Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Reagents
2.3. Fermentations
2.4. Extract Procedure
2.5. Yogurt Production
2.6. Physicochemical Properties
2.7. Bioactive Compounds and Antioxidant Activity
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fermentations
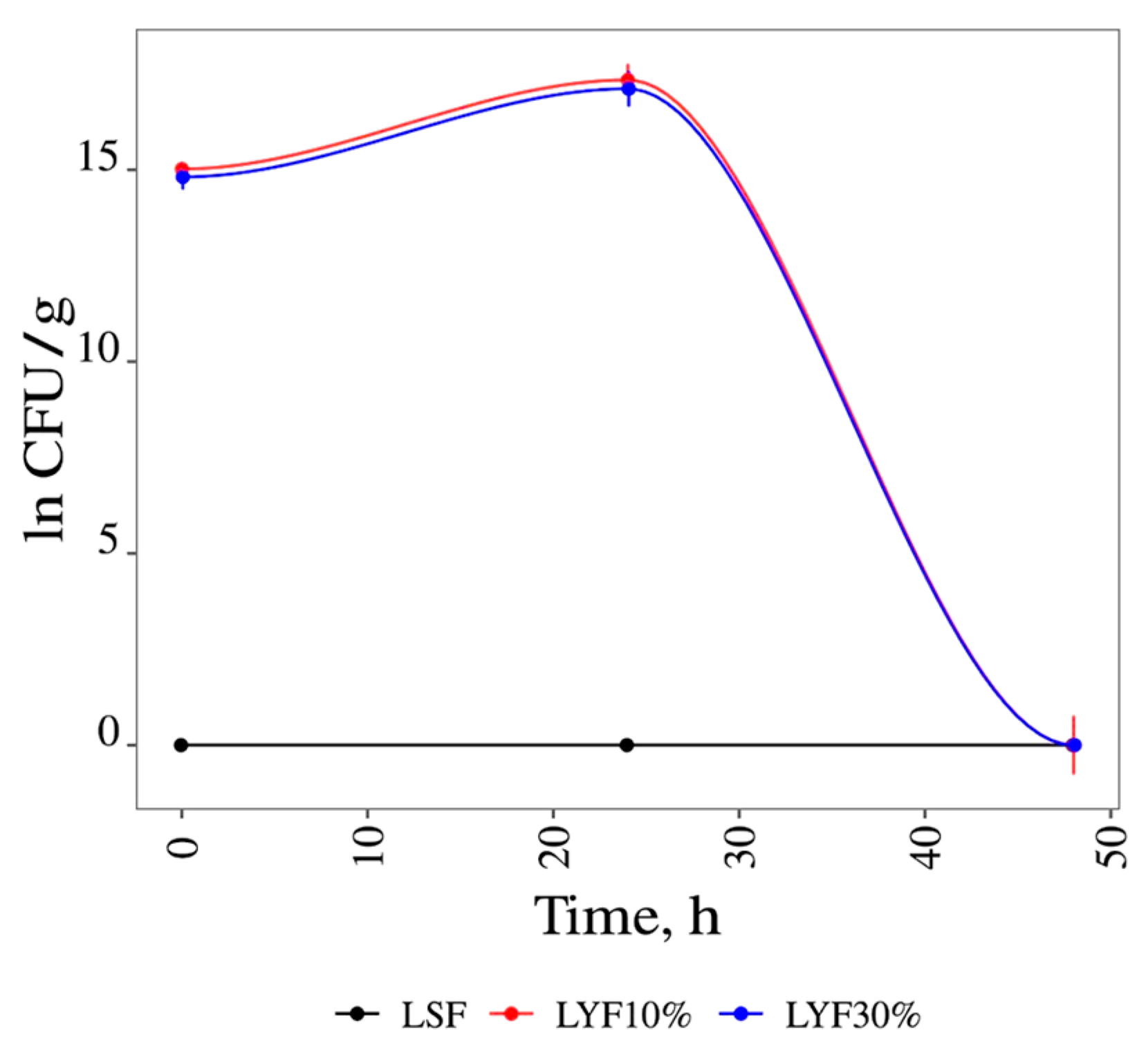
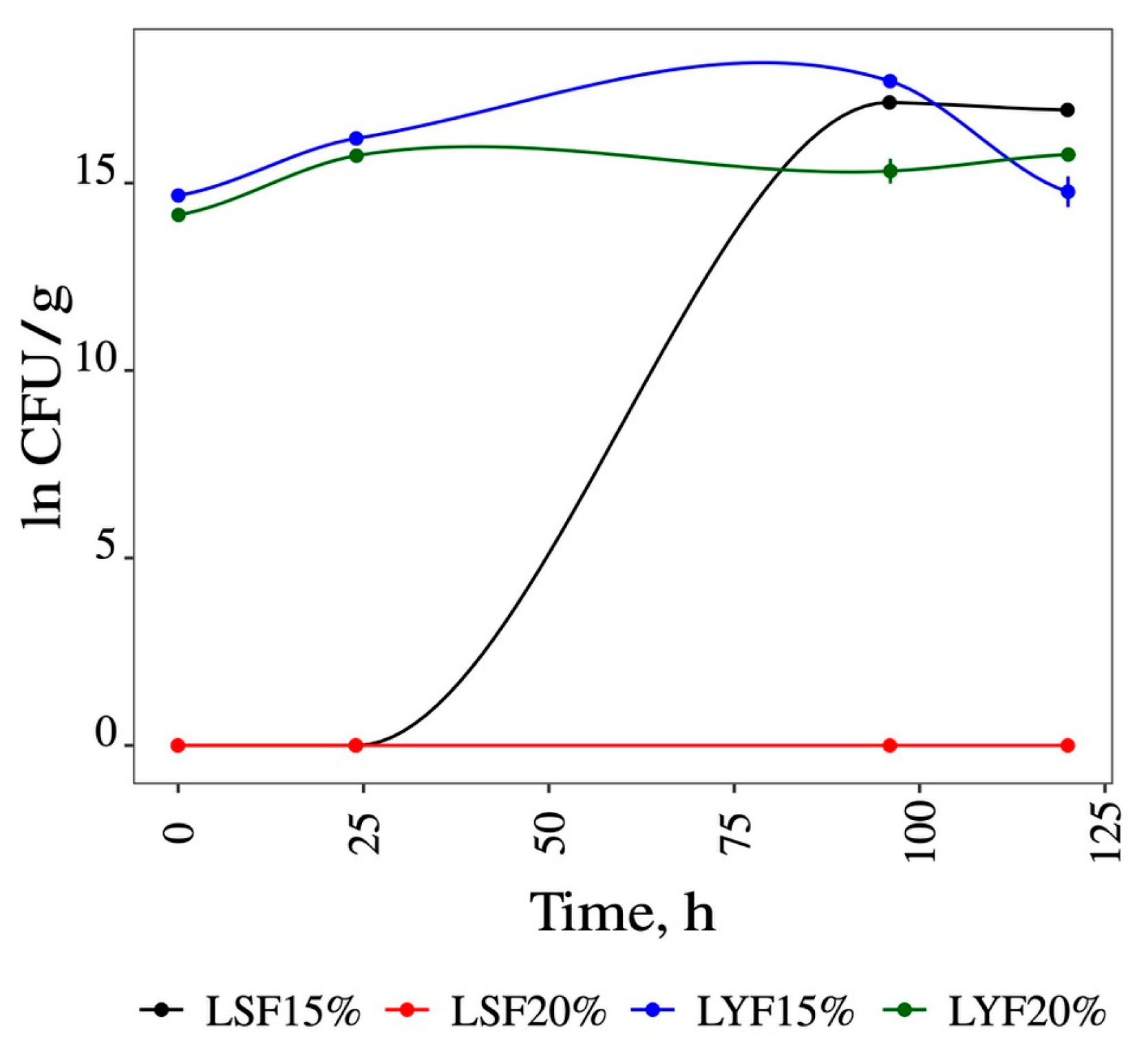
3.1.1. Liquid Media Fermentation
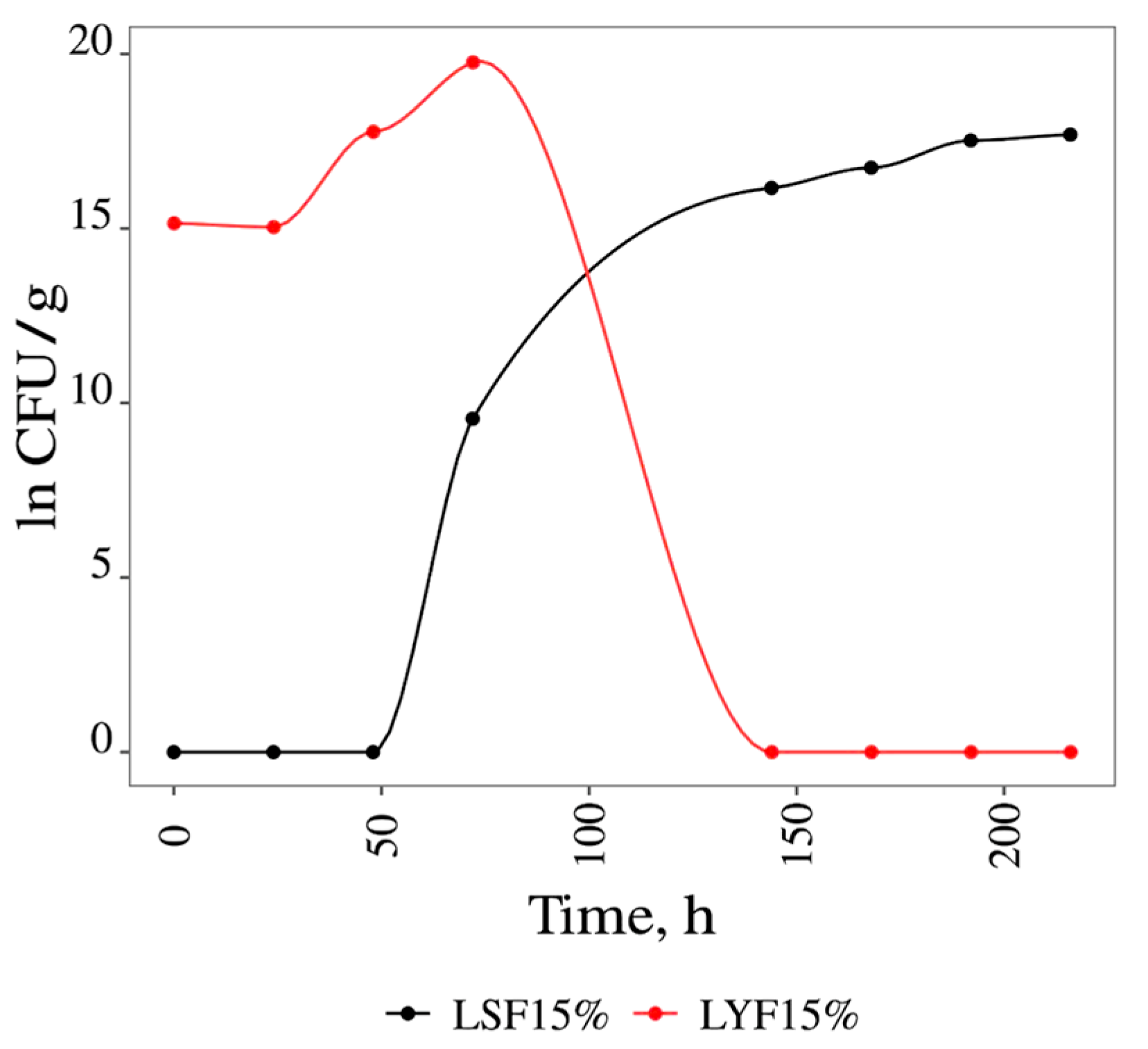
3.1.2. Solid Media Fermentation
3.2. Characterization of V. vinifera L. Leaves and Fermentation Products
3.2.1. Physicochemical Properties
3.2.2. Chemical Properties
