Active Biodegradable Starch/PBAT-poly(butylene adipate-co-terephthalate) Film with Eucalyptus citriodora Essential Oil Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Eucalyptus Citriodora Microencapsulation by Spray Drying
2.2.2. Production of Extruded Active Biodegradable Blown Film
2.2.3. Eucalyptus Citriodora CG-MS Analysis
2.2.4. Release of Total Phenolic Compounds—TPCs and ABTS Cation Scavenging Assays in Food Simulants
2.2.5. Mechanical Properties
2.2.6. Optical Properties
2.2.7. Swelling Index (SI) and Weight Loss in Water (WLW)
2.2.8. Water Vapor Permeability—WVP
2.2.9. Fourier Transform Infrared Spectroscopy—FT-IR
2.2.10. X-ray Diffraction—XRD
2.2.11. Scanning Electron Microscopy—SEM
2.3. Statistical Analysis
3. Results and Discussion
3.1. Eucalyptus Citriodora CG-MS Analysis
3.2. Release of Total Phenolic Compounds (TPCs) and ABTS Cation Scavenging Assays in Food Simulants
3.3. Mechanical Properties
3.4. Optical Properties
3.5. Swelling Index (SI) and Weight Loss in Water (WLW)
3.6. Water Vapor Permeability—WVP
3.7. Fourier Transform Infrared Spectroscopy—FT-IR
3.8. X-ray Diffraction—XRD
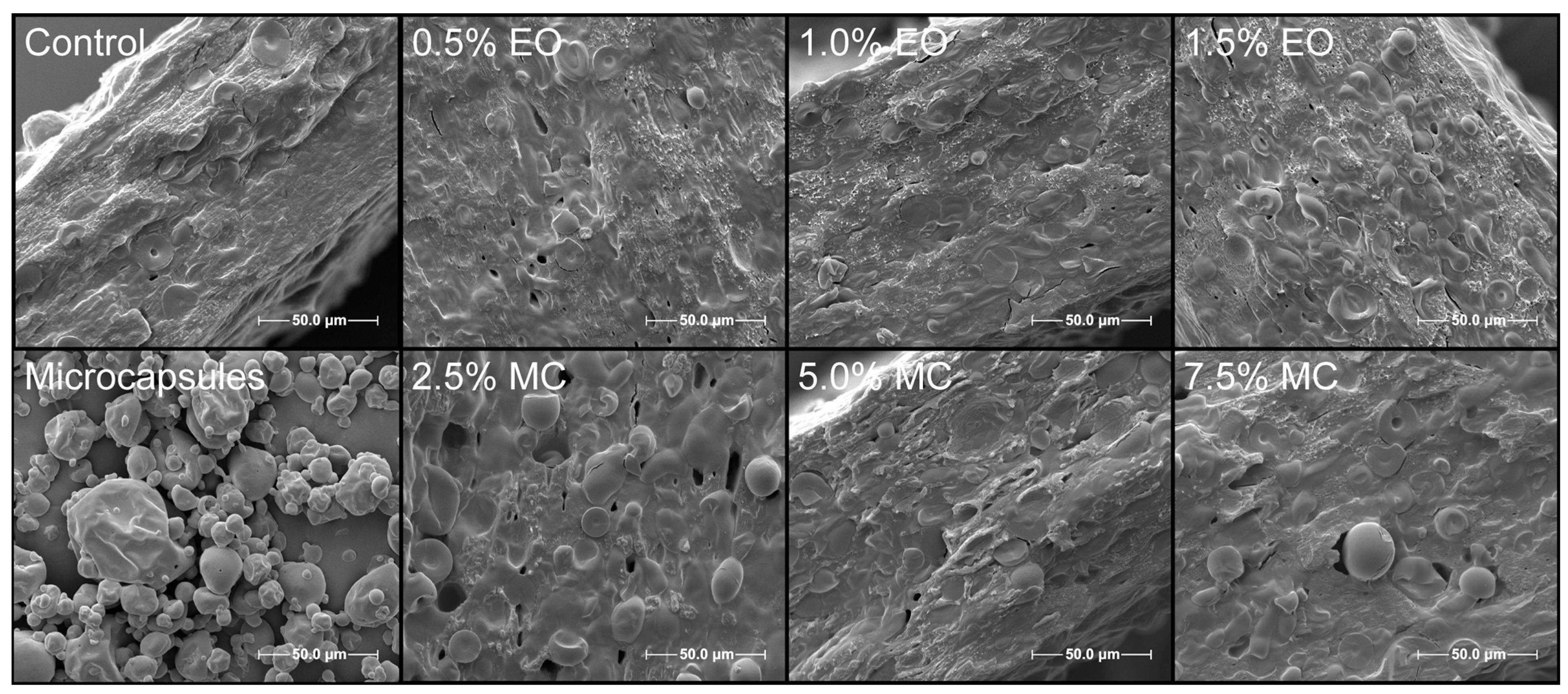
3.9. Scanning Electron Microscopy—SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkaczewska, J.; Jamróz, E.; Kasprzak, M.; Zając, M.; Pająk, P.; Grzebieniarz, W.; Nowak, N.; Juszczak, L. Edible Coatings Based on a Furcellaran and Gelatin Extract with Herb Addition as an Active Packaging for Carp Fillets. Food Bioprocess Technol. 2023, 16, 1009–1021. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of Mango Peel Extracts on the Biodegradable Films for Active Packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Acosta, P.P.d.S.; Latorres, J.M.; Martins, V.G. The Influence of Cinnamon and Litsea Cubeba Essential Oils on Methylcellulose Films. J. Appl. Polym. Sci. 2023, 140, e53342. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation Strategies to Enhance the Antibacterial Properties of Essential Oils in Food System. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of in Vitro Antioxidant Activity of Essential Oil of Eucalyptus Citriodora (Lemon-Scented Eucalypt; Myrtaceae) and Its Major Constituents. LWT Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Miguel, M.; Gago, C.; Antunes, M.; Lagoas, S.; Faleiro, M.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia Citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Bukvicki, D.; Gottardi, D.; Tabanelli, G.; Montanari, C.; Malik, A.; Guerzoni, M.E. Eucalyptus Essential Oil as a Natural Food Preservative: In Vivo and In Vitro Antiyeast Potential. Biomed. Res. Int. 2014, 2014, 969143. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Shi, J.; Kim, I.S. Fabrication of Electrospun PVA/Zein/Gelatin Based Active Packaging for Quality Maintenance of Different Food Items. Polymers 2023, 15, 2538. [Google Scholar] [CrossRef]
