Benzyl Isothiocyanate and Resveratrol Synergistically Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antioxidant Activity
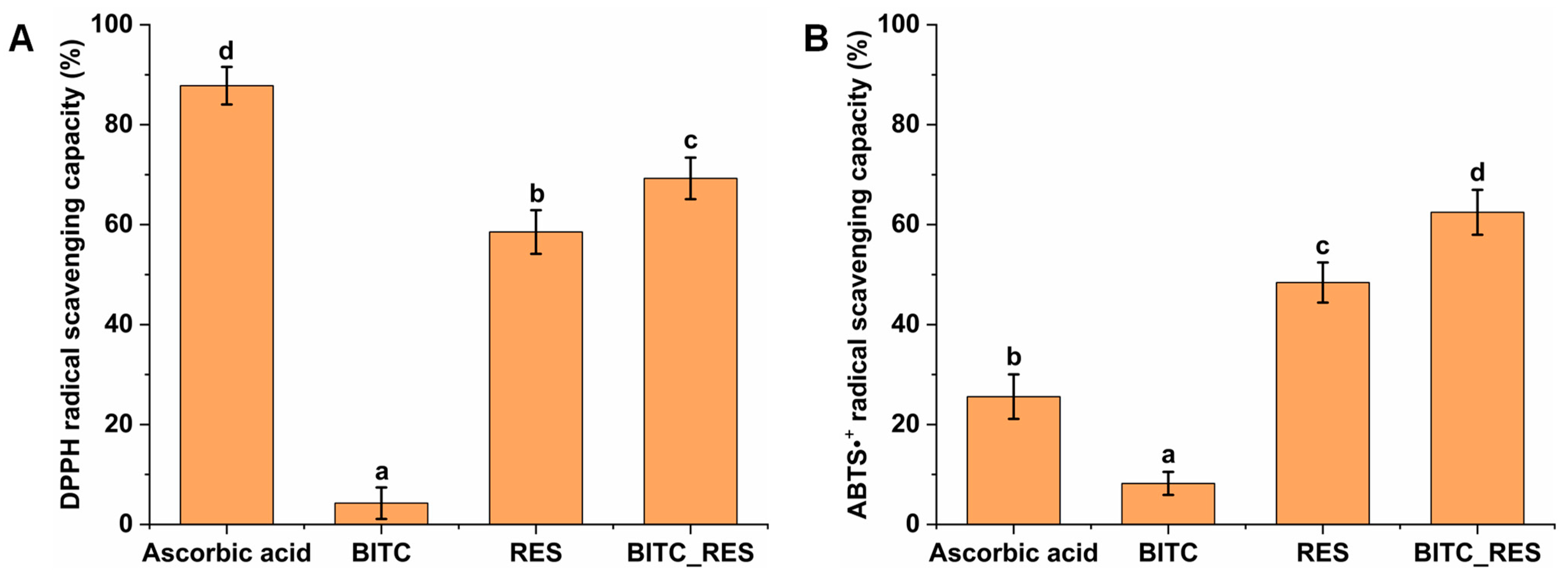
2.2.1. DPPH Radical Scavenging Activity Assay
2.2.2. ABTS•+ Scavenging Activity Assay
2.3. Experimental Animals
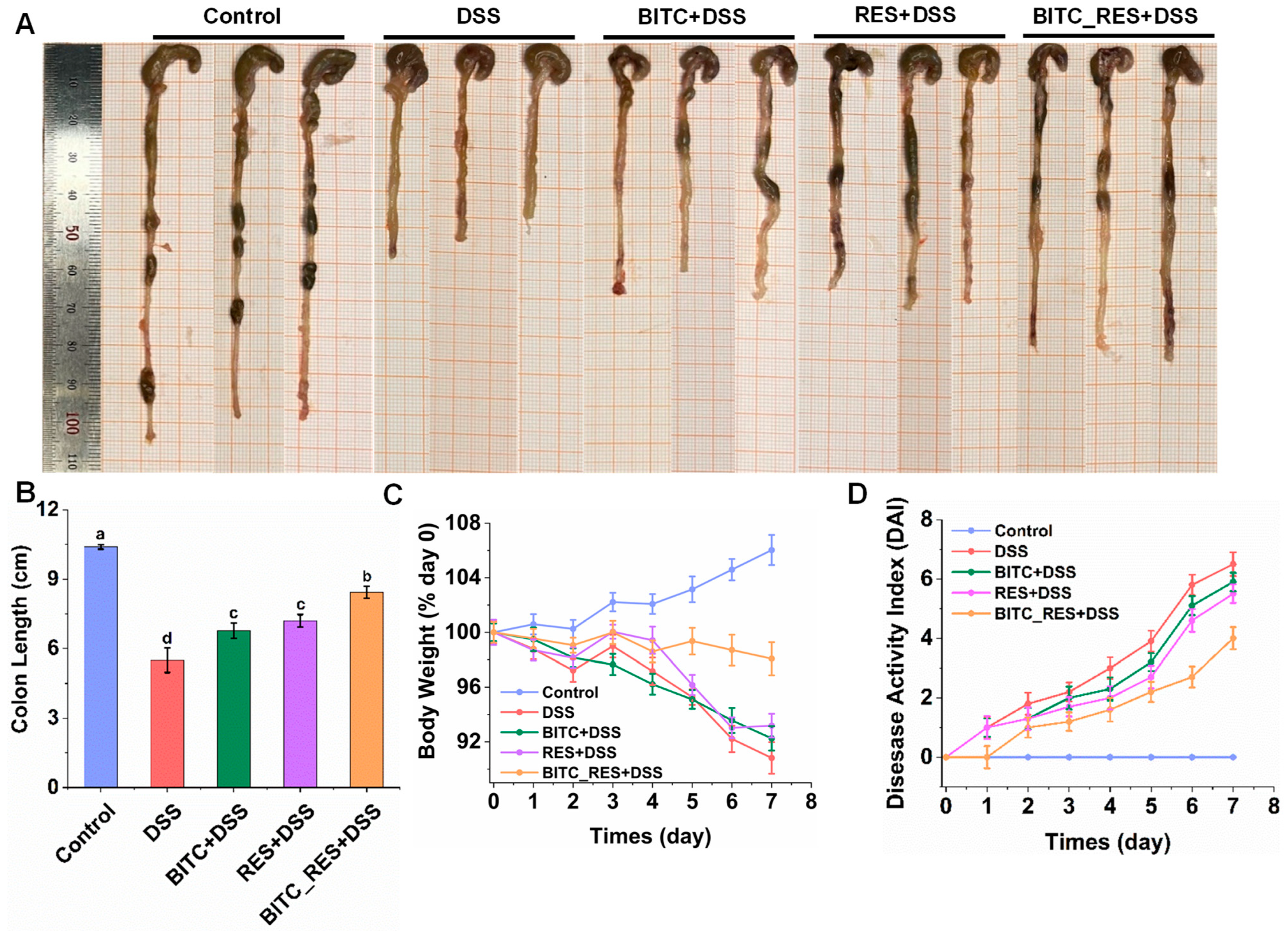
2.4. DSS-Induced Colitis in Mice
2.5. Assessment of the Disease Activity Index (DAI)
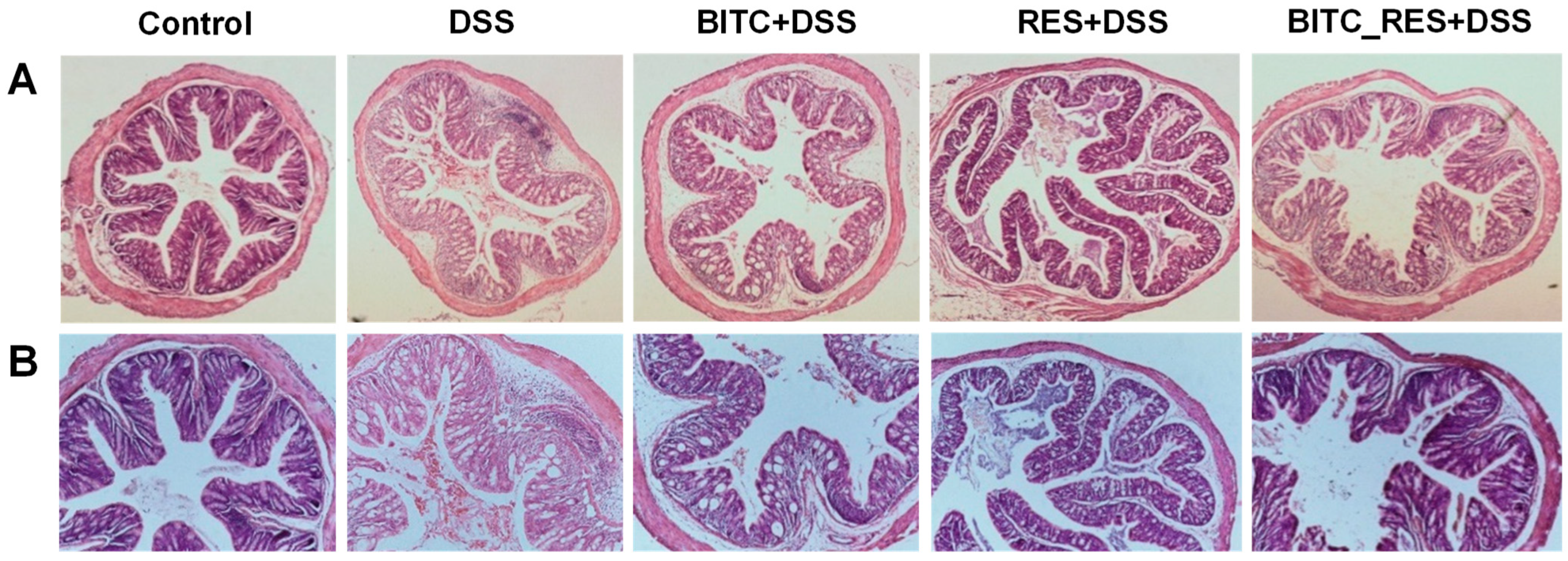
2.6. Histological Analysis
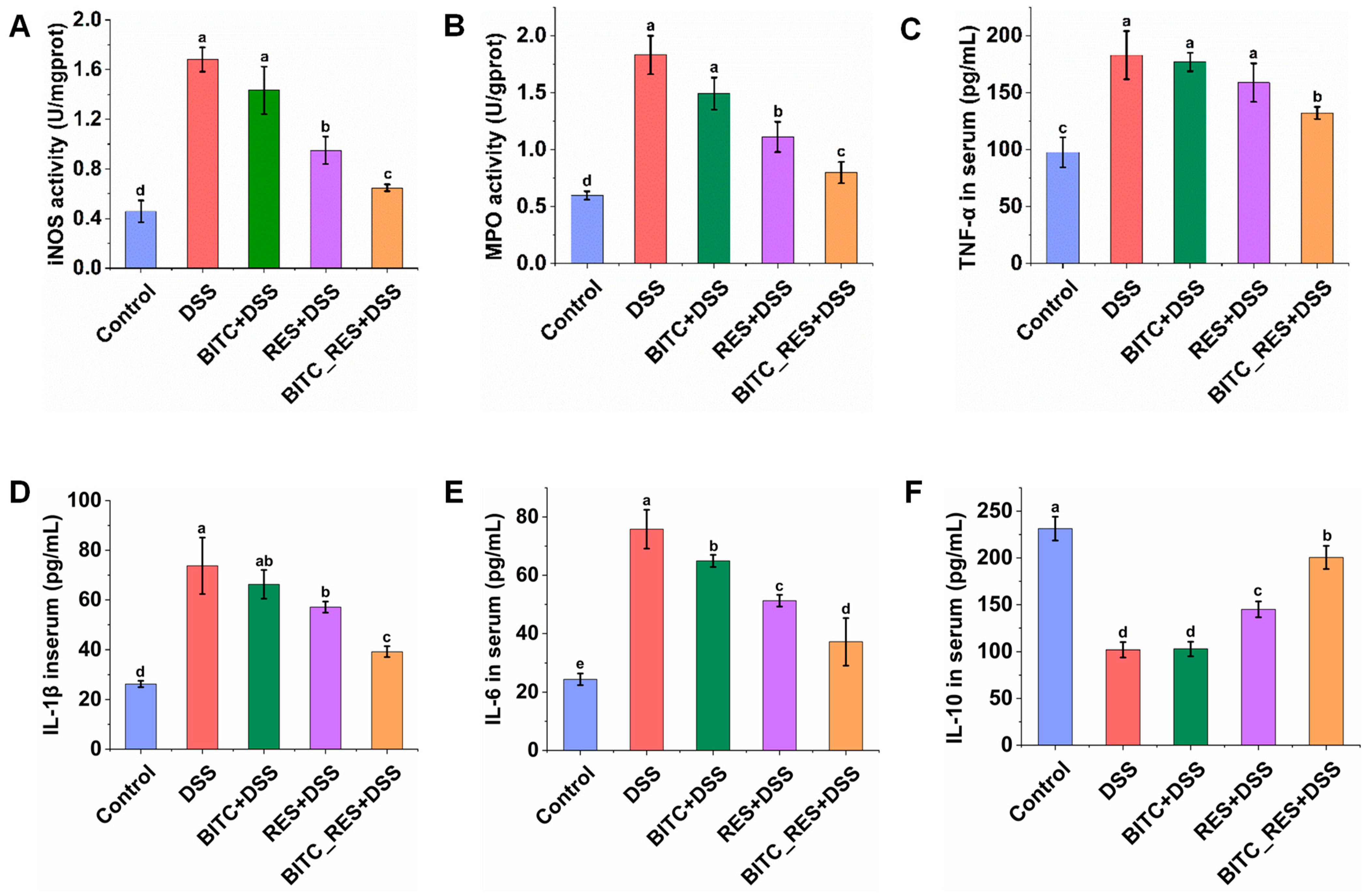
2.7. Inducible Nitric Oxide Synthase (iNOS) Level and Myeloperoxidase (MPO) Activity Assay
2.8. Determination of the Serum Proinflammatory Cytokine Concentration
2.9. Microbial Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synergistic Antioxidant Activities of BITC and RES
3.2. Synergistic Effects of BITC and RES on DSS-Induced Colitis Symptom Severity
3.3. Synergistic Effects of BITC and RES on Colonic Microinjury in DSS-Induced Colitis
3.4. Synergistic Effects of BITC and RES on iNOS and MPO Levels in Colonic Tissues and Cytokine Level in Serum
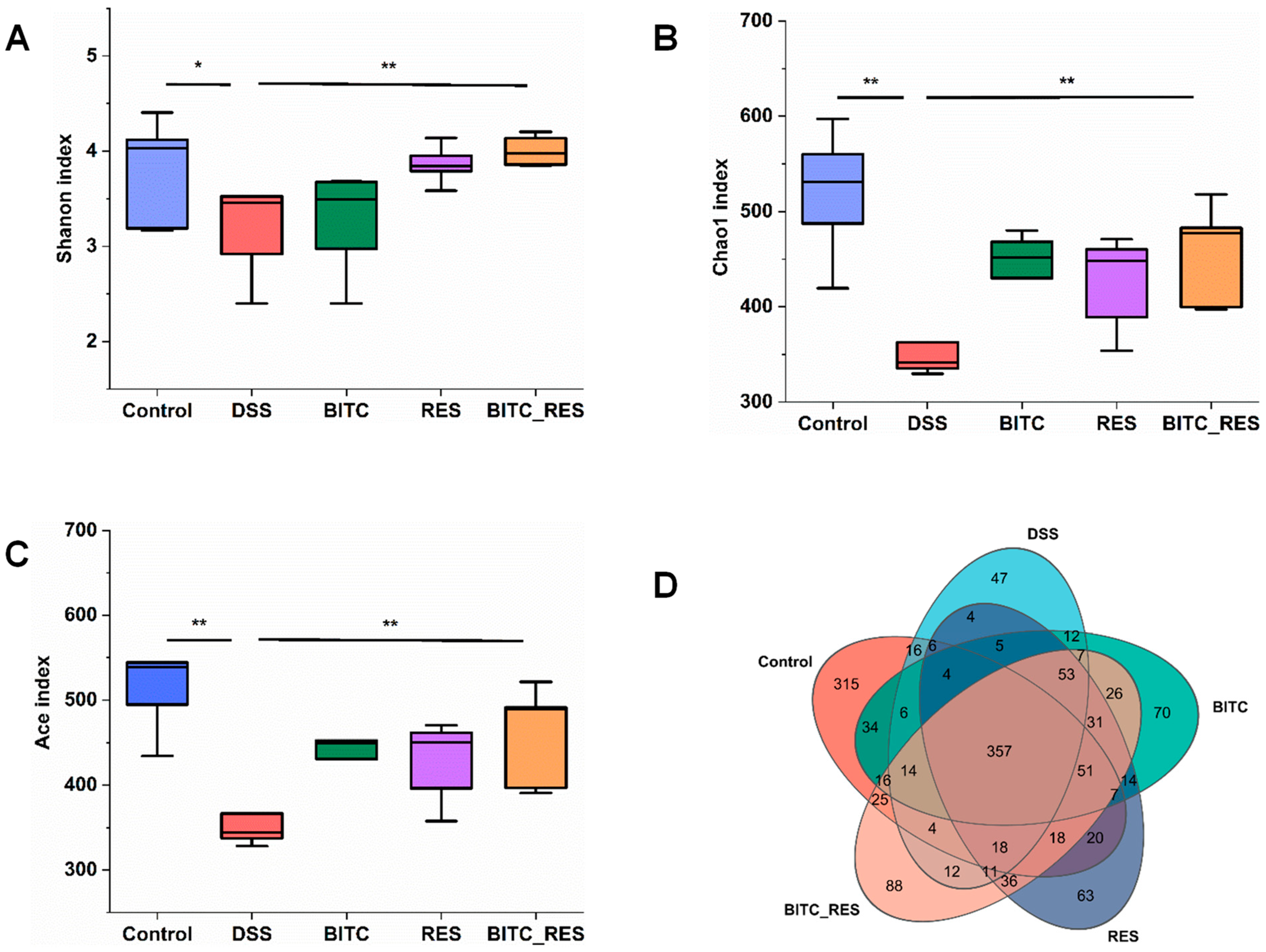
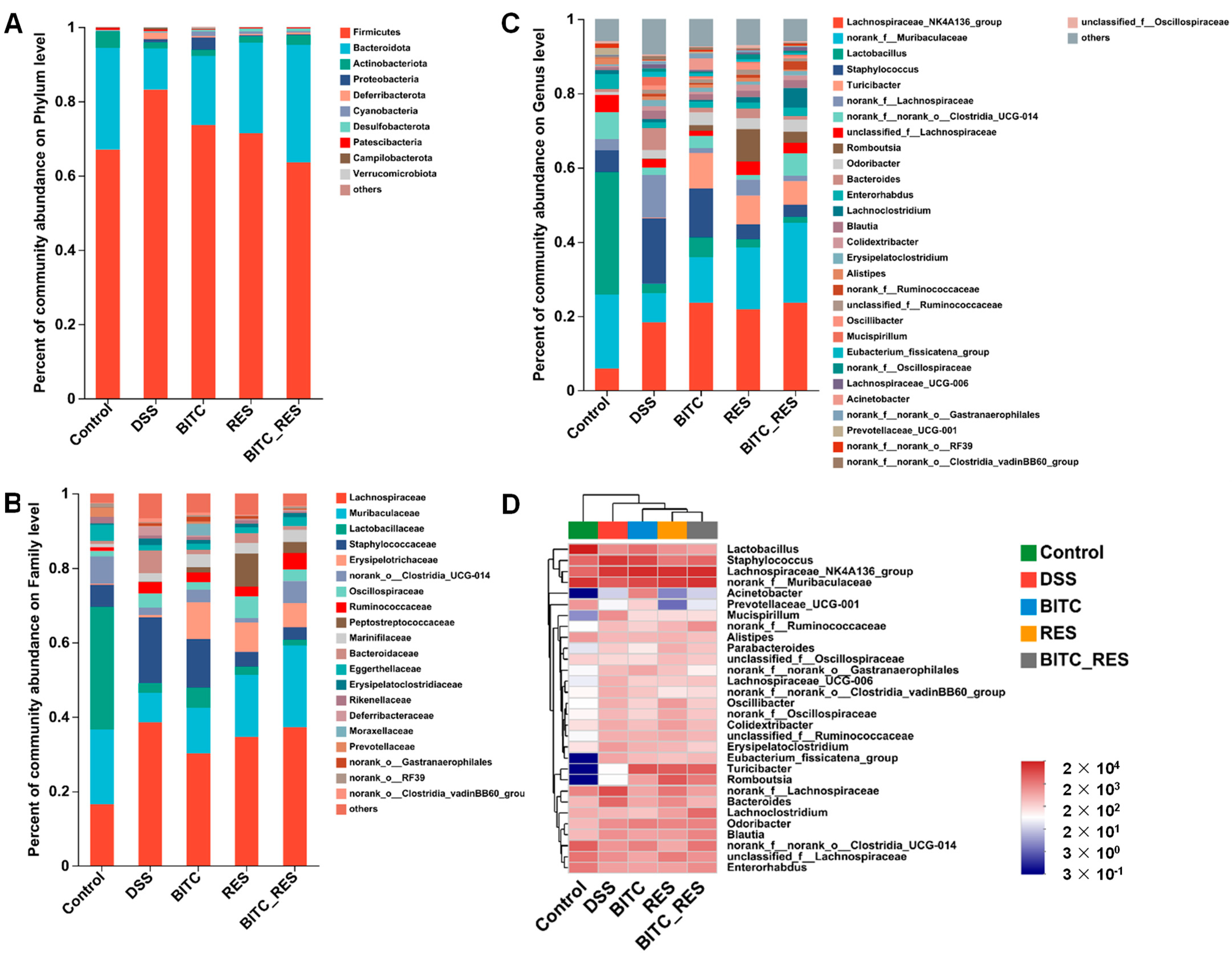
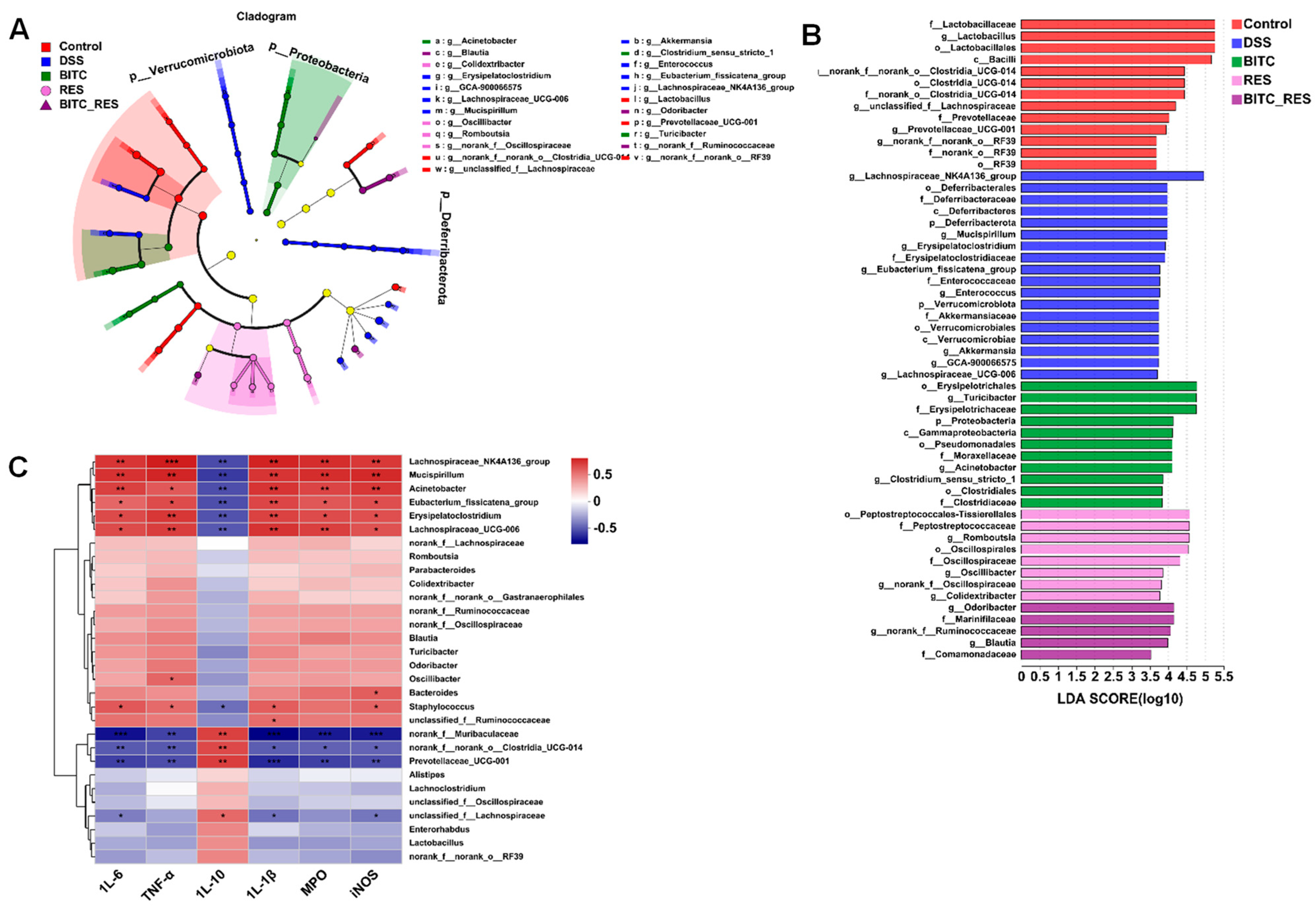
3.5. Synergistic Effects of BITC and RES on the Colon Microbiota of Mice with DSS-Induced Colitis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BITC | benzyl isothiocyanate |
| RES | resveratrol |
| BITC_RES | benzyl isothiocyanate and resveratrol |
| MPO | myeloperoxidase |
| DSS | dextran sodium sulfate |
| NOS | nitric oxide synthase |
| DAI | disease activity index |
| OUT | operational taxonomic unit |
References