3.2.3. Microbiological Analysis Results
3.3. Yogurt Analysis
3.3.1. Physicochemical Analysis Results of the Yogurt Samples
3.3.2. Chemical Analysis Results of the Yogurt Samples
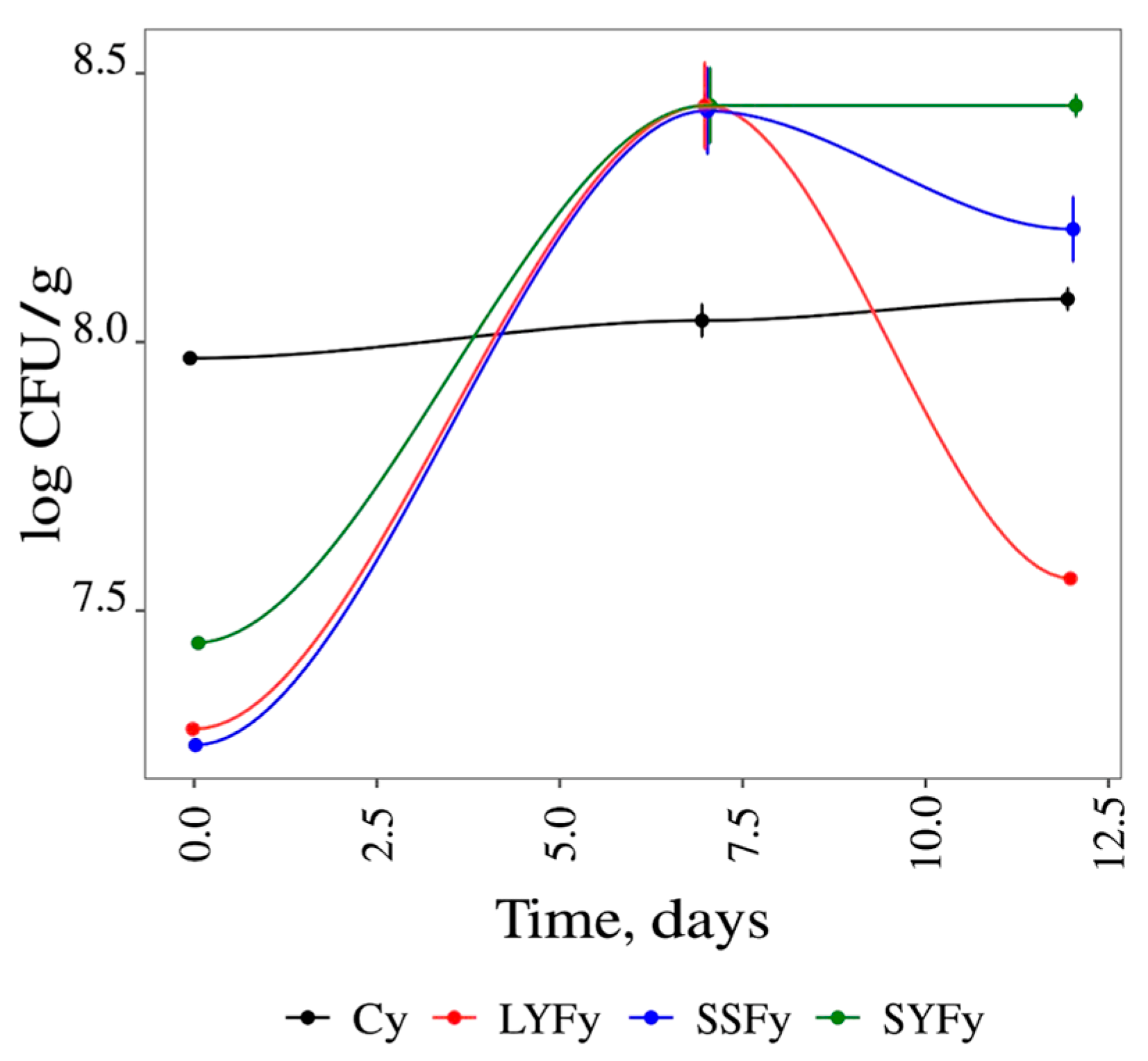
3.3.3. Microbiological Analysis Results of the Yogurt Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathias, T.R.S.; Carvalho Junior, I.C.; Carvalho, C.W.P.; Sérvulo, E.F.C. Rheological characterization of coffee-flavored yogurt with different types of thickener. Alim. Nutr. 2011, 22, 521–529. [Google Scholar]
- Lopes, R.P.; Mota, M.J.; Pinto, C.A.; Souza, S.; Silva, J.A.L.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Physicochemical and microbial changes in yogurts produced under different pressure and temperature conditions. LWT 2019, 99, 423–430. [Google Scholar] [CrossRef]
- Santos, J.V.R.; Miranda, E.S.M.; Oliveira, A.T.C.; Damaceno, M.N.; Silva, M.S.; Cavalcante, A.B.D. Cinética da fermentação de leite adicionado de farinha de banana verde na produção de iogurte. Res. Soc. Dev. 2020, 9, e295985316. [Google Scholar] [CrossRef]
- Kefi, B.B.; Baccouri, S.; Torkhani, R.; Koumba, S.; Martin, P.; M’hamdi, N. Application of response surface methodology to optimize solid-phase extraction of benzoic acid and sorbic acid from food drinks. Foods 2022, 11, 1257. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Xu, J.; Wang, L.; Zhou, K. Determination of nine preservatives in food samples by solid phase extraction coupled with capillary electrophoresis. Int. J. Electrochem. Sci. 2021, 16, 21022. [Google Scholar] [CrossRef]
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. 2022, 1, 100019. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. 2011. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32011R1129&from=es (accessed on 18 May 2024).