- Mohan, S.; Unnikrishnan, T.G.; Dubey, U.; Ramesh, M.; Panneerselvam, K. Development and Characterization of Mustard Oil Incorporated Biodegradable Chitosan Films for Active Food Packaging Applications. J. Polym. Environ. 2023, 31, 2190–2203. [Google Scholar] [CrossRef]
- Silva, L.R.C.; Rios, A.d.O.; Santana, R.M.C. Polymer Blends of Poly(Lactic Acid) and Starch for the Production of Films Applied in Food Packaging: A Brief Review. Polym. Renew. Resour. 2023, 14, 108–153. [Google Scholar] [CrossRef]
- Chan, M.-K.; Tang, T.-H. The Properties of Starch/Cellulose/Polyvinyl Alcohol Composite as Hydrodegradable Film. Polym. Polym. Compos. 2022, 30, 096739112211003. [Google Scholar] [CrossRef]
- Olivato, J.B.; Grossmann, M.V.E.; Yamashita, F.; Eiras, D.; Pessan, L.A. Citric Acid and Maleic Anhydride as Compatibilizers in Starch/Poly(Butylene Adipate-Co-Terephthalate) Blends by One-Step Reactive Extrusion. Carbohydr. Polym. 2012, 87, 2614–2618. [Google Scholar] [CrossRef]
- Araya, J.; Esquivel, M.; Jimenez, G.; Navia, D.; Poveda, L. Antimicrobial Activity and Physicochemical Characterization of Thermoplastic Films Based on Bitter Cassava Starch, Nanocellulose and Rosemary Essential Oil. J. Plast. Film Sheeting 2022, 38, 46–71. [Google Scholar] [CrossRef]
- Gao, S.; Zhai, X.; Cheng, Y.; Zhang, R.; Wang, W.; Hou, H. Starch/PBAT Blown Antimicrobial Films Based on the Synergistic Effects of Two Commercial Antimicrobial Peptides. Int. J. Biol. Macromol. 2022, 204, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, M.; Zhang, R.; Wang, W.; Hou, H. Extrusion-Blown Starch/PBAT Biodegradable Active Films Incorporated with High Retentions of Tea Polyphenols and the Release Kinetics into Food Simulants. Int. J. Biol. Macromol. 2023, 227, 851–862. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, H.W.; Son, D.; Han, J.H.; Kang, D.; Kang, S.I.; Lee, J.; Shim, J.K. In Situ Reactive Compatibilization of Thermoplastic Starch/Poly(Butylene Adipate- Co -Terephthalate) Blends with Robust Water Resistance Performance. ACS Appl. Polym. Mater. 2023, 5, 5445–5453. [Google Scholar] [CrossRef]