- Alsoud, D.; Verstockt, B.; Fiocchi, C.; Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 2021, 6, 589–595. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.F.; Hu, X.S.; Chen, F. Gut Microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Sun, L.; Ouyang, J.; Zeng, F.; Wu, S.Z. An AIEgen-based oral-administration nanosystem for detection and therapy of ulcerative colitis via 3D-MSOT/NIR-II fluorescent imaging and inhibiting NLRP3 inflammasome. Biomaterials 2022, 283, 121468. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Yan, S.; Li, X. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2188–2193. [Google Scholar] [CrossRef]
- Fatani, A.J.; Alrojayee, F.S.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Exp. Ther. Med. 2016, 12, 730–738. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Min, K.; Seungho, C.; Sun, K.; Yoon, Y.; Kang, J.H.; Oh, S. Allyl isothiocyanate ameliorates dextran sodium sulfate-induced colitis in mouse by enhancing tight junction and mucin expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef]
- Ramachandra, D.P.; Sudheer, P. Self-micro emulsifying drug delivery via intestinal lymphatics: A lucrative approach to drug targeting. Pharm. Nanotechnol. 2023, 11, 238–246. [Google Scholar] [CrossRef]
- Mayangsari, Y.; Suzuki, T. Resveratrol ameliorates intestinal barrier defects and inflammation in colitic mice and intestinal cells. J. Agric. Food Chem. 2018, 66, 12666–12674. [Google Scholar] [CrossRef]
