizing Pickering Emulsions. Foods 2019, 8, 479. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle Tissue Engineering in Fibrous Gelatin: Implications for Meat Analogs. NPJ Sci Food 2019, 3, 20. [Google Scholar] [CrossRef]
- Etxabide, A.; Mojío, D.; Guerrero, P.; De La Caba, K.; Gómez-Estaca, J. Chitin Nanowhisker-Containing Photo-Crosslinked Antimicrobial Gelatin Films. Food Hydrocoll. 2024, 147, 109371. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Liu, Z.; Huang, H. Enhanced Swelling and Multiple-Responsive Properties of Gelatin/Sodium Alginate Hydrogels by the Addition of Carboxymethyl Cellulose Isolated from Pineapple Peel. Cellulose 2018, 25, 593–606. [Google Scholar] [CrossRef]
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical Properties with Respect to Water Content of Gelatin Films in Glassy State. Polymer 2005, 46, 12577–12585. [Google Scholar] [CrossRef]
- Ma, M.; Ma, L.; Yu, W.; Zhang, X.; Shen, Y.; Zhang, Y. Research on Rapid Gelatinization of Rabbit Skin Collagen as Effect of Acid Treatment. Food Hydrocoll. 2018, 77, 945–951. [Google Scholar] [CrossRef]
- Al-Hassan, A.A. Gelatin from Camel Skins: Extraction and Characterizations. Food Hydrocoll. 2020, 101, 105457. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as Emulsifiers for Oil-in-Water Emulsions: Extraction, Chemical Composition, Molecular Structure, and Molecular Modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Sompie, M.; Surtijono, S.E.; Pontoh, J.H.W.; Lontaan, N.N. The Effects of Acetic Acid Concentration and Extraction Temperature on Physical and Chemical Properties of Pigskin Gelatin. Procedia Food Sci. 2015, 3, 383–388. [Google Scholar] [CrossRef]
- Chen, L.; Ma, L.; Zhou, M.; Liu, Y.; Zhang, Y. Effects of Pressure on Gelatinization of Collagen and Properties of Extracted Gelatins. Food Hydrocoll. 2014, 36, 316–322. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Guo, T.; Yu, Y.; Li, X.; Xia, W.; Zhang, Y. Effects of Freezing-Thawing Pretreatment Combined with Liquid Nitrogen and Dilute Acid on the Gelatinization of Collagen. Int. J. Biol. Macromol. 2018, 118, 435–441. [Google Scholar] [CrossRef]
- Feng, X.; Liu, T.; Ma, L.; Dai, H.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Tan, H.; Zhang, Y. A Green Extraction Method for Gelatin and Its Molecular Mechanism. Food Hydrocoll. 2022, 124, 107344. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of Sodium Alginate Coating Enriched with Horsemint (Mentha Longifolia) Essential Oil on the Quality of Bighead Carp Fillets during Storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E. Developing Multifunctional Edible Coatings Based on Alginate for Active Food Packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef]
- FDA. Title 21 of the CFR—Food and Drugs. Available online: https://www.ecfr.gov/current/title-21 (accessed on 4 May 2024).
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of an Edible Wheat Gluten Film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of Plasticiser on the Barrier, Mechanical and Grease Resistance Properties of Alginate Cast Films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative Studies on the Characterization and Antioxidant Properties of Biodegradable Alginate Films Containing Ginseng Extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Maizura, M.; Fazilah, A.; Norziah, M.H.; Karim, A.A. Antibacterial Activity and Mechanical Properties of Partially Hydrolyzed Sago Starch–Alginate Edible Film Containing Lemongrass Oil. J. Food Sci. 2007, 72, C324–C330. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L.; Rodrıguez-Montesinos, E. Pilot Plant Scale Extraction of Alginate from Macrocystis Pyrifera. 1. Effect of Pre-Extraction Treatments on Yield and Quality of Alginate. J. Appl. Phycol. 1998, 10, 507–513. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of Alginate Extraction Processes: Impact on Alginate Molecular Structure and Techno-Functional Properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Murano, E.; Paoletti, S. Alginate as Immobilization Material. II: Determination of Polyphenol Contaminants by Fluorescence Spectroscopy, and Evaluation of Methods for Their Removal. Biotechnol. Bioeng. 1989, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Egusa, M.; Matsukawa, S.; Miura, C.; Nakatani, S.; Yamada, J.; Endo, T.; Ifuku, S.; Kaminaka, H. Improving Nitrogen Uptake Efficiency by Chitin Nanofiber Promotes Growth in Tomato. Int. J. Biol. Macromol. 