Physicochemical Properties, Stability, and Functionality of Non-Covalent Ternary Complexes Fabricated with Pea Protein, Hyaluronic Acid and Chlorogenic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soluble Protein
2.3. Preparation of PP-HA Complexes
2.4. Turbidity and Zeta-Potential
2.5. Preparation of Ternary Complexes
2.6. Fluorescence Spectroscopy
2.7. Molecular Docking
2.8. Particle Size and Zeta-Potential
2.9. Stability Measurement
2.10. CA Retention Measurement
2.11. Emulsifying Properties
2.12. Antioxidant Activity
2.12.1. DPPH/ABTS Scavenging Capacity
2.12.2. Ferric Reducing Ability Power (FRAP)
2.13. Antimicrobial Activity
2.14. Statistical Analysis
3. Results and Discussion
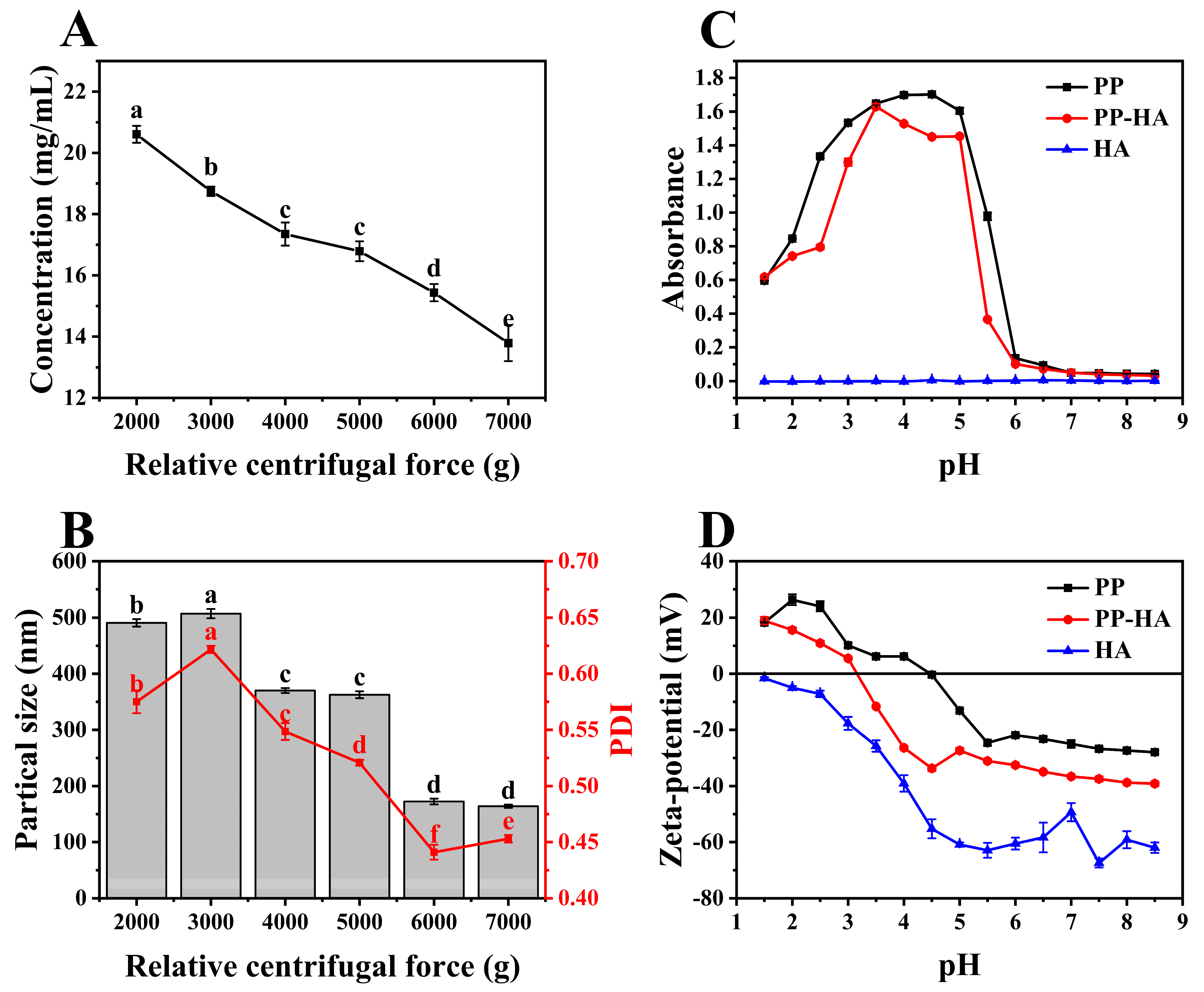
3.1. Selection of the Preparation Conditions for Soluable Pea Protein Solution
3.2. Preparation of PP-HA Mixture
3.3. Binding Ability of PP-HA to CA
3.4. Molecular Docking
3.5. Particle Size and Zeta-Potential of Ternary Complexes
3.6. Stability Analysis
3.6.1. Thermal Stability
3.6.2. UV Stability
3.7. Emulsifying Properties
3.8. Antioxidative Properties
3.9. Antimicrobial Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Z.H.; Huang, Q.R. Assembly of Protein−Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food. Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, X.H.; Xu, L.S.; Deng, C.J.; Jin, W.P.; Wang, X.H.; Kong, Y.R.; Duan, M.G.; Nei, Y.Y.; Zeng, J.; et al. Structures, fabrication mechanisms, and emulsifying properties of self-assembled and spray-dried ternary complexes based on lactoferrin, oat β-glucan and curcumin: A comparison study. Food Res. Int. 2020, 131, 109048. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y.P. The health benefits, functional properties, modifications, and applications of pea (L.) protein: Current status, challenges, and perspectives. Compr. Rev. 2020, 19, 1835–1876. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Huang, H.L. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Li, X.Q.; McClements, D.J.; Shi, M.R.; Shang, Q.; Liu, X.B.; Liu, F.G. Physicochemical and functional properties of lactoferrin−hyaluronic acid complexes: Effect of non−covalent and covalent interactions. LWT Food Sci. Technol. 2021, 151, 112121. [Google Scholar] [CrossRef]
- Zhong, W.G.; Li, J.T.; Wang, C.N.; Zhang, T.H. Formation, stability and digestion of curcumin loaded whey protein/hyaluronic acid nanoparticles: Ethanol desolvation vs. pH-shifting method. Food Chem. 2023, 414, 135684. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.N.; Varga, N.; Juhász, A.; Csapó, E. Serum protein−hyaluronic acid complex nanocarriers: Structural characterisation and encapsulation possibilities. Carbohydr. Polym. 2021, 251, 117047. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.Q.; Jiang, L.S.; Chu, Y.; Gao, S.; Jiang, X.Y.; Zhang, Y.H.; Chen, Y.; Luo, S.J.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, S.Y.; Yang, Y.; Wang, C.N.; Zhang, T.H. Formation and characterization of noncovalent ternary complexes based on whey protein concentrate, high methoxyl pectin, and phenolic acid. J. Dairy Sci. 2022, 105, 2963–2977. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wang, C.; Qi, Z.T.; Zhao, R.; Wang, C.N.; Zhang, T.H. Pea protein based nanocarriers for lipophilic polyphenols: Spectroscopic analysis, characterization, chemical stability, antioxidant and molecular docking. Food Res. Int. 2022, 160, 111713. