Licuri Kernel (Syagrus coronata (Martius) Beccari): A Promising Matrix for the Development of Fermented Plant-Based Kefir Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
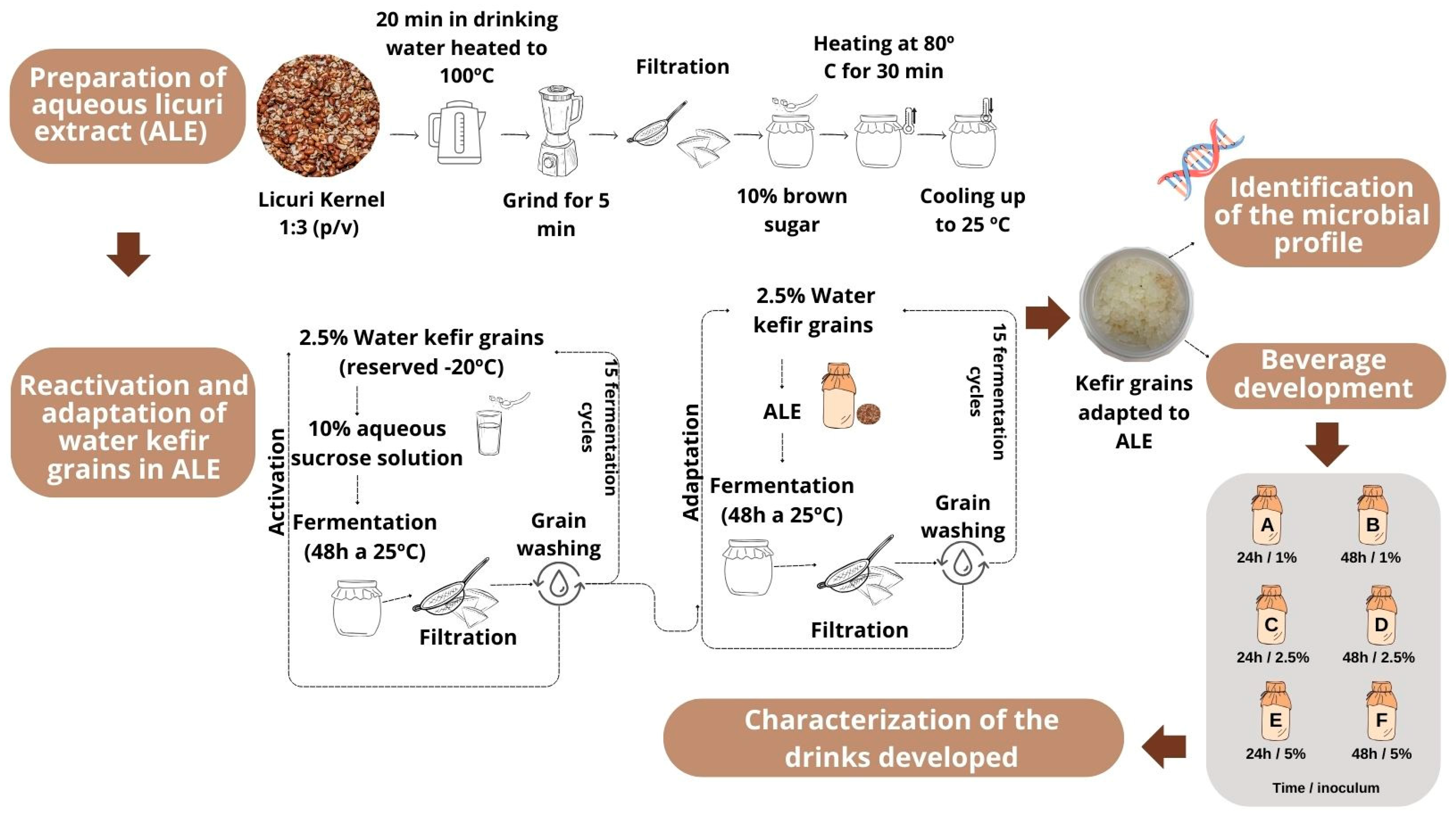
2.2.1. Preparation of Aqueous Licuri Extract
2.2.2. Reactivation and Adaptation of Water Kefir Grains in Aqueous Licuri Extract
2.2.3. Identification of the Microbial Profile of Kefir Grains Adapted in Aqueous Licuri Extract
2.3. Preparation of a Fermented Plant-Based Kefir Beverage Made from Licuri Kernel
Growth of Kefir Grains
2.4. Analysis of Quality Parameters in Fermented Plant-Based Kefir Beverages Made from Licuri during Refrigerated Storage