- Sat, I.G.; Sengul, M.; Keles, F. Use of grape leaves in canned food. Pak. J. Nutr. 2002, 1, 257–262. [Google Scholar]
- Lima, A.F. Caracterização da Bioatividade de Folhas de Diferentes Castas de Videira Quando Sujeitas a Processamento Alimentar. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2015. [Google Scholar]
- Nzekoue, F.K.; Nguefang, M.L.K.; Alessandroni, L.; Mustafa, A.M.; Vittori, S.; Caprioli, G. Grapevine leaves (Vitis vinifera): Chemical characterization of bioactive compounds and antioxidant activity during leave development. Food Biosci. 2022, 50, 102120. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, D.; Shukla, R. Bioactive compounds and reported pharmacological activities of Vitis vinifera L.—An overview. World J. Pharm. Res. 2023, 12, 27–38. [Google Scholar]
- Caleja, C.S.G. Incorporação de Ingredientes Naturais em Diferentes Matrizes Alimentares como Potenciadores de Conservação e Promotores de Saúde. Ph.D. Thesis, Porto University, Porto, Portugal, 2018. [Google Scholar]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Lee, B.H. Fundamentos de Biotecnología de los Alimentos; Editorial Acribia: Zaragoza, Spain, 1996; p. 475. ISBN 84-200-0922-9. [Google Scholar]
- Rhodes, A.; Fletcher, D.L. Principles of Industrial Microbiology; Pergamon Press: Oxford, UK, 1975; p. 320. ISBN 0080119050. [Google Scholar]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- Xu, L.; Guo, S.; Zhang, S. Effects of solid-state fermentation with three higher fungi on the total phenol contents and antioxidante properties of diverse cereal grains. FEMS Microbiol. Lett. 2018, 365, fny163. [Google Scholar] [CrossRef] [PubMed]
- García, L.R.P.; Bianchi, V.L.D. Efeito da fermentação fúngica no teor de compostos fenólicos em casca de café robusta. Semin. Ciências Agrárias 2015, 36, 777–786. [Google Scholar] [CrossRef]
- Kosar, M.; Küpeli, E.; Malyer, H.; Uylaser, V.; Türkben, C.; Baser, K.H.C. Effect of brining on biological activity of leaves of Vitis vinífera L. (Cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.M.; Ferreira, A.M.; Benevides, C.M.J.; Melo, D.; Costa, A.S.G.; Faia, A.M.; Oliveira, M.B.P.P. Effect of controlled microbial fermentation on nutritional and functional characteristics of cowpea bean flours. Foods 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Paula, V.M.B. Caraterização Química e Biológica do Própolis da “Serra de Bornes” por TLC. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2012. [Google Scholar]
- Celik, O.F.; Temiz, H. Lactobacilli isolates as potential aroma producer starter cultures: Effects on the chemical, physical, microbial, and sensory properties of yogurt. Food Biosci. 2022, 48, 101802. [Google Scholar] [CrossRef]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-time monitoring of yogurt fermentation process by aquaphotomics near-infrared spectroscopy. Sensors 2020, 21, 177. [Google Scholar] [CrossRef]