- Paulo, A.F.S.; Balan, G.C.; Ströher, G.R.; Yamashita, F.; Bittencourt, P.R.S.; Sakanaka, L.S.; Katsuda, M.S.; Shirai, M.A. Influence of Free and Microencapsulated Oregano Oil on Starch and Poly (Butylene Co-Terephthalate Adipate) Active Film Properties. Polym. Bull. 2022, 79, 4859–4877. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; de Morais, S.M.; Sampaio, C.D.G.; Pérez-jiménez, J.; Saura-Colixto, F.D. Metodologia Científica: Determinação Da Atividade Antioxidante Total Em Frutas Pela Captura Do Radical Livre. Comun. Técnico Line 2007, 128, 3. [Google Scholar]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of Polyphenols from Starch-Chitosan Based Films Containing Thyme Extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zanela, J.; Casagrande, M.; Reis, M.O.; Grossmann, M.V.E.; Yamashita, F. Biodegradable Sheets of Starch/Polyvinyl Alcohol (PVA): Effects of PVA Molecular Weight and Hydrolysis Degree. Waste Biomass Valorization 2017, 10, 319–326. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, Characterization and Antibacterial Activity of Biodegradable Starch/PVA Composite Films Reinforced with Cellulosic Fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Olivato, J.B.; Grossmann, M.V.E.; Bilck, A.P.; Yamashita, F. Effect of Organic Acids as Additives on the Performance of Thermoplastic Starch/Polyester Blown Films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial Activity of Essential Oils from Eucalyptus and of Selected Components against Multidrug-Resistant Bacterial Pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal Activity of Myrtaceae Essential Oils and Their Components against Three Phytopathogenic Fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Mann, T.S.; Babu, G.D.K.; Guleria, S.; Singh, B. Variation in the Volatile Oil Composition of Eucalyptus Citriodora Produced by Hydrodistillation and Supercritical Fluid Extraction Techniques. Nat. Prod. Res. 2013, 27, 675–679. [Google Scholar] [CrossRef]
- Manika, N.; Mishra, P.; Kumar, N.; Chanotiya, C.S.; Bagchi, G.D. Effect of Season on Yield and Composition of the Essential Oil of Eucalyptus Citriodora Hook. Leaf Grown in Sub-Tropical Conditions of North India. J. Med. Plants Res. 2012, 6, 2875–2879. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Mocan, A.; Sepúlveda, B. High Resolution Metabolite Fingerprinting of the Resin of Baccharis Tola Phil. from the Atacama Desert and Its Antioxidant Capacities. Ind. Crops Prod. 2016, 94, 368–375. [Google Scholar] [CrossRef]