2020, 151, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; An, S.; Yin, X. Nanochitin Whisker Enhances Insecticidal Activity of Chemical Pesticide for Pest Insect Control and Toxicity. J. Nanobiotechnol. 2021, 19, 49. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin Nanocrystals and Nanofibers as Nano-Sized Fillers into Thermoplastic Starch-Based Biocomposites Processed by Melt-Mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Andrés, M.A.; Fernandes, S.C.M.; Labidi, J. The Antifungal Activity of Functionalized Chitin Nanocrystals in Poly (Lactid Acid) Films. Materials 2017, 10, 546. [Google Scholar] [CrossRef]
- Satam, C.C.; Irvin, C.W.; Lang, A.W.; Jallorina, J.C.R.; Shofner, M.L.; Reynolds, J.R.; Meredith, J.C. Spray-Coated Multilayer Cellulose Nanocrystal—Chitin Nanofiber Films for Barrier Applications. ACS Sustain. Chem. Eng. 2018, 6, 10637–10644. [Google Scholar] [CrossRef]
- Hai, L.; Choi, E.S.; Zhai, L.; Panicker, P.S.; Kim, J. Green Nanocomposite Made with Chitin and Bamboo Nanofibers and Its Mechanical, Thermal and Biodegradable Properties for Food Packaging. Int. J. Biol. Macromol. 2020, 144, 491–499. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation of Multifunctional Chitin Nanowhiskers/ZnO-Ag NPs and Their Effect on the Properties of Carboxymethyl Cellulose-Based Nanocomposite Film. Carbohydr. Polym. 2017, 169, 467–479. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti Khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A.; Noshirvani, N.; Hosseini, M. The Optimization of Gelatin-CMC Based Active Films Containing Chitin Nanofiber and Trachyspermum Ammi Essential Oil by Response Surface Methodology. Carbohydr. Polym. 2019, 208, 457–468. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Maté, J.I.; Gómez-Estaca, J. Characterization of Glucose-Crosslinked Gelatin Films Reinforced with Chitin Nanowhiskers for Active Packaging Development. LWT 2022, 154, 112833. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of Corn Oil on Physical, Thermal, and Antifungal Properties of Gelatin-Based Nanocomposite Films Containing Nano Chitin. LWT—Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of Gelatin Bionanocomposite Films Containing Chitin and ZnO Nanoparticles. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Li, Y.; Ma, J.; Lu, Y.; Pang, J.; Wu, C. Preparation and Characterization of Citric Acid Crosslinked Konjac Glucomannan/Surface Deacetylated Chitin Nanofibers Bionanocomposite Film. Int. J. Biol. Macromol. 2020, 164, 2612–2621. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of Chitin Nano-Whiskers on the Antibacterial and Physicochemical Properties of Maize Starch Films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Xu, J.; Deng, X.; Dong, Y.; Zhou, Z.; Zhang, Y.; Yu, J.; Cai, J.; Zhang, Y. High-Strength, Transparent and Superhydrophobic Nanocellulose/Nanochitin Membranes Fabricated via Crosslinking of Nanofibers and Coating F-SiO2 Suspensions. Carbohydr. Polym. 2020, 247, 116694. [Google Scholar] [CrossRef]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Salehi, E.A.; Soltani, M. Study of Structural and Antibacterial Properties of Edible Coating of Zein/Nano Chitin Composite. J. Innov. Food Sci. Technol. 2021, 13, 135–142. [Google Scholar]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of Chitosan/Curcumin Nanoparticles Based Zein and Potato Starch Composite Films for Schizothorax prenati Fillet Preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Joseph, B.; Mavelil Sam, R.; Balakrishnan, P.; Maria, H.J.; Gopi, S.; Volova, T.; Fernandes, S.C.M.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Rouhi, J.; Mahmud, S.; Naderi, N.; Ooi, C.R.; Mahmood, M.R. Physical Properties of Fish Gelatin-Based Bio-Nanocomposite Films Incorporated with ZnO Nanorods. Nanoscale Res. Lett. 2013, 8, 364. [Google Scholar] [CrossRef]
- Zeppa, C.; Gouanvé, F.; Espuche, E. Effect of a Plasticizer on the Structure of Biodegradable Starch/Clay Nanocomposites: Thermal, Water-Sorption, and Oxygen-Barrier Properties. J. Appl. Polym. Sci. 2009, 112, 2044–2056. [Google Scholar] [CrossRef]