2.4.1. Nutritional and Technological Characterization of Fermented Plant-Based Kefir Beverages Made from Licuri Kernel
2.4.2. Microbiological Safety Analysis of Plant-Based Fermented Kefir Beverages Made from Licuri
2.4.3. Sensory Analysis—Acceptance Test and Ideal Scale of Fermented Plant-Based Kefir Beverages Made from Licuri Kernel
2.5. Data Processing
3. Results and Discussion
3.1. Microbial Profile of Water Kefir Grains Adapted in Aqueous Licuri Extract
3.2. Growth of Water Kefir Grains Adapted to Aqueous Licuri Extract
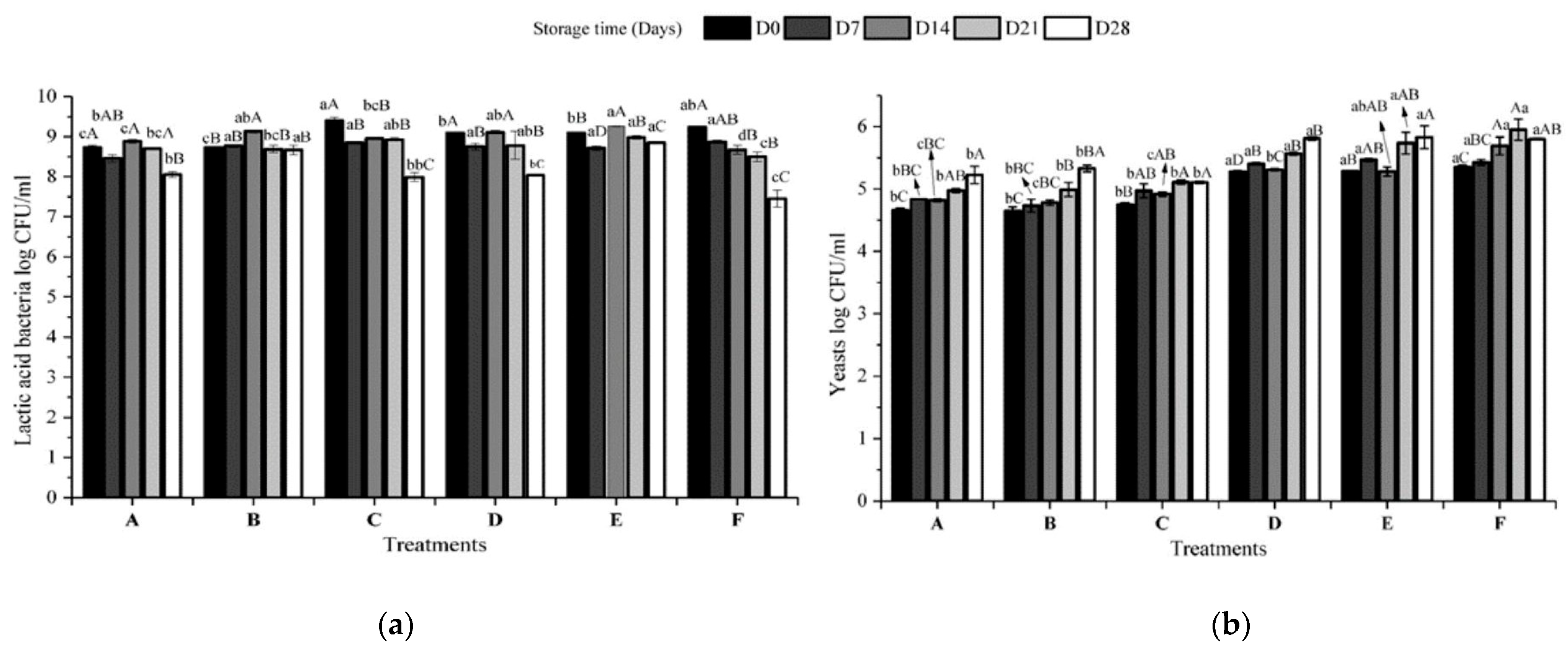
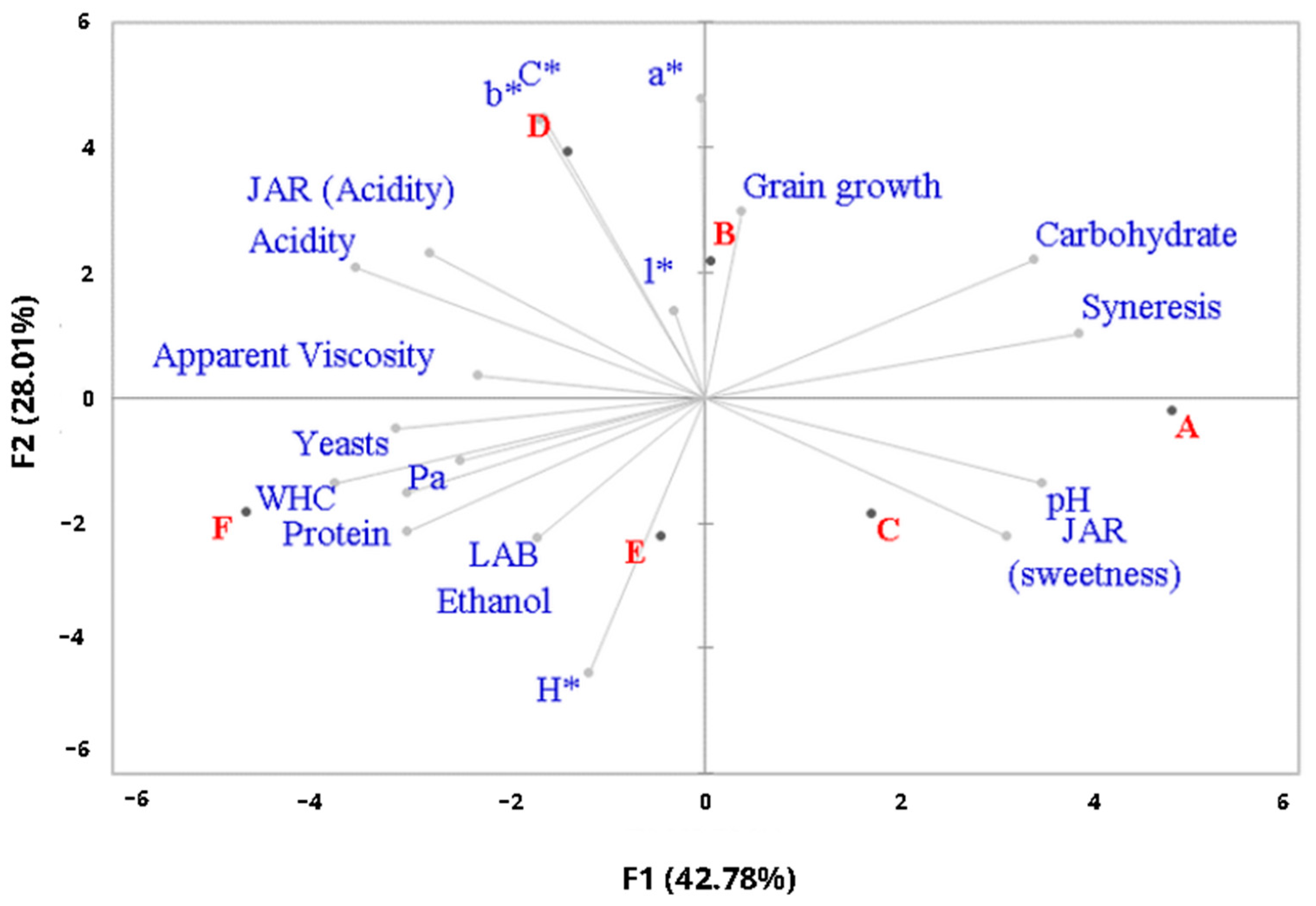
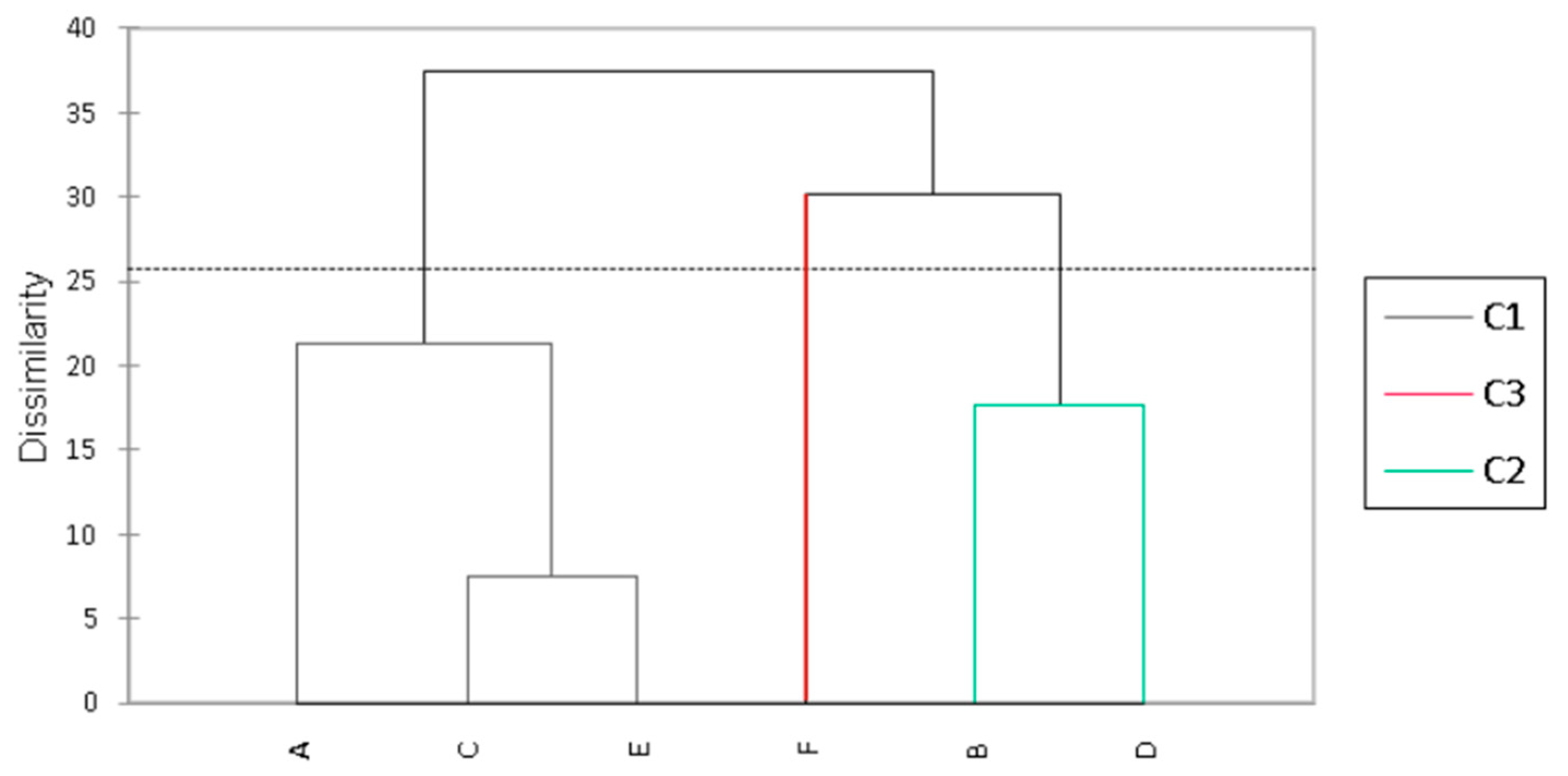
3.3. Analysis of Quality Parameters in Fermented Plant-Based Kefir Beverages Made from Licuri during Refrigerated Storage