- Cui, X.; Jin, Y.; Hofseth, A.B.; Pena, E.; Habiger, J.; Chumanevich, A.; Poudyal, D.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Resveratrol Suppresses Colitis and Colon Cancer Associated with Colitis. Cancer Prev. Res. 2010, 3, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Wu, H.Y.; Liu, J.N.; Hao, H.S.; Bi, J.R.; Hou, H.M.; Zhang, G.L. Synergistic effects of benzyl isothiocyanate and resveratrol against Listeria monocytogenes and their application in chicken meat preservation. Food Chem. 2023, 419, 135984. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; Mcclements, D.J. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, S.D.; Sui, X.D.; Guo, L.Y.; Liu, X.M.; Li, H.M.; Gao, L.M.; Cai, S.S.; Li, Y.R.; Wang, T.T.; et al. Root Extract of Polygonum cuspidatum Siebold & Zucc. Ameliorates DSS-induced Ulcerative colitis by affecting NF-kappaB signaling pathway in a mouse model via synergistic effects of polydatin, resveratrol, and emodin. Front. Pharmacol. 2018, 9, 347. [Google Scholar]
- Cuggino, S.G.; Bascon-Villegas, I.; Rincon, F.; Maria, A.; Guiomar, P.; Javier, M.; Cristina, P.C.; Fernando, P.R. Modelling the combined effect of chlorine, benzyl isothiocyanate, exposure time and cut size on the reduction of Salmonella in fresh-cut lettuce during washing process. Food Microbiol. 2020, 86, 103346. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, M.; Hertig, I. Benzyl isothiocyanate disturbs lipid metabolism in rats in a way independent of its thyroid impact following in vivo long-term treatment and in vitro adipocytes studies. J. Physiol. Biochem. 2013, 69, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dey, M. Dietary phenethyl isothiocyanate protects mice from colitis associated colon cancer. J. Turbul. 2017, 90, 1908. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Dig. World Core Med. J. 2006, 55, 205–211. [Google Scholar]