- Dai, H.; Lv, T.; Dai, D.; Luo, Y.; Ma, L.; Zhang, Y. Preparation and Physicochemical Properties of Nanocellulose Lightweight Porous Materials: The Regulating Effect of Gelatin. Food Chem. 2023, 426, 136497. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Sarbon, N.M. A Comparative Study: Physical, Mechanical and Antibacterial Properties of Bio-Composite Gelatin Films as Influenced by Chitosan and Zinc Oxide Nanoparticles Incorporation. Food Biosci. 2021, 43, 101250. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The Impact of Cross-Linking Mode on the Physical and Antimicrobial Properties of a Chitosan/Bacterial Cellulose Composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef]
- Yeamsuksawat, T.; Liang, J. Characterization and Release Kinetic of Crosslinked Chitosan Film Incorporated with α-Tocopherol. Food Packag. Shelf Life 2019, 22, 100415. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yu, H.; Zain-ul-Abdin; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent Progress on Synthesis, Property and Application of Modified Chitosan: An Overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the Physical Properties and Biological Activity of Chitosan Films Grafted with Gallic Acid and Caffeic Acid: A Comparison Study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Águila-Almanza, E.; Salgado-Delgado, R.; Vargas-Galarza, Z.; García-Hernández, E.; Hernández-Cocoletzi, H. Enzymatic Depolimerization of Chitosan for the Preparation of Functional Membranes. J. Chem. 2019, 2019, e5416297. [Google Scholar] [CrossRef]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.D.A. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes Sapidus) Waste and Pectin from Orange (Citrus Sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant Potential of Eugenol and Ginger Essential Oils with Gelatin/Chitosan Films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of Poly(Lactic Acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of Bioactive Composite Films from Chitosan and Carboxymethyl Cellulose Using Glutaraldehyde, Cinnamon Essential Oil and Oleic Acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical Properties of the Edible Films from the Blends of High Methoxyl Apple Pectin and Chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Shu, Z. Development of Biodegradable Composite Chitosan-Based Films Incorporated with Xylan and Carvacrol for Food Packaging Application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Huang, M.; Yang, Y.; Shen, X.; Tang, X. Cinnamon and Clove Essential Oils to Improve Physical, Thermal and Antimicrobial Properties of Chitosan-Gum Arabic Polyelectrolyte Complexed Films. Carbohydr. Polym. 2019, 217, 116–125. [Google Scholar] [CrossRef]
- Li, S.; Yan, Y.; Guan, X.; Huang, K. Preparation of a Hordein-Quercetin-Chitosan Antioxidant Electrospun Nanofibre Film for Food Packaging and Improvement of the Film Hydrophobic Properties by Heat Treatment. Food Packag. Shelf Life 2020, 23, 100466. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-Chemical and Antilisterial Properties of Nisin-Incorporated Chitosan/Carboxymethyl Chitosan Films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers 2019, 11, 1418. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Kariminejad, M.; Mortazavian, A.M.; Sadeghi, E.; Hasanvand, S. Physico-Mechanical and Structural Properties of Eggshell Membrane Gelatin- Chitosan Blend Edible Films. Int. J. Biol. Macromol. 2018, 107, 406–412. [Google Scholar] [CrossRef]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and Characterization of Acid Soluble Collagen from Fish Waste: Development of Collagen-Chitosan Blend as Food Packaging Film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Batista, J.T.S.; Araújo, C.S.; Peixoto Joele, M.R.S.; Silva, J.O.C.; Lourenço, L.F.H. Study of the Effect of the Chitosan Use on the Properties of Biodegradable Films of Myofibrillar Proteins of Fish Residues Using Response Surface Methodology. Food Packag. Shelf Life 2019, 20, 100306. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative Analysis of Blend and Bilayer Films Based on Chitosan and Gelatin Enriched with LAE (Lauroyl Arginate Ethyl) with Antimicrobial Activity for Food Packaging Applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of Active Packaging Based on Chitosan-Gelatin Blend Films Functionalized with Chinese Hawthorn (Crataegus pinnatifida) Fruit Extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Samsalee, N.; Sothornvit, R. Development and Characterization of Porcine Plasma Protein-Chitosan Blended Films. Food Packag. Shelf Life 2019, 22, 100406. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ibresam, O.T.; Al-Hatim, R.R.; Al-Ali, R.M.; Maslekar, N.; Yao, Y.; Agarwal, V. Development of Chitosan/Whey Protein Hydrolysate Composite Films for Food Packaging Application. J. Compos. Sci. 2023, 7, 94. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined Antioxidant and Sensory Effects of Active Chitosan/Zein Film Containing α-Tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De La Caba, K. Characterization and Antimicrobial Analysis of Chitosan-Based Films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; De Toledo Guimarães, J.; Fallah, M.; Mohammadi, R. Bioactive Edible Films: Development and Characterization of Gelatin Edible Films Incorporated with Casein Phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; De La Caba, K. Citric Acid-Incorporated Fish Gelatin/Chitosan Composite Films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Yin, D.; Zeng, X. A Comparative Study on Chitosan/Gelatin Composite Films with Conjugated or Incorporated Gallic Acid. Carbohydr. Polym. 2017, 173, 473–481. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating Nisin and Grape Seed Extract in Chitosan-Gelatine Edible Coating and Its Effect on Cold Storage of Fresh Pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn Starch and Gelatin-Based Films Added with Guabiroba Pulp for Application in Food Packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and Physical Reinforcement of Hydrophilic Gelatin Film with Di-Aldehyde Nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Boughriba, S.; Souissi, N.; Jridi, M.; Li, S.; Nasri, M. Thermal, Mechanical and Microstructural Characterization and Antioxidant Potential of Rhinobatos Cemiculus Gelatin Films Supplemented by Titanium Dioxide Doped Silver Nanoparticles. Food Hydrocoll. 2020, 103, 105695. [Google Scholar] [CrossRef]
- Ahmed, S. Alginates: Applications in the Biomedical and Food Industries; John Wiley & Sons: New York, NY, USA, 2019; ISBN 978-1-119-48791-3. [Google Scholar]
- Abdel Aziz, M.S.; Salama, H.E.; Saad, G.R. Diglycidyl Ether of Bisphenol A/Chitosan-Graft-Polyaniline Composites with Electromagnetic Interference Shielding Properties: Synthesis, Characterization, and Curing Kinetics. Polym. Eng. Sci. 2019, 59, 372–381. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, X.; Jia, R.; Fang, Y.; Qin, Z. Development of Antibacterial Film Based on Alginate Fiber, and Peanut Red Skin Extract for Food Packaging. J. Food Eng. 2022, 330, 111106. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and Bioactivities of Biopolymeric Films Incorporated with Cellulose, Sodium Alginate and Copper Oxide Nanoparticles for Food Packaging Application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Daneshvar, H.; Seyed Dorraji, M.S.; Ghasempour, Z.; Panahi-Azar, V.; Ehsani, A. Starch/Alginate/ Cu-g-C3N4 Nanocomposite Film for Food Packaging. Mater. Chem. Phys. 2021, 267, 124583. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Ben Akacha, B.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants 2022, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/Sodium Alginate Multilayer Composite Film Crosslinked with Green Tea Extract for Active Food Packaging Application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131013. [Google Scholar] [CrossRef]
- Tong, W.Y.; Ahmad Rafiee, A.R.; Leong, C.R.; Tan, W.-N.; Dailin, D.J.; Almarhoon, Z.M.; Shelkh, M.; Nawaz, A.; Chuah, L.F. Development of Sodium Alginate-Pectin Biodegradable Active Food Packaging Film Containing Cinnamic Acid. Chemosphere 2023, 336, 139212. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, H.-J.; Rhim, J.-W. Effect of Sulfur Nanoparticles on Properties of Alginate-Based Films for Active Food Packaging Applications. Food Hydrocoll. 2021, 110, 106155. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of Sodium Alginate-Based Films Incorporated with Thymol for Fresh-Cut Apple Packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Sun, J.; Lv, S. Bioactive Edible Sodium Alginate Films Incorporated with Tannic Acid as Antimicrobial and Antioxidative Food Packaging. Foods 2022, 11, 3044. [Google Scholar] [CrossRef] [PubMed]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate Sensing of Chitin and Chitosan. PLoS Pathog. 2013, 9, e1003080. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef]