- Amri, I.; Khammassi, M.; Ben Ayed, R.; Khedhri, S.; Mansour, M.B.; Kochti, O.; Pieracci, Y.; Flamini, G.; Mabrouk, Y.; Gargouri, S.; et al. Essential Oils and Biological Activities of Eucalyptus Falcata, E. Sideroxylon and E. Citriodora Growing in Tunisia. Plants 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, L.M.; Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Waksmundzka-Hajnos, M. Antioxidant Synergism and Antagonism between Selected Monoterpenes Using the 2,2-Diphenyl-1-Picrylhydrazyl Method. Flavour Fragr. J. 2016, 31, 412–419. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; González-Martínez, C.; Chiralt, A.; Desobry, S. Study of the Release of Limonene Present in Chitosan Films Enriched with Bergamot Oil in Food Simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched With Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch Stärke 2018, 70, 1700238. [Google Scholar] [CrossRef]
- de Medeiros, J.A.S.; Blick, A.P.; Galindo, M.V.; Alvim, I.D.; Yamashita, F.; Ueno, C.T.; Shirai, M.A.; Grosso, C.R.F.; Corradini, E.; Sakanaka, L.S. Incorporation of Oregano Essential Oil Microcapsules in Starch-Poly (Butylene Adipate Co-Terephthalate) (PBAT) Films. Macromol. Symp. 2019, 383, 1800052. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of Starch Film Containing Thyme Essential Oil Microcapsules on Physicochemical Activity of Mango. LWT 2020, 131, 109700. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Eugenol Incorporation into Thermoprocessed Starch Films Using Different Encapsulating Materials. Food Packag. Shelf Life 2019, 21, 100326. [Google Scholar] [CrossRef]
- Zanela, J.; Casagrande, M.; Radaelli, J.C.; Dias, A.P.; Wagner Júnior, A.; Malfatti, C.R.M.; Yamashita, F. Active Biodegradable Packaging for Foods Containing Baccharis Dracunculifolia Leaf as Natural Antioxidant. Food Bioprocess Technol. 2021, 14, 1301–1310. [Google Scholar] [CrossRef]
- Balan, G.C.; Paulo, A.F.S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of Wheat Flour/PBAT Active Films Incorporated with Oregano Oil Microparticles and Its Application in Fresh Pastry Conservation. Food Bioprocess Technol. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Jafari, R.; Zandi, M.; Ganjloo, A. Characterization of Alginate-Gelatin Edible Film Containing Anise (Pimpinella anisum L.) Essential Oil. J. Polym. Environ. 2023, 31, 1568–1583. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Zhang, R.; Wang, X.; Wang, J.; Chen, M. Preparation, Characterization and Application of Poly(Lactic Acid)/Corn Starch/Eucalyptus Leaf Essential Oil Microencapsulated Active Bilayer Degradable Film. Int. J. Biol. Macromol. 2022, 195, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Akhir, M.A.M.; Zubir, S.A.; Mariatti, J. Effect of Different Starch Contents on Physical, Morphological, Mechanical, Barrier, and Biodegradation Properties of Tapioca Starch and Poly(Butylene Adipate-co-terephthalate) Blend Film. Polym. Adv. Technol. 2023, 34, 717–730. [Google Scholar] [CrossRef]