- Li, M.; Li, P.; Tang, R.; Lu, H. Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways. Food Sci. Hum. Wellness 2022, 11, 22–31. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 1. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.H.; Le, G.W. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Lelis, D.D.F.; Souza, L.A.A.; Brito, R.V.J.; Andrade, M.C.; Nobre, S.A.W.; Guimaraes, A.L.S.; de Paula, A.M.B.; de Lima, J.P.; Hilzendeger, A.M. Lactococcus lactis and resveratrol decrease body weight and increase benefic gastrointestinal microbiota in mice. Protein Pept. Lett. 2021, 28, 761–768. [Google Scholar]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z.Y. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Zhang, L.; Khea, W.; Brittany, L.G.; Peter, K.; Kristin, M.; Richar, C.; Susan, N.; Michael, S.J. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J. Funct. Foods 2018, 47, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.L.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043. [Google Scholar] [CrossRef]
- Ibrahim, A.; Al-Hizab, F.A.; Abushouk, A.I.; Abdel-Daim, M.M. Nephroprotective effects of benzyl isothiocyanate and resveratrol against cisplatin-induced oxidative stress and inflammation. Front. Pharmacol. 2018, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.Y.; Saleem, M.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.F.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2019, 64, 103641. [Google Scholar] [CrossRef]