- Dušková-smrčková, M.; Dušek, K. Processes and States during Polymer Film Formation by Simultaneous Crosslinking and Solvent Evaporation. J. Mater. Sci. 2002, 37, 4733–4741. [Google Scholar] [CrossRef]
- Gulin-Sarfraz, T.; Grøvlen, M.S.; Rosqvist, E.; Pettersen, M.K.; Peltonen, J.; Sarfraz, J. Optimized Multilayer Coating Using Layer-by-Layer Assembly Method for Excellent Oxygen Barrier of Poly(Lactic Acid) Based Film. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131155. [Google Scholar] [CrossRef]
- Del Hoyo-Gallego, S.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Laza, J.M.; Vila-Vilela, J.L. Construction of Antibacterial Poly(Ethylene Terephthalate) Films via Layer by Layer Assembly of Chitosan and Hyaluronic Acid. Carbohydr. Polym. 2016, 143, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhang, J.; Du, K.; Zhang, Y.; Gao, J.; Su, J.; Zhang, S. Reclaimed Lignosulfonate-Modified Bamboo Fibers Prepared via Layer-by-Layer Self-Assembly to Reinforce Poly(3-Hydroxybutyrate) Biocomposite. Ind. Crops Prod. 2023, 205, 117449. [Google Scholar] [CrossRef]
- Fraga, A.N.; Williams, R.J.J. Thermal Properties of Gelatin Films. Polymer 1985, 26, 113–118. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Arivazhagan, V.; Xie, J.; Yang, Z.; Hang, P.; Parvathi, M.M.; Xiao, K.; Cui, C.; Yang, D.; Yu, X. Vacuum Co-Deposited CH3NH3PbI3 Films by Controlling Vapor Pressure for Efficient Planar Perovskite Solar Cells. Sol. Energy 2019, 181, 339–344. [Google Scholar] [CrossRef]
- Wang, S.; Ono, L.K.; Leyden, M.R.; Kato, Y.; Raga, S.R.; Lee, M.V.; Qi, Y. Smooth Perovskite Thin Films and Efficient Perovskite Solar Cells Prepared by the Hybrid Deposition Method. J. Mater. Chem. A 2015, 3, 14631–14641. [Google Scholar] [CrossRef]
- Otalora, C.; Botero, M.A.; Mantilla, M.A.; Petit, J.F.; Ospina, R.; Gordillo, G. Hybrid Perovskite Films Deposited by Thermal Evaporation from a Single Source. J. Mater. Sci. Mater. Electron. 2021, 32, 12151–12163. [Google Scholar] [CrossRef]
- WWF. The Hidden Cost of Plastic. Available online: https://www.wwfdrc.org/?36252/The-hidden-cost-of-plastic (accessed on 12 June 2024).
| Ingredients | Properties Improvement | Reference |
|---|---|---|
| Bamboo cellulose nanofiber | Mechanical properties: tensile strength increased by 204%, Young’s modulus increased by 33%, elongation at break increased by 152%; Biodegradable time: fully degraded within a week; Antimicrobial properties: Escherichia coli (E. coli) and Listeria monocytogenes (L. monocytogenes). | [141] |
| Carboxymethyl cellulose, ZnO-Ag | Mechanical properties: tensile strength increased by 132%, elastic modulus increased by 101%; Water vapor permeability: decreased by 21%; UV light barrier; Thermal stability: mid-point temperature of the degradation increased by 15 °C. | [142] |
| Gelatin | Mechanical properties: elongation at break increased by 152%, elastic modulus increased by 140%; UV light barrier. | [113] |
| Gelatin, anthocyanins | Mechanical property: elongation at break increased by 23%; UV light barrier: light transmittance rate at 560 nm decreased by 60.18%; Oxygen barrier: decreased by 35%; Water vapor permeability: decreased by 14%; Antioxidant property: the maximum radical scavenging rate could be close to 100%. | [143] |
| Gelatin, carboxymethyl cellulose, ajowan essential oil | Mechanical properties: ultimate tensile strength significantly decreased, and strain at break increased; Water vapor barrier: water contact angle increased and water vapor permeability decreased by 40%; Antibacterial properties: Staphylococcus aureus (S. aureus) and E. coli. | [144] |
| Glucose-crosslinked gelatin | Thermal stability: degradation temperature increased; UV light barrier; Mechanical properties: Young’s modulus increased by 98%, ultimate tensile strength increased by 83%; Antioxidant release: absorbance in the UV region increased and reduced the occurrence of photo-oxidation reactions in foods. | [145] |
| Gelatin, corn oil | Mechanical properties: elastic modulus decreased by 82% and elongation at break increased by 887%; Moisture content: decreased by 51%; Water vapor barrier: solubility and water contact angle increased by 33%, and water vapor permeability decreased by 21%; Antimicrobial property: Aspergillus niger. | [146] |