- Olivato, J.B.; Marini, J.; Pollet, E.; Yamashita, F.; Grossmann, M.V.E.; Avérous, L. Elaboration, Morphology and Properties of Starch/Polyester Nano-Biocomposites Based on Sepiolite Clay. Carbohydr. Polym. 2015, 118, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of Cinnamon Essential Oil on the Physical, Mechanical, Structural and Thermal Properties of Cassava Starch-Based Edible Films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Structure, Functionality, and Active Release of Nanoclay–Soy Protein Films Affected by Clove Essential Oil. Food Bioprocess Technol. 2016, 9, 1937–1950. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of Ginger (Zingiber officinale) Extract by Spray Drying Technology. LWT 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Burhan, A.M.; Abdel-Hamid, S.M.; Soliman, M.E.; Sammour, O.A. Optimisation of the Microencapsulation of Lavender Oil by Spray Drying. J. Microencapsul. 2019, 36, 250–266. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of Active Films Poly (Butylene Adipate Co-Terephthalate)—PBAT Incorporated with Oregano Essential Oil and Application in Fish Fillet Preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]

| Film | ABTS (µmol of TEAC g Film−1) | |||
|---|---|---|---|---|
| Water | Ethanol 10% | Ethanol 50% | Acetic Acid 3% | |
| MC | 35.96 ± 2.45 | 34.32 ± 2.59 | 35.07 ± 0.41 | 23.60 ± 1.56 |
| 0.5% EO | 0.92 ± 0.16 bC * | 2.65 ± 0.19 bA | 2.74 ± 0.20 bA | 1.87 ± 0.04 cB |
| 1.0% EO | 0.91 ± 0.27 bC | 3.64 ± 0.76 aA | 2.77 ± 0.08 bB | 2.13 ± 0.13 bB |
| 1.5% EO | 1.23 ± 0.10 abD | 2.93 ± 0.39 abB | 3.49 ± 0.09 aA | 2.18 ± 0.07 bC |
| 2.5% MC | 0.94 ± 0.07 bD | 2.41 ± 0.05 bB | 2.67 ± 0.03 bA | 2.19 ± 0.08 bC |
| 5.0% MC | 1.21 ± 0.13 abC | 2.93 ± 0.07 abAB | 3.54 ± 0.59 aA | 2.38 ± 0.05 aB |
| 7.5% MC | 1.52 ± 0.34 aC | 2.66 ± 0.04 bA | 2.95 ± 0.14 abA | 2.18 ± 0.06 bB |
| Film | TPCs (µg GAE g film−1) | |||
| Water | Ethanol 10% | Ethanol 50% | Acetic acid 3% | |
| MC | 1296 ± 208 | 1393 ± 198 | 722 ± 134 | 894 ± 84 |
| 0.5% EO | 20.36 ± 2.78 dBC | 21.86 ± 3.35 cB | 42.77 ± 2.03 dA | 12.72 ± 6.73 bC |
| 1.0% EO | 24.75 ± 1.19 dB | 23.47 ± 3.49 cB | 54.41 ± 8.55 cdA | 10.44 ± 5.34 bC |
| 1.5% EO | 53.41 ± 4.42 aB | 57.01 ± 7.28 aB | 142.74 ± 12.19 aA | 36.24 ± 3.33 aC |
| 2.5% MC | 32.36 ± 2.31 cBC | 36.68 ± 3.29 bB | 53.61 ± 7.20 cdA | 21.46 ± 9.00 bC |
| 5.0% MC | 44.69 ± 1.71 bB | 47.81 ± 7.18 abB | 74.15 ± 6.05 bcA | 42.16 ± 5.16 aB |
| 7.5% MC | 57.52 ± 2.62 aB | 59.45 ± 6.06 aB | 93.15 ± 15.60 bA | 47.33 ± 2.87 aB |
| Film | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Apparent Opacity (%) | Color Difference (ΔE*) |