- Water, J.V.; Naiyanetr, P. Yoghurt and immunity: The health benefits of fermented milk products that contain lactic acid bacteria. In Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; pp. 113–144. [Google Scholar]
- AOAC. Association of Official Analytical Chemistry: Official Methods of Analysis, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012; pp. 1–3000. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Savi, P.R.S.; Santos, L.; Gonçalves, A.M.; Biesek, S.; Lima, C.P. Análise de flavonoides totais presentes em algumas frutas e hortaliças convencionais e orgânicas mais consumidas na região sul do Brasil. Demetra Aliment. Nutr. Saúde 2017, 12, 275–287. [Google Scholar] [CrossRef]
- Santos, M.S.; Estevinho, L.M.; Carvalho, C.A.L.; Morais, J.S.; Conceição, A.L.S.; Paula, V.B.; Guedes, K.M.; Almeida, R.C.C. Probiotic yogurt with brazilian red propolis: Physicochemical and bioactive properties, stability, and shelf life. J. Food Sci. 2019, 84, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Liofilchem. Rose Bengal CAF Agar. Selective Medium for Detection of Yeasts and Moulds from Food and Environmental Materials. 2015. Available online: http://www.liofilchem.net/login/pd/ifu/10034_IFU.pdf (accessed on 10 October 2023).
- Dai, Z.; Huang, M.; Chen, Y.; Siewers, V.; Nielsen, J. Global rewiring of cellular metabolism renders Saccharomyces cerevisiae Crabtree negative. Nat. Commun. 2018, 9, 3059. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Ferreira, A.; Sampaio-Marques, B.; Barbosa, C.; Rodrigues, F.; Costa, V.; Mendes-Faia, A.; Ludovico, P.; Leao, C. Accumulation of non-superoxide anion reactive oxygen species mediates nitrogen-limited alcoholic fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol 2010, 76, 7918–7924. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.E. Influência da Fonte de Nitrogênio no Perfil Fermentativo, Transcriptômico, e na Produção de Álcoois Superiores em Saccharomyces cerevisiae. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil, 2012. [Google Scholar]
- Pereira, A.F. Suplementação de Nitrogênio Sobre a Fermentação Alcoólica para Produção de Cachaça, Cerveja e Vinho. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil, 2007. [Google Scholar]
- Ruiz, J.; Celis, M.; Toro, M.; Mendes-Ferreira, A.; Rauhut, D.; Santos, A.; Belda, I. Phenotypic and transcriptional analysis of Saccharomyces cerevisiae during wine fermentation in response to nitrogen nutrition and co-inoculation with Torulaspora delbrueckii. Int. Food Res. 2020, 137, 109663. [Google Scholar] [CrossRef]