| Gelatin, zinc oxide nanoparticles | Mechanical properties: ultimate tensile strength increased by 2%, elongation at the break increased by 194%, elastic modulus increased by 175%; Water vapor permeability: decreased by 44%; Thermal stability: temperature of melting point increased by 25% and enthalpy increased by 1150%; Antimicrobial property: A. niger. | [147] |
| Konjac glucomannan, citric acid | Mechanical properties: tensile strength increased by 191.86%, elongation at break increased by 444%; Water vapor permeability: 42% reduction; Antibacterial properties: S. aureus and E. coli. | [148] |
| Maize starch | Mechanical property: tensile strength increased by 45%; Water vapor permeability: decreased by 58%; Antibacterial properties: L. monocytogenes and E. coli. | [149] |
| Nanocellulose, nano-SiO2 | Mechanical property: tensile strength increased by 80%; Light transmittance: showed 30.7% lower; Super hydrophobicity: the water contact angle becomes larger, increasing by 106.4°. | [150] |
| Starch | Mechanical properties: tensile strength increased by 216%, toughness had a 270% reduction, Young’s modulus increased by 239%; Moisture absorption: moisture absorption dropped 13%. | [151] |
| Zein | Antibacterial properties. | [152] |
| Zein, potato starch | Mechanical properties: tensile strength increased by 66%, elongation at break increased by 163%; Barrier properties: water vapor permeability decreased by 10%, oxygen permeability decreased by 17%; Antioxidant characteristic. | [153] |
| Ingredients | Properties Improvement | Reference |
|---|---|---|
| Acetic acid, glycerin | Antibacterial properties: Pseudomonas aeruginosa (P. aeruginosa), Pantoea ananatis, and E. coli; Antioxidant property: DPPH reduction barely exceeds 10%. | [166] |
| Carboxymethyl cellulose, glutaraldehyde, cinnamon essential oil, oleic acid | Mechanical property: elongation at break increased by 62%; Solubility: 32% reduction; Antibacterial properties: P. aeruginosa and L. monocytogenes; Antioxidant property. | [171] |
| Carvacrol and xylan | Mechanical properties: tensile strength increased by 41%, elongation at break increased by 14%; Thermal stability: mass loss rate decreased by 14.5%/°C. | [174] |
| Eugenol, ginger essential oils, gelatin | Mechanical properties: tensile strength increased by 106%, elongation at break increased by 822%; UV barrier: transmittance was null until 300 nm and showed a high barrier from 300–450 nm. | [168] |
| Gum, cinnamon, and clove essential oils | Mechanical property: elongation at break increased by 28%; Antibacterial properties: S. aureus and E. coli; Water vapor permeability: 36% reduction. | [175] |
| Gelatin, tapioca starch, zinc oxide nanoparticles | Mechanical properties: tensile strength increased by 10%; Thermal stability: melting temperature increased by 4%; Antibacterial properties: S. aureus and E. coli. | [158] |
| High-methoxyl apple pectin | Mechanical properties: tensile strength increased by 432%, elongation at break increased by 62%, Young’s modulus increased by 358%; Water barrier: water vapor permeability decreased, and water contact angle increased; Transparency: opacity increased by 345%. | [173] |
| Hordein, quercetin | Water resistance: water contact angle increased; Antioxidant activity: Slowing down the enzymatic browning rate of food. | [176] |
| Mango leaf extract | Mechanical properties: tensile strength increased by 27%, elastic modulus increased by 23%; Water barrier: water vapor permeability decreased by 52%, water contact angle increased by 17.1°; Antioxidant activity: opacity increased by 78%. | [93] |
| Nisin | Mechanical properties: tensile strength increased by 23%, elongation at break increased by 211%; Thermal stability: endotherm peak temperatures increased by 8.8 °C; Antibacterial property: L. monocytogenes. | [177] |
| Potato starch, citric acid | Mechanical properties: tensile strength increased by 7%, elongation at break increased by 57%; Water barrier: water contact angle increased by 17.26°, water vapor permeability decreased by 30%; Antimicrobial properties: E. coli and S. aureus. | [178] |