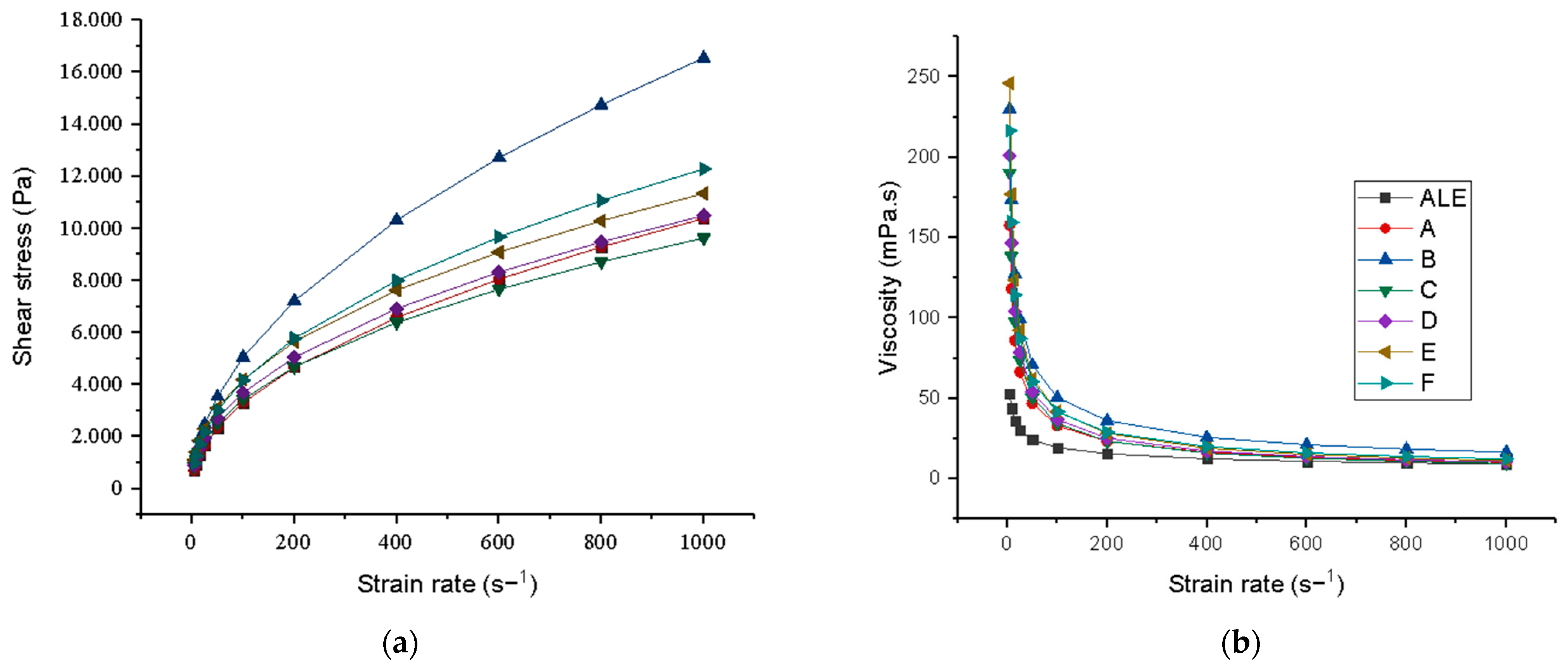
3.4. Nutritional and Technological Characterization of Fermented Plant-Based Kefir Beverages Made from Licuri
3.5. Microbiological Safety Analysis of Fermented Plant-Based Kefir Beverages of Licuri Kernel
3.6. Sensory Analysis—Acceptance Test and Ideal Scale of Fermented Plant-Based Kefir Beverages of Licuri Kernel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allied Market Research—Global Insights, Custom Reports, and Expert Consulting for Strategic Business Success. Available online: https://www.alliedmarketresearch.com/plant-based-beverage-market (accessed on 10 August 2023).