- Câmara, G.B.; Oliveira, T.K.B.; Macedo, C.D.S.; Leite, D.D.D.F.; Soares, T.D.C.; Lima, A.R.N.; Vasconcelos, S.H.; Soares, T.C.; Barbosa, M.L.; Trigueiro, L.S.D.L. Physico-chemical, toxicological and nutritional characterization of dry and in natura Moringa oleifera Lam leaves. Res. Soc. Dev. 2019, 8, e178111450. [Google Scholar] [CrossRef]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinífera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative analysis of chemical composition, antioxidant and antiproliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinífera L. varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Afiyah, D.N.; Sarbini, R.N.; Huda, M.S. Analysis of the yogurt nutrient content and antioxidant activity by adding Podang Urang Mango juice (Mangifera indica L.). J. Ternak 2022, 13, 47–52. [Google Scholar] [CrossRef]
- Haq, F.U.; Sameen, A.; Zaman, Q.U.; Mushtaq, B.S.; Hussain, M.B.; Javed, A.; Plygun, S.; Korneeva, O.; Shariati, M.A. Development and evalutation of yogurt supplemented with lentil flour. JMBFS. 2019, 8, 1005–1009. [Google Scholar] [CrossRef]
- Fagnani, R.; Boniatti, P.M.S. Formulação de iogurte concentrado enriquecido com farinha de semente de uva: Atividade antioxidante e cinética de fermentação. Ensaios 2020, 24, 189–193. [Google Scholar] [CrossRef]
- Lopes, R.P. Effects of high hydrostatic pressure on yogurt production. Master’s Thesis, Departamento de Química, Universidade de Aveiro, Aveiro, Portugal, 2013. [Google Scholar]
- Duarte, M.C.K.H.; Cortez, N.M.S.; Cortez, M.A.S.; Franco, R.M.; Macedo, N. Ação antagonista de Lactobacillus acidophilus frente a estirpes patogênicas inoculadas em leite fermentado. J. Bioenergy Food Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Anari, H.N.B.; Majdinasab, M.; Shaghaghian, S.; Khalesi, M. Development of a natamycin-based non-migratory antimicrobial active packaging for extending shelf-life of yogurt drink (Doogh). Food Chem. 2022, 366, 130606. [Google Scholar] [CrossRef]
- De Andrade, R.B.; Machado, B.A.S.; Barreto, G.d.A.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 50, 1–26. [Google Scholar]

| Liquid Medium Fermentation | ||||
|---|---|---|---|---|
| Sample | Lag Phase (h) | µc (h−1) | DT (h) | FB (CFU/mL) |
| LSF15% | 24 | 0.095 | 7.27 | 4.80 × 107 ± 0.00 |
| LYF10% | 0 | 0.097 | 7.18 | 3.40 × 107 ± 0.04 |
| LYF15% | 0 | 0.130 | 5.32 | 3.80 × 108 ± 0.04 |
| LYF20% | 0 | 0.133 | 5.21 | 3.40 × 107 ± 0.05 |
| LYF30% | 0 | 0.096 | 7.23 | 2.70 × 107 ± 0.32 |
| Assay | Ash (%) | Fat (%) |
|---|---|---|
| Leaf | 5.86 ± 0.69 a | 3.30 ± 0.03 a |
| SSF | 1.21 ± 0.83 b | 0.85 ± 0.15 b |
| SYF | 2.22 ± 0.33 b | 0.80 ± 0.26 b |
| LYF | 37.19 ±1.01 c | 0.48 ± 0.28 b |
| Total Phenols mg GAE/g | Total Flavonoids mg QE/g | DPPH mg TroloxE/g | FRAP mg Fe(II)E/g | |
|---|---|---|---|---|
| Leaves of V. vinifera | 32.32 ± 3.64 | 17.41 ± 2.68 | 37.65 ± 2.18 | 79.34 ± 6.74 |
| Assay | Total Phenol Content mg GAE/g |
|---|---|
| Leaf | 32.32 ± 3.64 a |
| SSF | 41.62 ± 0.57 b |
| SYF | 52.58 ± 0.35 c |
| LYF | 39.23 ± 1.85 b |
| Days | Yogurt | Mold and Yeast (log CFU/mL) |
|---|---|---|
| 1 | Leaf | 1.00 ± 0.07 a |
| SSF | 1.00 ± 0.01 a | |
| SYF | 2.16 ± 0.08 b | |
| LYF | 3.85 ± 0.17 c | |
| 3 | SSF | 2.26 ± 0.07 b |
| SYF | 4.11 ± 0.21 c | |
| LYF | 4.87 ± 0.55 c | |
| 9 | SSF | 1.18 ± 0.20 a |