| Starch, nano titanium dioxide, clove oil | Mechanical properties: tensile strength increased by 19%, elongation at break increased by 45%; Water barrier: water contact angle increased by 23.5°, water vapor permeability decreased by 25%; Antioxidant activity: DPPH radical scavenging activity increased by 222%, the ABTS radical scavenging activity increased by 130%. | [179] |
| Ingredients | Properties Improvement | Reference |
|---|---|---|
| Eggshell membrane gelatin | Mechanical property: elongation at break increased by 1031%; Water vapor permeability: 84% reduction; UV barrier. | [180] |
| Fish collagen, pomegranate peel extract | Antibacterial properties: Bacillus saprophyticus LNB 333 F5, Bacillus subtilis NCIM 2635, Salmonella typhimurium (S. typhimurium) NCIM 2501, E. coli NCIM 2832. | [181] |
| Fish residue myofibrillar proteins | Mechanical properties: tensile strength increased by 28%, elongation at break increased by 179%; Solubility: 66% reduction; UV barrier: except for the wavelength at 280 nm, all transmittance values were significantly lower; Thermal stability: the melting temperature was higher. | [182] |
| Gelatin, lauroyl arginate ethyl | Mechanical properties: tensile strength increased by 258%, elongation at break increased by 9%, elastic modulus increased by 72%; Water vapor permeability: 25% reduction; UV barrier; Antibacterial properties: L. monocytogenes, E. coli, S. typhimurium, Campylobacter jejuni (C. jejuni). | [183] |
| Polyphenols | Mechanical properties: tensile strength increased by 21%, elongation at break increased by 171%; Water vapor permeability: 54% reduction; Antioxidant activity: DPPH radical scavenging activity increased by 2070%. | [184] |
| Porcine plasma protein | Mechanical properties: tensile strength increased by 651%, elongation at break increased by 163%; Water solubility; Water vapor permeability: 50% reduction; Thermal ability: 1st peak temperature and 2nd peak temperature increased by 3 °C. | [185] |
| Whey protein | Mechanical property: tensile strength increased by 141%; Antioxidant activity: DPPH activity increased by 113%. | [186] |
| Zein, α-tocopherol | Oxygen barrier: oxygen permeability decreased by 61%; Water vapor permeability: 50% reduction Antioxidant activity: inhibit the browning of mushrooms, which may be related to the inhibition of POD and PPO activities. | [187] |
| Ingredients | Properties Improvement | Reference |
|---|---|---|
| Anthocyanins, nanochitin | UV light barrier: light transmittance rate at 560 nm decreased by 60.18%; Oxygen barrier: decreased by 35%; Water vapor permeability: 14% reduction; Antioxidant property: the maximum radical scavenging rate could be close to 100%. | [143] |
| Casein phosphopeptides | Mechanical properties: tensile strength increased by 89%, elongation at break increased by 260%; Water vapor permeability: 35% reduction; UV barrier; Antimicrobial properties: Bacillus cereus (B. cereus) and S. aureus; Antioxidant activity. | [189] |
| Carboxymethyl cellulose, nanochitin, ajowan essential oil | Mechanical properties: ultimate tensile strength significantly decreased, and strain at break increased; Water vapor barrier: water contact angle increased, and water vapor permeability decreased by 40%; Antibacterial properties: S. aureus and E. coli. | [144] |
| Chitosan, citric acid | Swelling: swelling values decreased below 600% after 24 h with the addition of citric acid; UV Barrier: films provided a UV light barrier from 200 to 250 nm; Antibacterial property: E. coli; Hydrophobic surfaces. | [190] |
| Chitosan, gallic acid | Mechanical property: Young’s modulus increased by 103%; Water vapor permeability: 12% reduction; Antioxidant property; Antimicrobial properties: B. cereus, S. aureus, E. coli, and S. typhimurium. | [191] |
| Chitosan, nisin, frape seed extract | Antioxidant activity. | [192] |
| Chitosan, lauroyl arginate ethyl | Mechanical properties: tensile strength increased by 258%, elongation at break increased by 9%, elastic modulus increased by 72%; Water vapor permeability: 25% reduction; UV barrier; Antibacterial properties: L. monocytogenes, E. coli, S. typhimurium, C. jejuni. | [183] |
| Corn starch, guabiroba pulp | Mechanical property: tensile strength achieved highest at gelatin: corn starch = 2:1, elongation at break reached highest at gelatin: corn starch = 1:2; Water vapor permeability: achieved lowest at gelatin: corn starch = 1:1. | [193] |