- UN-BR. 17 Objetivos para Transformar Nosso Mundo. Transformando Nosso Mundo: A Agenda 2030 para o Desenvolvimento Sustentável. 2015. Available online: https://nacoesunidas.org/pos2015/agenda2030/ (accessed on 8 November 2023).
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Cai, J.-S.; Feng, J.-Y.; Ni, Z.-J.; Ma, R.-H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Verni, M.; Demarinis, C.; Rizzello, C.G.; Baruzzi, F. Design and characterization of a novel fermented beverage from lentil grains. Foods 2020, 9, 893. Available online: https://www.mdpi.com/2304-8158/9/7/893 (accessed on 25 November 2023). [CrossRef]
- Zhu, Y.-Y.; Thakur, K.; Feng, J.-Y.; Cai, J.-S.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. B-vitamin enriched fermented soymilk: A novel strategy for soy-based functional foods development. Trends Food Sci. Technol. 2020, 105, 43–55. [Google Scholar] [CrossRef]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. The water kefir grain inoculum determines the characteristics of the resulting water kefir fermentation process. J. Appl. Microbiol. 2017, 122, 719–732. [Google Scholar] [CrossRef]
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, Á. Water kefir, a fermented beverage containing probiotic microorganisms: From ancient and artisanal manufacture to industrialized and regulated commercialization. Future Foods 2022, 5, 100123. [Google Scholar] [CrossRef]
- Waldherr, F.W.; Doll, V.M.; Meißner, D.; Vogel, R.F. Identification and characterization of a glucan-producing enzyme from Lactobacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol. 2010, 27, 672–678. [Google Scholar] [CrossRef]
- Xu, D.; Fels, L.; Wefers, D.; Behr, J.; Jakob, F.; Vogel, R.F. Lactobacillus hordei dextrans induce Saccharomyces cerevisiae aggregation and network formation on hydrophilic surfaces. Int. J. Biol. Macromol. 2018, 115, 236–242. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Martínez-Torres, A.; Gutiérrez-Ambrocio, S.; Heredia-del-Orbe, P.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Inferring the role of microorganisms in water kefir fermentations. Int. J. Food Sci. Technol. 2017, 52, 559–571. [Google Scholar] [CrossRef]
- Bueno, R.S.; Ressutte, J.B.; Hata, N.N.Y.; Henrique-Bana, F.C.; Guergoletto, K.B.; de Oliveira, A.G.; Spinosa, W.A. Quality and shelf life assessment of a new beverage produced from water kefir grains and red pitaya. LWT—Food Sci. Technol. 2021, 140, 110770. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; de Foy, J.-M.P.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Cui, X.-H.; Chen, S.-J.; Wang, Y.; Han, J.-R. Fermentation conditions of walnut milk beverage inoculated with kefir grains. LWT—Food Sci. Technol. 2013, 50, 349–352. [Google Scholar] [CrossRef]
- Gocer, E.M.C.; Koptagel, E. Production and evaluation of microbiological & rheological characteristics of kefir beverages made from nuts. Food Biosci. 2023, 52, 102367. [Google Scholar] [CrossRef]
- dos Santos, D.C.; Filho, J.G.d.O.; Santana, A.C.A.; de Freitas, B.S.M.; Silva, F.G.; Takeuchi, K.P.; Egea, M.B. Optimization of soymilk fermentation with kefir and the addition of inulin: Physicochemical, sensory and technological characteristics. LWT—Food Sci. Technol. 2019, 104, 30–37. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy Kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Atalar, I. Functional kefir production from high pressure homogenized hazelnut milk. LWT—Food Sci. Technol. 2019, 107, 256–263. [Google Scholar] [CrossRef]
- Atik, D.S.; Gürbüz, B.; Bölük, E.; Palabıyık, İ. Development of vegan kefir fortified with Spirulina platensis. Food Biosci. 2021, 42, 101050. [Google Scholar] [CrossRef]
- Kabakcı, S.A.; Türkyılmaz, M.; Özkan, M. Changes in the quality of kefir fortified with anthocyanin-rich juices during storage. Food Chem. 2020, 326, 126977. [Google Scholar] [CrossRef]
- Alves, V.; Scapini, T.; Camargo, A.F.; Bonatto, C.; Stefanski, F.S.; de Jesus, E.P.; Diniz, L.G.T.; Bertan, L.C.; Maldonado, R.R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. LWT—Food Sci. Technol. 2021, 145, 111364. [Google Scholar] [CrossRef]
- Laureys, D.; Leroy, F.; Hauffman, T.; Raes, M.; Aerts, M.; Vandamme, P.; De Vuyst, L. The type and concentration of inoculum and substrate as well as the presence of oxygen impact the water Kefir fermentation process. Front. Microbiol. 2021, 12, 628599. [Google Scholar] [CrossRef]
- BAHIA (Estado). Lei n° 13.908 de 29 de Janeiro de 2018. Estabelece Como Patrimônio Biocultural as Espécies Do LICURI, Do Ariri E Do Umbu, Torna Essas Espécies Imunes Ao Corte E Dá Outras Providências. Available online: http://www.legislabahia.ba.gov.br/documentos/lei-no-13908-de-29-de-janeiro-de-2018 (accessed on 21 December 2023).
- Crepaldi, I.C.; Almeida-Muradian, L.B.D.E.; Rios, M.D.G.; Penteado, M.D.E.V.C.; Salatino, A. Composição nutricional do fruto de licuri (Syagrus coronata (Martius) Beccari). Braz. J. Bot. 2001, 24, 155–159. [Google Scholar] [CrossRef]
- Rodrigues, I. In Vitro Anti-Leishmania Amazonensis Activity of the Polymeric Procyanidin-Rich Aqueous Extract from Syagrus coronata. J. Med. Plants Res. 2011, 5, 3781–3790. Available online: http://www.academicjournals.org/JMPR (accessed on 21 December 2023).