- Kong, Y.; Yan, T.; Tong, Y.; Deng, H.T.; Tan, C.; Wan, M.Z.; Wang, M.Y.; Meng, X.J.; Wang, Y.H. Gut microbiota modulation by polyphenols from aronia melanocarpa of LPS-induced liver diseases in rats. J. Agric. Food Chem. 2021, 69, 3312–3325. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.Y.; Li, Z.Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2019, 11, 1063–1073. [Google Scholar] [CrossRef]
- Larrosa, M.J.; Yañéz-Gascón, M.; Selma, M.A.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Dolara, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2005, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, S.; Chen, Y. Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother. Res. 2021, 35, 5708–5719. [Google Scholar]
- Aaron, S.M.; Jesus, D.L.; Yamamoto-Furusho, K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 9. [Google Scholar]
- Chen, Y.; Su, W.; Tie, S.; Cui, W.; Yu, X.Y.; Zhang, L.J.; Hua, Z.; Tan, M. Orally deliverable sequence-targeted astaxanthin nanoparticles for colitis alleviation. Biomaterials 2023, 293, 121976. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, J.Y.; Liu, L.; Zeng, W.S.; Li, Y.X.; Xun, A.Y.; Zhao, L.; Jia, C.H.; Feng, J.L.; Wei, X.X.; et al. Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Medica 2011, 77, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Goosen, T.C.; Mills, D.E.; Hollenberg, P.F. Effects of benzyl isothiocyanate on rat and human cytochromes P450: Identification of metabolites formed by P450 2B1. J. Pharmacol. Exp. Ther. 2011, 296, 198–206. [Google Scholar]