|---|---|---|---|---|---|
| Control | 6.8 ± 0.6 b * | 501 ± 53 ab | 42.5 ± 14.9 b | 64.0 ± 0.7 ab | 11.4 ± 0.8 ab |
| 0.5% EO | 7.5 ± 0.7 a | 563 ± 37 a | 37.1 ± 7.6 b | 64.2 ± 0.5 ab | 11.4 ± 0.7 ab |
| 1.0% EO | 6.1 ± 0.3 cd | 319 ± 65 d | 50.0 ± 17.0 ab | 63.3 ± 2.3 b | 10.5 ± 1.0 b |
| 1.5% EO | 6.3 ± 0.4 bcd | 467 ± 52 bc | 37.7 ± 3.5 b | 66.6 ± 1.5 a | 11.4 ± 0.4 ab |
| 2.5% MC | 6.8 ± 0.4 bc | 468 ± 69 bc | 50.0 ± 7.7 ab | 65.2 ± 1.1 ab | 12.7 ± 1.3 a |
| 5.0% MC | 6.1 ± 0.3 d | 413 ± 44 c | 51.9 ± 10.0 ab | 63.4 ± 1.9 b | 11.8 ± 0.8 ab |
| 7.5% MC | 5.7 ± 0.6 d | 427 ± 47 c | 57.7 ± 11.3 a | 64.3 ± 1.2 ab | 12.4 ± 0.3 a |
| Film | Swelling Index (%) | Weight Loss in Water (%) | Water Vapor Permeability (g·m−1·s−1·kPa−1) (×10−10) | Crystallinity Index (%) |
|---|---|---|---|---|
| Control | 32.4 ± 2.0 a * | 14.6 ± 0.6 c | 6.9 ± 0.5 ns ** | 28.2 |
| 0.5% EO | 26.2 ± 0.9 bc | 14.9 ± 0.5 c | 6.9 ± 0.3 | 24.7 |
| 1.0% EO | 21.9 ± 0.6 d | 15.6 ± 0.4 bc | 7.3 ± 0.4 | 23.0 |
| 1.5% EO | 19.4 ± 1.3 d | 15.4 ± 0.6 c | 7.7 ± 0.2 | 24.6 |
| 2.5% MC | 29.6 ± 2.0 a | 15.9 ± 0.4 bc | 6.6 ± 0.6 | 27.0 |
| 5.0% MC | 29.3 ± 1.2 ab | 17.1 ± 0.9 ab | 6.8 ± 0.5 | 24.0 |
| 7.5% MC | 22.7 ± 1.5 cd | 18.1 ± 0.6 a | 7.3 ± 0.3 | 26.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanela, J.; Shirai, M.A.; Olivato, J.B.; Casagrande, M.; Canonico, C.M.; Wagner Júnior, A.; Yamashita, F. Active Biodegradable Starch/PBAT-poly(butylene adipate-co-terephthalate) Film with Eucalyptus citriodora Essential Oil Incorporation. Foods 2024, 13, 2104. https://doi.org/10.3390/foods13132104
Zanela J, Shirai MA, Olivato JB, Casagrande M, Canonico CM, Wagner Júnior A, Yamashita F. Active Biodegradable Starch/PBAT-poly(butylene adipate-co-terephthalate) Film with Eucalyptus citriodora Essential Oil Incorporation. Foods. 2024; 13(13):2104. https://doi.org/10.3390/foods13132104
Chicago/Turabian StyleZanela, Juliano, Marianne Ayumi Shirai, Juliana Bonametti Olivato, Maira Casagrande, Cristian Medrado Canonico, Américo Wagner Júnior, and Fabio Yamashita. 2024. "Active Biodegradable Starch/PBAT-poly(butylene adipate-co-terephthalate) Film with Eucalyptus citriodora Essential Oil Incorporation" Foods 13, no. 13: 2104. https://doi.org/10.3390/foods13132104
APA StyleZanela, J., Shirai, M. A., Olivato, J. B., Casagrande, M., Canonico, C. M., Wagner Júnior, A., & Yamashita, F. (2024). Active Biodegradable Starch/PBAT-poly(butylene adipate-co-terephthalate) Film with Eucalyptus citriodora Essential Oil Incorporation. Foods, 13(13), 2104. https://doi.org/10.3390/foods13132104