- Belviso, S.; Ghirardello, D.; Giordano, M.; Ribeiro, G.S.; Alves, J.d.S.; Parodi, S.; Risso, S.; Zeppa, G. Phenolic composition, antioxidant capacity and volatile compounds of licuri (Syagrus coronata (Martius) Beccari) fruits as affected by the traditional roasting process. Food Res. Int. 2013, 51, 39–45. [Google Scholar] [CrossRef]
- Hughes, A.F.d.S.; de Lima, F.G.; Lucchese, A.M.; Neto, A.G.; Uetanabaro, A.P.T. Antimicrobial activity of Syagrus coronata (Martius) Beccari. Braz. Arch. Biol. Technol. 2013, 56, 269–274. [Google Scholar] [CrossRef]
- Antoniassi, R.; Bizzo, H.R.; Matsuura, M.D.S.; Bispo, E.; da Cruz, I.C.M.; Chiaro, R.S. Esteróis de gordura de licuri (Syagrus Coronata). In Congresso Brasileiro De Ciência E Tecnologia De Alimentos; CBCTA: Curitiba, Brazil, 2006. [Google Scholar]
- Teixeira, S.L.S.K.; Meneghetti, S.M.P.; Ferreira, L.S.W.; Meneghetti, M.R.; dos Santos, I.C.F.; da Silva, J.P.V.; de Carvalho, S.H.V.; Soletti, J.I. Characterization of Syagrus coronata (Mart.) Becc. oil and properties of methyl esters for use as biodiesel. Ind. Crops Prod. 2010, 32, 518–521. [Google Scholar] [CrossRef]
- Cibele, M.A.D.S.B.; Rodrigo, S.D.N.; Renata, C.C.A.; José, M.A.; Ana, P.S.A.S.; Alexandre, G.D.S.; Vera, L.D.M.L.; Josean F., T.; Luís, C.N.D.S.; Marcia, V.D.S.; et al. Syagrus coronata seed oils have antimicrobial action against multidrug-resistant Staphylococcus aureus. J. Med. Plants Res. 2016, 10, 310–317. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. Microbial species diversity, community dynamics, and metabolite kinetics of water Kefir fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Brasiel, P.G.d.A.; Medeiros, J.D.; Machado, A.B.F.; Ferreira, M.S.; Peluzio, M.D.C.G.; Luquetti, S.C.P.D. Microbial community dynamics of fermented kefir beverages changes over time. Int. J. Dairy. Technol. 2021, 74, 324–331. [Google Scholar] [CrossRef]
- Gamba, R.R.; Koyanagi, T.; Peláez, A.L.; De Antoni, G.; Enomoto, T. Changes in Microbiota during multiple fermentation of Kefir in different sugar solutions revealed by high-throughput sequencing. Curr. Microbiol. 2021, 78, 2406–2413. [Google Scholar] [CrossRef]
- Magalhães, K.T.; Pereira, M.A.; Nicolau, A.; Dragone, G.; Domingues, L.; Teixeira, J.A.; Silva, J.B.d.A.; Schwan, R.F. Production of fermented cheese whey-based beverage using kefir grains as starter culture: Evaluation of morphological and microbial variations. Bioresour. Technol. 2010, 101, 8843–8850. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; En: PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar]
- Schmidt, P.A.; Bálint, M.; Greshake, B.; Bandow, C.; Römbke, J.; Schmitt, I. Illumina metabarcoding of a soil fungal community. Soil. Biol. Biochem. 2013, 65, 128–132. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- AOAC; Horwitz, W. International A: Official Methods of Analysis of the AOAC International; The Association: Arlington County, VA, USA, 2000. [Google Scholar]
- American Public Health Association (APHA). Compendium of Methods for the Microbiological Examination of Foods; APHA Press: Washington, DC, USA, 2015. [Google Scholar]
- Atwater, W.O.; Bosque, C.D. Bulletin 28, U.S. Department of Agriculture, Chemical Composition of American Food Materials; Government Printing Office: Washington, DC, USA, 1896; pp. 1–47.
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef]
- Jamali, S.; McKinley, G.H.; Armstrong, R.C. Microstructural rearrangements and their rheological implications in a model thixotropic elastoviscoplastic fluid. Phys. Rev. Lett. 2017, 118, 048003. [Google Scholar] [CrossRef]
- CODEX STAN 243-2003; Codex Standard for Fermented Milks. Codex Alimentarius Commission: Rome, Italy, 2003. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B243-2003%252FCXS_243e.pdf/ (accessed on 10 August 2023).
- Brasil. Ministério da Saúde. Conselho Nacional de Saúde. Resolução n.º 196, de 10 de Outubro de 1996. Dispõe Sobre Diretrizes e Normas Regulamentadoras de Pesquisas Envolvendo seres Humanos. Diário Oficial da União, Brasília, 16 out. 1996. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/cns/1996/res0196_10_10_1996.html (accessed on 10 November 2023).
- Li, B.; Hayes, J.E.; Ziegler, G.R. Just-about-right and ideal scaling provide similar insights into the influence of sensory attributes on liking. Food Qual. Prefer. 2014, 37, 71–78. [Google Scholar] [CrossRef]
- Rothman, L.; Parker, M. Just-about-Right (JAR) Scales; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- LUMIVERO XLSTAT, Version 2023.1.5. Statistical and Date Analysis Solution. LUMIVERO: New York, NY, USA, 2023. Available online: https://www.xlstat.com (accessed on 21 August 2023).
- ORIGINPRO, Version 2017; OriginLab Corporation: Northampton, MA, USA, 2016.