- Wong, K.W.; Teh, S.S.; Law, K.P.; Ismail, I.S.; Sato, K.; Mase, N.Y.; Mah, S.H. Synthesis of benzylated amine-substituted xanthone derivatives and their antioxidant and anti-inflammatory activities. Arch. Pharm.-Chem. Life Sci. 2023, 356, 2200418. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wen, X.L. Gut microbiota and inflammatory bowel disease: The current status and perspectives. World J. Clin. Cases 2021, 9, 13. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikur, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Liu, G.; Wen, X.; Ding, S.J.; Jiang, H.M.; Ma, Y.; Wang, H.; Fang, J. Effects of IRW and IQW on oxidative stress and gut microbiota in dextran sodium sulfate-induced colitis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, Z. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019, 10, 6873–6881. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, W.; Jiang, Y.; Lin, Q.R.; Peng, X.N.; Hu, H.; Ye, Z.M.; Liu, G.; Yu, Y.G. Dextran-sulfate-sodium-induced colitis-ameliorating effect of aqueous phyllanthus emblica L. Extract through regulating colonic cell gene expression and gut microbiomes. J. Agric. Food Chem. 2023, 71, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, M.; Kong, Y.; Wan, M.Z.; Deng, H.T.; Tong, Y.Q.; Lyu, C.M.; Meng, X.J. Anti-inflammatory and intestinal microbiota modulation properties of high hydrostatic pressure treated cyanidin-3-glucoside and blueberry pectin complexes on dextran sodium sulfate-induced ulcerative colitis mice. Food Funct. 2022, 68, 12295–12309. [Google Scholar]
- Moissl-Eichinger, C.; Willing, B.P.; Burke, C.M.; Lukiw, W. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in alzheimers disease. Front. Microbiol. 2017, 7, 1544. [Google Scholar]