| SYF | 6.36 ± 0.38 d | |
| LYF | 7.36 ± 0.64 d |
| Day | Yogurt | Ash (%) | Fat (%) | Protein (%) | pH | TA g of LA/100 g |
|---|---|---|---|---|---|---|
| 1 | Cy | 0.75 ± 0.04 | 2.14 ± 0.04 | 2.63 ± 0.05 | 4.34 ± 0.04 | 1.08 ± 0.02 |
| SSFy | 0.69 ± 0.15 | 2.07 ± 0.17 | 2.51 ± 0.08 | 4.18 ± 0.10 | 0.98 ± 0.05 | |
| SYFy | 0.94 ± 0.07 | 2.00 ± 0.12 | 2.15 ± 0.04 | 4.24 ± 0.02 | 0.93 ± 0.08 | |
| LYFy | 0.69 ± 0.01 | 2.12± 0.07 | 2.48 ± 0.04 | 4.26 ± 0.01 | 1.05 ± 0.07 | |
| 7 | Cy | 0.77 ± 0.02 | 2.09 ± 0.05 | 2.48 ± 0.11 | 4.34 ± 0.05 | 0.95 ± 0.07 |
| SSFy | 0.71 ± 0.05 | 2.12 ± 0.13 | 2.48 ± 0.13 | 4.20 ± 0.03 | 0.95 ± 0.07 | |
| SYFy | 0.58 ± 0.11 | 2.20 ± 0.06 | 2.51± 0.07 | 4.25 ± 0.03 | 0.98 ± 0.05 | |
| LYFy | 0.71 ± 0.02 | 2.24 ± 0.01 | 2.43 ± 0.10 | 4.19 ± 0.07 | 0.93 ± 0.06 | |
| 12 | Cy | 0.69 ± 0.10 | 2.15 ± 0.03 | 2.53 ± 0.02 | 4.28 ± 0.07 | 0.94 ± 0.00 |
| SSFy | 0.72 ± 0.01 | 2.27 ± 0.02 | 2.84 ± 0.06 | 4.16 ± 0.05 | 1.00 ± 0.04 | |
| SYFy | 0.72 ± 0.12 | 2.16 ± 0.10 | 2.93 ± 0.10 | 4.20 ± 0.00 | 0.90 ± 0.04 | |
| LYFy | 0.75 ± 0.03 | 2.25 ± 0.14 | 2.80 ± 0.07 | 4.19 ± 0.00 | 0.97 ± 0.04 |
| Days | Yogurt | Total Phenols mg GAE/g | DPPH mg TroloxE/g | FRAP mg Fe(II)E/g |
|---|---|---|---|---|
| 1 | Cy | 1.23 ± 0.05 a | 1.19 ± 0.34 a | 4.22 ± 0.16 a |
| SSFy | 1.60 ± 0.13 b | 1.23 ± 0.06 a | 5.06 ± 0.19 b | |
| SYFy | 1.87 ± 0.01 b | 1.51 ± 0.07 a | 5.28 ± 0.25 b | |
| LYFy | 1.34 ± 0.13 ab | 1.19 ± 0.13 a | 3.80 ± 0.27 a | |
| 12 | Cy | 1.44 ± 0.07 ab | 1.01 ± 0.06 a | 4.00 ± 0.36 a |
| SSFy | 1.94 ± 0.14 b | 1.33 ± 0.06 a | 5.30 ± 0.12 b | |
| SYFy | 2.16 ± 0.28 c | 1.34 ± 0.08 a | 5.77 ± 0.21 c | |
| LYFy | 1.43 ± 0.11 ab | 1.38 ± 0.06 a | 4.32 ± 0.33 a |
| Days | Yogurt | Lactic Bacteria log CFU/mL |
|---|---|---|
| 1 | Cy | 7.97 ± 0.01 b |
| SSFy | 7.25 ± 0.01 c | |
| SYFy | 7.44 ± 0.02 c | |
| LYFy | 7.28 ± 0.01 c | |
| 7 | Cy | 8.04 ± 0.07 b |
| SSFy | 8.43 ± 0.19 a | |
| SYFy | 8.44 ± 0.15 a | |
| LYFy | 8.44 ± 0.18 a | |
| 12 | Cy | 8.08 ± 0.05 b |
| SSFy | 8.21 ± 0.13 ab | |
| SYFy | 8.44 ± 0.05 a | |
| LYFy | 7.56 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, L.; Sousa-Dias, M.; Paula, V.B.; Dias, L.G.; Estevinho, L.M. Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt. Foods 2024, 13, 2053. https://doi.org/10.3390/foods13132053
Freitas L, Sousa-Dias M, Paula VB, Dias LG, Estevinho LM. Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt. Foods. 2024; 13(13):2053. https://doi.org/10.3390/foods13132053
Chicago/Turabian StyleFreitas, Lais, Miguel Sousa-Dias, Vanessa B. Paula, Luís G. Dias, and Leticia M. Estevinho. 2024. "Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt" Foods 13, no. 13: 2053. https://doi.org/10.3390/foods13132053
APA StyleFreitas, L., Sousa-Dias, M., Paula, V. B., Dias, L. G., & Estevinho, L. M. (2024). Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt. Foods, 13(13), 2053. https://doi.org/10.3390/foods13132053