- Zanirati, D.F.; Abatemarco, M.J.; de Cicco Sandes, S.H.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, M. The microbial flora of sugary kefir grain (the gingerbeer plant): Biosynthesis of the grain from Lactobacillus hilgardii producing a polysaccharide gel. World J. Microbiol. Biotechnol. 1989, 5, 223–238. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. Oxygen and diverse nutrients influence the water kefir fermentation process. Food Microbiol. 2018, 73, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, M.; Brillouet, J.M.; Quemener, B. Characterization of the polysaccharides from a Lactobacillus brevis and from sugary kefir grains. Biotechnol. Lett. 1988, 10, 415–420. [Google Scholar] [CrossRef]
- Pourramezan, Z.; Oloomi, M.; Kermanshahi, R.K.; Rezadoost, H. Extraction and isolation of antioxidant-antibacterial compounds from Lactobacillus casei strain K1C by thin-layer chromatography. Jundishapur J. Nat. Pharm. Prod. 2021, 16, 1. [Google Scholar] [CrossRef]
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Review: Adaptation of beneficial propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Carasi, P.; Jacquot, C.; Romanin, D.E.; Elie, A.M.; De Antoni, G.L.; Urdaci, M.C.; Serradell, M.d.L.A. Safety and potential beneficial properties of Enterococcus strains isolated from kefir. Int. Dairy J. 2014, 39, 193–200. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. Shotgun metagenomics of a water Kefir fermentation ecosystem reveals a novel Oenococcus species. Front. Microbiol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. The buffer capacity and calcium concentration of water influence the microbial species diversity, grain growth, and metabolite production during water Kefir fermentation. Front. Microbiol. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Tavares, P.P.L.G.; Silva, M.R.; Santos, L.F.P.; Nunes, I.L.; Magalhães-Guedes, K.T. Production of quinoa (Chenopodium quinoa) kefir fermented beverage flavored with cocoa (Theobroma cacao) powder. Braz. J. Agric. Sci. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo, P.G.V.; Thomaz-Soccol, V.; Medeiros, A.P.; Rakshit, S.K.; Soccol, C.R. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. LWT—Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]
- Tavares, P.P.L.G.; Mamona, C.T.P.; Nascimento, R.Q.; dos Anjos, E.A.; de Souza, C.O.; Almeida, R.C.d.C.; Mamede, M.E.d.O.; Magalhães-Guedes, K.T. Non-conventional sucrose-based substrates: Development of non-dairy Kefir beverages with probiotic potential. Fermentation 2023, 9, 384. [Google Scholar] [CrossRef]
- Andrade, A.C.; Marinho, J.F.U.; de Souza, A.C.; de Sousa Tavares, T.; Dias, D.R.; Schwan, R.F.; Nunes, C.A.; Bastos, S.C. Prebiotic potential of pulp and kernel cake from Jerivá (Syagrus romanzoffiana) and Macaúba palm fruits (Acrocomia aculeata). Food Res. Int. 2020, 136, 109595. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y. Response surface optimization of millet milk fermented by Lactobacillus kefir. J. Food Process. Preserv. 2022, 46, 7. [Google Scholar] [CrossRef]
- Sabokbar, N.; Moosavi-Nasab, M.; Khodaiyan, F. Preparation and characterization of an apple juice and whey based novel beverage fermented using kefir grains. Food Sci. Biotechnol. 2015, 24, 2095–2104. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 6. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Śmietana, N.; Paradowska, D.; Drozłowska, E. Black cumin (Nigella sativa L.) seed press cake as a novel material for the development of new non-dairy beverage fermented with Kefir grains. Microorganisms 2022, 10, 300. [Google Scholar] [CrossRef]
- Fioravante, M.B.; Hiane, P.A.; Neto, J.A.B. Elaboration, sensorial acceptance and characterization of fermented flavored drink based on water-soluble extract of baru almond. Cienc. Rural. 2017, 47, 9. [Google Scholar] [CrossRef]
- Soumya, S.A.; Parameswaran, R.; Nampoothiri, K.M. Physico-chemical and organoleptic evaluation of probiotic plant-milk yogurt-type beverages as a functional alternative to dairy yogurts. Biocatal. Agric. Biotechnol. 2024, 57, 103060. [Google Scholar] [CrossRef]
- Rincon, L.; Botelho, R.B.A.; de Alencar, E.R. Development of novel plant-based milk based on chickpea and coconut. LWT—Food Sci. Technol. 2020, 128, 109479. [Google Scholar] [CrossRef]
- Zardo, D.M.; Dantas, A.P.; Vanz, R.; Wosiacki, G.; Nogueira, A. Intensity of red pigmentation in apples and its influence onphenolic compounds content and antioxidant activity. Food Sci. Technol. 2009, 29, 148–154. [Google Scholar] [CrossRef]
- Chandra, R.D.; Prihastyanti, M.N.U.; Lukitasari, D.M. Effects of pH, high pressure processing, and ultraviolet light on carotenoids, chlorophylls, and anthocyanins of fresh fruit and vegetable juices. eFood 2021, 2, 113–124. [Google Scholar] [CrossRef]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis investigations of lacto-fermented sodium caseinate in a mixed model system. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef]
- Arab, M.; Yousefi, M.; Khanniri, E.; Azari, M.; Ghasemzadeh-Mohammadi, V.; Mollakhalili-Meybodi, N. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. J. Food Sci. Technol. 2023, 60, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Baria, B.; Agarwal, K.; Ravi, R.; Singh, A.K.; Shetty, P.H. Functional enhancement of yoghurt through incorporation of glucan exopolysaccharide from Enterococcus hirae OL616073 of food origin. J. Food Meas. Charact. 2024, 1–15. [Google Scholar] [CrossRef]
- Silva, K.; Machado, A.; Cardoso, C.; Silva, F.; Freitas, F. Rheological behavior of plant-based beverages. Food Sci. Technol. 2020, 40, 258–263. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Yu, Z.; Gao, Z.; Mu, G.; Wu, X. Influence on physical properties and digestive characters of fermented coconut milk with different loading proportion of skimmed coconut drink using Lactiplantibacillus plantarum MWLp-4 from human milk mixing with commercial bacteria. Food Biosci. 2023, 53, 102598. [Google Scholar] [CrossRef]
- Gamli, O.F.; Atasoy, A.F. Physico-chemical and sensorial properties of groundnut milk and it’s yoghurt. J. Food Meas. Charact. 2018, 12, 1997–2004. [Google Scholar] [CrossRef]
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Regulamento Técnico de Identidade e Qualidade de Leites Fermentados. Instrução Normativa n° 46, de 23 de Outubro de 2007. Available online: https://www.cidasc.sc.gov.br/inspecao/files/2019/09/instru%C3%A7%C3%A3o-normativa-n-46-de-23-de-outubro-de-2007-Leites-Fermentados.pdf (accessed on 21 May 2023).