- Zhou, Y.; Zhi, F. Lower level of bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. BioMed Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.H.; Liao, W.Y.; Li, Q.R.; Zhang, H.M.; Liu, Z.H.; Zheng, X.J.; Zheng, L.; Feng, X. Interactions between resveratrol and gut microbiota affect the development of hepatic steatosis: A fecal microbiota transplantation study in high-fat diet mice. J. Funct. Foods 2020, 67, 103883. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.Q.; Liang, X.; Bu, Y.S.; Gong, P.; Liu, T.J.; Zhang, L.W.; Xia, Y.; et al. Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef]
- Liu, H.; Liao, C.; Wu, L.; Tang, J.; Chen, J.; Lei, C.; Zheng, L.; Zhang, C.; Liu, Y.Y.; Xavier, J. Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 2022, 16, 2040–2055. [Google Scholar] [CrossRef]
- Smith, B.; Miller, R.; Schmidt, T. Muribaculaceae genomes assembled from metagenomes suggest genetic drivers of differential response to acarbose treatment in mice. MSphere 2021, 6, e0085121. [Google Scholar] [CrossRef]
- María, A.M.; Ramos, S. Impact of dietary flavanols on microbiota, immunity and inflammation in metabolic diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Satokari, R. Novel odoribacter splanchnicus strain and its outer membrane vesicles exert immunoregulatory effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Xu, Y.; Kong, X.; Wang, J.; Zha, X.; Wang, Y. Probiotic Bacillus cereus alleviates dextran sulfate sodium-induced colitis in mice through improvement of the intestinal barrier function, anti-Inflammation, and gut microbiota modulation. J. Agric. Food Chem. 2021, 49, 69. [Google Scholar] [CrossRef] [PubMed]