| Di-aldehyde nanocellulose | Mechanical Properties: tensile strength enhanced 275%; Hydrophilic property. | [194] |
| Nanochitin | Mechanical properties: elongation at break increased by 152%, elastic modulus increased by 140%; UV light barrier. | [113] |
| Titanium dioxide doped silver nanoparticles | Thermal stability: glass transition temperature increased by 82 °C, film is thermally stable until approximately 800 °C; Antioxidant property: DPPH radical scavenging activity increased by 25%. | [195] |
| Tapioca starch, nanochitin | Mechanical property: tensile strength increased by 10%; Thermal stability: melting temperature increased by 4%; Antibacterial properties: S. aureus and E. coli. | [158] |
| Additives | Properties Improvement | Reference |
|---|---|---|
| Aloe vera, garlic oil | Mechanical properties: tensile strength increased by 51%, elongation at break increased by 316%; Water vapor permeability: 45% reduction; UV light barrier: transmittance of UVC, UVB, and UVA decreased by 97%, 96%, and 96%, respectively; Antimicrobial properties: S. aureus, E. coli, and S. racemosum. | [125] |
| Carboxymethyl cellulose, chitosan, CaCl2 | Mechanical properties: tensile strength increased by 81%, elongation at break increased by 46%; Water vapor permeability: 4% reduction; Antimicrobial properties: S. aureus and E. coli. | [198] |
| Cellulose nanofibers, peanut red skin extract | Mechanical property: tensile strength increased by 1033%; Antioxidant property: the maximum ABTS scavenging activity was 99.28%; Antibacterial properties: E. coli, S. typhimurium, S. aureus, and L. monocytogenes. | [199] |
| Cellulose nanowhisker, copper oxide nanoparticles | Antimicrobial properties: S. aureus, E. coli, Salmonella spp., C. albicans, Trichoderma spp.; Antioxidant property: DPPH scavenging increased by 41.55%. | [200] |
| Cu-deposited graphitic carbon nitride (g-C3N4) nanoparticles, starch | Mechanical property: tensile strength increased by 55%; Water vapor permeability: 59% reduction; Antimicrobial properties: S. aureus and E. coli. | [201] |
| Gelatin, aqueous beetroot peel extract | Mechanical properties: tensile strength increased by 158%, elongation at break increased by 49%; Antimicrobial properties: S. aureus, L. monocytogenes, S. enterica, and E. coli; Antioxidant property: DPPH scavenging ability increased by 133%. | [202] |
| Gelatin, green tea extract | Mechanical property: tensile strength increased; Water vapor permeability: 35% reduction; Oxygen barrier: oxygen permeability reduced by 58%; UV light barrier; Antimicrobial properties: S. aureus and E. coli. | [203] |
| Pectin, cinnamic acid | Mechanical properties: tensile strength increased by 9%, elongation at break increased by 13%; Antimicrobial activity: B. subtilis, MRSA, S. aureus, Proteus mirabilis (P. mirabilis), S. typhimurium, E. coli, A. anitratus, Yersinia enterocolitica (Y. enterocolitica), and Pseudomonas aeruginosa (P. aeruginosa). | [204] |
| Sulfur nanoparticles | Mechanical properties: tensile strength increased by 12%, elastic modulus increased by 107%; Water vapor permeability: 41% reduction; UV light barrier; Antibacterial properties: E. coli and L. monocytogenes. | [205] |
| Thymol | Mechanical properties: tensile strength increased by 15%, elongation at break increased by 111%; Water vapor permeability: 17% reduction; Water solubility: 60% reduction; Antibacterial properties: S. aureus and E. coli;. | [206] |
| Tannic acid | Water vapor permeability: 56% reduction; UV barrier; Antioxidant property: DPPH radical scavenging activity increased from 0 to 89.2%. | [207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Z.; Feng, Y.; Zhao, J.; Qiao, M.; Jin, Q. Valorization of Seafood Waste for Food Packaging Development. Foods 2024, 13, 2122. https://doi.org/10.3390/foods13132122
Zhan Z, Feng Y, Zhao J, Qiao M, Jin Q. Valorization of Seafood Waste for Food Packaging Development. Foods. 2024; 13(13):2122. https://doi.org/10.3390/foods13132122
Chicago/Turabian StyleZhan, Zhijing, Yiming Feng, Jikai Zhao, Mingyu Qiao, and Qing Jin. 2024. "Valorization of Seafood Waste for Food Packaging Development" Foods 13, no. 13: 2122. https://doi.org/10.3390/foods13132122
APA StyleZhan, Z., Feng, Y., Zhao, J., Qiao, M., & Jin, Q. (2024). Valorization of Seafood Waste for Food Packaging Development. Foods, 13(13), 2122. https://doi.org/10.3390/foods13132122