- Sommo, M.; de Aguiar, L.A.; Raposo, A.; Saraiva, A.; Teixeira-Lemos, E.; Chaves, C.; Romão, B. Development and Rapid Sensory Descriptive Characterization of Cereal Bars Made with Brazilian Licuri Nut (Syagrus coronata). Foods 2024, 13, 502. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, D.; Clausen, M.P.; Jaeger, S.R. Understanding barriers to consumption of plant-based foods and beverages: Insights from sensory and consumer science. Curr. Opin. Food Sci. 2022, 48, 100919. [Google Scholar] [CrossRef]
- Farah, J.S.; Araujo, C.B.; Melo, L. Analysis of yoghurts’, whey-based beverages’ and fermented milks’ labels and differences on their sensory profiles and acceptance. Int. Dairy J. 2017, 68, 17–22. [Google Scholar] [CrossRef]
| Formulations | Variables | |
|---|---|---|
| Fermentation Time (h) | Inoculum (%) | |
| Aqueous licuri extract | 0 | 0 |
| A | 24 | 1 |
| B | 48 | 1 |
| C | 24 | 2.5 |
| D | 48 | 2.5 |
| E | 24 | 5 |
| F | 48 | 5 |
| Group | Genus/Species | DNA Sequences | Species (%) | Kingdom (%) |
|---|---|---|---|---|
| Lactic acid bacteria | Lactobacillus Hilgardii | 87525 | 89.24 | Bacteria 58.51 |
| Lentilactobacillus sp. | 50360 | 5.13 | ||
| Lacticaseibacillus sp. | 19930 | 2.03 | ||
| Lacticaseibacillus casei | 13160 | 1.34 | ||
| Lactobacillus farraginis | 73700 | 0.75 | ||
| Lactobacillus diolivorans | 29900 | 0.30 | ||
| Enterococcus hirae | 1400 | 0.01 | ||
| Acetic acid bacteria | Acetobacter sp. | 603 | 0.61 | |
| Acetobacter orientalis | 548 | 0.56 | ||
| Acetobacter peroxydans | 8 | 0.01 | ||
| Yeast | Brettanomyces bruxellensis | 62917 | 59.49 | Fungi 41.49 |
| Pichiaceae sp. | 18608 | 17.59 | ||
| Brettanomyces anomalus | 11268 | 10.65 | ||
| Saccharomyces sp. | 90370 | 8.54 | ||
| Brettanomyces sp. | 3776 | 3.57 | ||
| Meyerozyma carpophila | 6500 | 0.06 | ||
| Pichia kudriavzevii | 57 | 0.05 | ||
| Pichia manshurica | 2400 | 0.02 | ||
| Starmerela apicola | 600 | 0.01 |
| Parameter | Formulation | D0 | D7 | D14 | D21 | D28 |
|---|---|---|---|---|---|---|
| Titratable acidity lactic acid (mg/100 mL) | ALE | 0.09 ± 0.00 g | ** | |||
| A | 0.33 ± 0.00 f D | 0.46 ± 0.00 eC | 0.46 ± 0.00 f BC | 0.47 ± 0.00 eB | 0.52 ± 0.00 dA | |
| B | 0.76 ± 0.00 cB | 0.80 ± 0.00 bA | 0.76 ± 0.00 cB | 0.73 ± 0.00 bC | 0.71 ± 0.00 bD | |
| C | 0.55 ± 0.00 eD | 0.62 ± 0.00 dC | 0.65 ± 0.00 eB | 0.65 ± 0.00 dB | 0.68 ± 0.00 cA | |
| D | 0.88 ± 0.00 aA | 0.81 ± 0.00 bC | 0.86 ± 0.00 aB | 0.80 ± 0.00 aC | 0.81 ± 0.00 aC | |
| E | 0.64 ± 0.00 dD | 0.69 ± 0.00 cC | 0.69 ± 0.00 dC | 0.72 ± 0.00 cA | 0.70 ± 0.00 bB | |
| F | 0.83 ± 0.00 bB | 0.83 ± 0.00 aB | 0.84 ± 0.00 bA | 0.81 ± 0.00 aC | 0.81 ± 0.00 aC | |
| pH | ALE | 5.93 ± 0.11 a | ** | |||
| A | 4.29 ± 0.04 bA | 3.93 ± 0.05 aB | 3.87 ± 0.00 aBC | 3.80 ± 0.01 aC | 3.71 ± 0.01 aD | |
| B | 3.56 ± 0.01 dA | 3.55 ± 0.01 cA | 3.56 ± 0.00 cA | 3.54 ± 0.01 cA | 3.48 ± 0.02 bB | |
| C | 3.75 ± 0.01 cA | 3.65 ± 0.01 bB | 3.64 ± 0.01 bB | 3.60 ± 0.00 bcC | 3.48 ± 0.01 bD | |
| D | 3.52 ± 0.01 dA | 3.50 ± 0.01 cA | 3.49 ± 0.01 dA | 3.58 ± 0.12 bcA | 3.47 ± 0.05 bA | |
| E | 3.76 ± 0.05 cA | 3.65 ± 0.01 bAB | 3.58 ± 0.01 cB | 3.66 ± 0.09 abAB | 3.45 ± 0.01 bC | |
| F | 3.52 ± 0.01 dAB | 3.53 ± 0.03 cA | 3.48 ± 0.02 dB | 3.51 ± 0.01 cAB | 3.51 ± 0.02 bAB | |
| Ethanol content (% v/v) | A | * | * | * | * | * |
| B | * | * | * | * | * | |
| C | * | * | * | * | * | |
| D | * | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | |
| E | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | 0.1 ± 0.00 aA | |
| F | 0.6 ± 0.00 aA | 0.6 ± 0.00 aA | 0.4 ± 0.00 aA | 0.4 ± 0.00 aA | 0.3 ± 0.01 aA | |
| Formulations | Moisture (g/100) | Lipids (g/100) | Carbohydrates (g/100) | Proteins (g/100) | Ashes (g/100) | Energy Value (Kcal/100 g) |
|---|---|---|---|---|---|---|
| ALE | 77.08 ± 0.13 a | 10.97 ± 0.18 a | 9.98 ± 0.16 ab | 1.80 ± 0.37 b | 0.23 ± 0.00 a | 125.47 ± 29.52 a |
| A | 76.42 ± 0.41 a | 10.36 ± 1.20 a | 11.51 ± 1.26 a | 1.46 ± 0.24 c | 0.25 ± 0.00 a | 122.11 ± 21.50 a |
| B | 77.10 ± 1.37 a | 12.27 ± 0.45 a | 9.06 ± 0.50 ab | 1.37 ± 0.33 c | 0.19 ± 0.01 a | 134.04 ± 21.86 a |
| C | 76.98 ± 0.10 a | 10.99 ± 1.04 a | 10.32 ± 1.07 ab | 1.43 ± 0.24 c | 0.27 ± 0.00 a | 125.31 ± 19.62 a |
| D | 76.22 ± 0.28 a | 11.44 ± 0.13 a | 10.50 ± 0.08 a | 1.55 ± 0.31 c | 0.29 ± 0.00 a | 130.20 ± 30.62 a |
| E | 76.88 ± 1.07 a | 13.72 ± 0.42 a | 7.66 ± 0.41 bc | 1.49 ± 0.02 c | 0.25 ± 0.00 a | 144.76 ± 25.46 a |
| F | 75.97 ± 0.30 a | 13.94 ± 2.70 a | 5.86 ± 0.19 c | 2.16 ± 0.84 a | 0.27 ± 0.01 a | 145.81 ± 7.96 a |
| Formulations | L* | a* | b* | C* | H° | ∆E* |