- Herp, S.; Durai Raj, A.C.; Salvado Silva, M.; Woelfel, S.; Stecher, B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Med. Microbiol. Immunol. 2021, 210, 173–179. [Google Scholar] [CrossRef]
- Fei, Y.; Zhang, S.; Han, S.; Qiu, B.; Lu, Y.M.; Huang, W.X.; Li, F.; Chen, D.Y.; Berglund, B.; Xiao, H. The role of dihydroresveratrol in enhancing the synergistic effect of Ligilactobacillus salivarius liol and resveratrol in ameliorating colitis in mice. Research 2022, 16, 9863845. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Q.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Benzyl Isothiocyanate and Resveratrol Synergistically Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice. Foods 2024, 13, 2078. https://doi.org/10.3390/foods13132078
Liu J, Zhang Q, Hao H, Bi J, Hou H, Zhang G. Benzyl Isothiocyanate and Resveratrol Synergistically Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice. Foods. 2024; 13(13):2078. https://doi.org/10.3390/foods13132078
Chicago/Turabian StyleLiu, Jianan, Qian Zhang, Hongshun Hao, Jingran Bi, Hongman Hou, and Gongliang Zhang. 2024. "Benzyl Isothiocyanate and Resveratrol Synergistically Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice" Foods 13, no. 13: 2078. https://doi.org/10.3390/foods13132078
APA StyleLiu, J., Zhang, Q., Hao, H., Bi, J., Hou, H., & Zhang, G. (2024). Benzyl Isothiocyanate and Resveratrol Synergistically Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice. Foods, 13(13), 2078. https://doi.org/10.3390/foods13132078