|---|---|---|---|---|---|---|
| ALE | 75.45 ± 0.02 e | 2.59 ± 0.01 a | 11.29 ± 0.02 g | 11.59 ± 0.02 f | 77.05 ± 0.04 f | - |
| A | 81.64 ± 0.04 b | 1.60 ± 0.00 d | 11.58 ± 0.01 e | 11.69 ± 0.01 e | 82.14 ± 0.01 d | 6.27 ± 0.04 c |
| B | 80.41 ± 0.00 d | 1.66 ± 0.00 c | 12.50 ± 0.00 b | 12.61 ± 0.00 b | 82.46 ± 0.00 c | 5.18 ± 0.00 f |
| C | 81.63 ± 0.01 b | 1.40 ± 0.01 f | 11.43 ± 0.02 f | 11.51 ± 0.02 g | 83.02 ± 0.03 b | 6.29 ± 0.01 b |
| D | 82.84 ± 0.01 a | 1.96 ± 0.00 b | 13.33 ± 0.01 a | 13.48 ± 0.01 a | 81.64 ± 0.01 e | 7.69 ± 0.00 a |
| E | 81.46 ± 0.01 c | 1.40 ± 0.01 f | 11.67 ± 0.01 d | 11.76 ± 0.01 d | 83.15 ± 0.03 a | 6.13 ± 0.01 e |
| F | 81.48 ± 0.01 c | 1.44 ± 0.01 e | 12.01 ± 0.01 c | 12.10 ± 0.01 c | 83.13 ± 0.02 a | 6.18 ± 0.01 d |
| Formulation | Syneresis (%) | WHC (%) | ||||
|---|---|---|---|---|---|---|
| N | (mPa∙s) | K (Pa.s n) | R2 | |||
| ALE | 70.43 ± 1.61 a | 30.13 ± 1.55 cd | 0.67 ± 0.01 a | 30.39 ± 1.57 e | 85.79 ± 6.07 c | 0.99 |
| A | 70.81 ± 1.24 a | 29.25 ± 0.13 d | 0.49 ± 0.02 bc | 66.50 ± 5.24 d | 337.72 ± 47.83 b | 0.99 |
| B | 66.86 ± 0.43 ab | 32.17 ± 0.11 bcd | 0.51 ± 0.04 b | 99.41 ± 8.52 a | 481.65 ± 95.02 ab | 0.99 |
| C | 65.20 ± 1.13 b | 33.91 ± 0.87 bc | 0.45 ± 0.01 cd | 73.82 ± 2.88 cd | 436.55 ± 26.86 ab | 0.98 |
| D | 63.64 ± 0.51 bc | 34.63 ± 0.67 b | 0.45 ± 0.01 cd | 78.73± 1.67 bcd | 457.37 ± 22.06 ab | 0.97 |
| E | 63.62 ± 1.13 bc | 34.54 ± 1.27 b | 0.43 ± 0.03 d | 92.41 ± 11.46 ab | 582.82 ± 130.50 a | 0.98 |
| F | 59.87 ± 1.15 c | 39.56 ± 1.59 a | 0.47 ± 0.00 bcd | 87.13± 0.82 abc | 481.21 ± 3.59 ab | 0.97 |
| Formulations | Appearance | Aroma | Flavor | Texture | Global Impression |
|---|---|---|---|---|---|
| A | 6.34 ± 1.96 a | 5.89 ± 1.96 a | 6.84 ± 1.89 a | 7.16 ± 1.74 a | 6.95 ± 1.64 a |
| B | 6.31 ± 1.87 a | 5.35 ± 2.07 a | 6.17 ± 2.20 a | 6.71 ± 1.89 a | 6.31 ± 1.90 a |
| C | 6.42 ± 1.86 a | 5.61 ± 1.95 a | 6.54 ± 1.96 a | 6.51 ± 1.99 a | 6.51 ± 1.67 a |
| D | 6.44 ± 1.67 a | 5.59 ± 2.02 a | 6.20 ± 2.28 a | 6.7 ± 1.97 a | 6.36 ± 1.98 a |
| E | 6.17 ± 1.94 a | 5.55 ± 2.01 a | 6.62 ± 1.94 a | 6.74 ± 1.97 a | 6.66 ± 1.55 a |
| F | 6.49 ± 2.00 a | 5.53 ± 1.94 a | 6.19 ± 2.22 a | 6.82 ± 1.73 a | 6.44 ± 1.87 a |
| Formulations | Ideal Scale | Penalty Analysis (% of Consumers and Mean Drop) | ||||
|---|---|---|---|---|---|---|
| Acidity | Sweetness | Acidity | Sweetness | |||
| Insufficient | Too Much | Insufficient | Too Much | |||
| A | 3.52 ± 1.00 b | 4.52 ± 1.4 ab | 35.00 (3.84) | - | - | 44.00 (2.31) |
| B | 4.62 ± 1.70 a | 4.09 ± 1.21 bc | - | 50.00 (3.34) | 22.00 (2.94) | 30.00 (2.40) |
| C | 3.51 ± 1.23 b | 4.64 ± 1.27 a | 43.00% (3.64) | - | - | 47.00 (3.90) |
| D | 4.36± 1.30 a | 4.02± 1.08 c | 20.00 (2.04) * | 41.00 (1.52) * | 22.00 (7.05) * | 23.00 (4.59) |
| E | 3.34 ± 1.22 b | 4.58 ± 1.20 a | 48 (2.88) | - | - | 47 (3.68) |
| F | 4.80 ± 1.3 a | 3.81 ± 1.02 c | - | 58 (3.41) | 30.00 (4.52) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Alves, J.; de Souza, C.O.; de Matos Santos, L.; Viana, S.N.A.; de Jesus Assis, D.; Tavares, P.P.L.G.; Requião, E.d.R.; Ferro, J.M.R.B.d.S.; Roselino, M.N. Licuri Kernel (Syagrus coronata (Martius) Beccari): A Promising Matrix for the Development of Fermented Plant-Based Kefir Beverages. Foods 2024, 13, 2056. https://doi.org/10.3390/foods13132056
de Carvalho Alves J, de Souza CO, de Matos Santos L, Viana SNA, de Jesus Assis D, Tavares PPLG, Requião EdR, Ferro JMRBdS, Roselino MN. Licuri Kernel (Syagrus coronata (Martius) Beccari): A Promising Matrix for the Development of Fermented Plant-Based Kefir Beverages. Foods. 2024; 13(13):2056. https://doi.org/10.3390/foods13132056
Chicago/Turabian Stylede Carvalho Alves, Janaína, Carolina Oliveira de Souza, Livia de Matos Santos, Suelen Neris Almeida Viana, Denilson de Jesus Assis, Pedro Paulo Lordelo Guimarães Tavares, Elis dos Reis Requião, Jéssica Maria Rio Branco dos Santos Ferro, and Mariana Nougalli Roselino. 2024. "Licuri Kernel (Syagrus coronata (Martius) Beccari): A Promising Matrix for the Development of Fermented Plant-Based Kefir Beverages" Foods 13, no. 13: 2056. https://doi.org/10.3390/foods13132056
APA Stylede Carvalho Alves, J., de Souza, C. O., de Matos Santos, L., Viana, S. N. A., de Jesus Assis, D., Tavares, P. P. L. G., Requião, E. d. R., Ferro, J. M. R. B. d. S., & Roselino, M. N. (2024). Licuri Kernel (Syagrus coronata (Martius) Beccari): A Promising Matrix for the Development of Fermented Plant-Based Kefir Beverages. Foods, 13(13), 2056. https://doi.org/10.3390/foods13132056