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lu, Y.C.; Yang, Y.; Li, S.Y.; Wang, C.; Wang, C.N.; Zhang, T.H. Comparison of non-covalent binding interactions between three whey proteins and chlorogenic acid: Spectroscopic analysis and molecular docking. Food Biosci. 2021, 41, 101035. [Google Scholar] [CrossRef]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.E.; Maugard, T. Complex coacervation of pea protein isolate and tragacanth gum: Comparative study with commercial polysaccharides. Innov. Food Sci. Emerg. Technol. 2021, 69, 102641. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Wang, W.N.; Wang, L.Q.; Jiang, L.Z.; Yu, D.Y.; Xie, F.Y. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021, 79, 105758. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, X.F.; Wang, J.Q.; Sun, X.M.; Wang, C.N. Systematical characterization of functional and antioxidative properties of heat-induced polymerized whey proteins. Food Sci. Biotechnol. 2018, 27, 1619–1626. [Google Scholar] [CrossRef]
- Duan, X.; Li, M.; Ma, H.J.; Xu, X.M.; Jin, Z.Y.; Liu, X.B. Physicochemical properties and antioxidant potential of phosvitin−resveratrol complexes in emulsion system. Food Chem. 2016, 206, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Fu, W.F.; Li, D.; Dong, C.; Bao, Z.X.; Wang, C.N. Structure and functionality of whey protein, pea protein, and mixed whey and pea proteins treated by pH shift or high-intensity ultrasound. J. Dairy Sci. 2024, 107, 726–741. [Google Scholar] [CrossRef]
- Zhi, Z.J.; Yan, L.; Li, H.; Dewettinck, K.; Van der Meeren, P.; Liu, R.; Van Bockstaele, F. A combined approach for modifying pea protein isolate to greatly improve its solubility and emulsifying stability. Food Chem. 2022, 380, 131832. [Google Scholar] [CrossRef]
- Djoullah, A.; Djemaoune, Y.; Husson, F.; Saurel, R. Native−state pea albumin and globulin behavior upon transglutaminase treatment. Process Biochem. 2015, 50, 1284–1292. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.T.; Wu, X.L. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β−carotene protection and delivery. Food Hydrocolloids 2021, 113, 106497. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, Q.Y.; Chen, K.W.; Li, D.; Liang, L. Water−soluble myofibrillar protein−pectin complex for enhanced physical stability near the isoelectric point: Fabrication, rheology and thermal property. Int. J. Biol. Macromol. 2020, 142, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shu, X.; Su, J.Q.; Li, Q.K.; Tong, Z.; Yuan, F.; Mao, L.K.; Gao, Y.X. Interfacial properties and antioxidant capacity of pickering emulsions stabilized by high methoxyl pectin-surfactant-pea protein isolate-curcumin complexes: Impact of different types of surfactants. LWT Food Sci. Technol. 2022, 153, 112453. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeganathan, B.; Dong, H.M.; Chen, L.Y.; Vasanthan, T. Effect of sodium chloride on the thermodynamic, rheological, and microstructural properties of field pea protein isolate/chitosan complex coacervates. Food Chem. 2021, 344, 128569. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Doublier, J.L.; Garnier, C.; Renard, D.; Sanchez, C. Protein-polysaccharide interactions. Curr. Opin. Colloid Interface Sci. 2000, 5, 202–214. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Bohidar, H.B. pH and ionic strength induced complex coacervation of Pectin and Gelatin A. Food Hydrocolloids 2018, 74, 132–138. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.D.; Sun, J.; Guo, H.Y.; Ding, Q.B.; Liu, R.H.; Ren, F.Z. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Li, M.; Ma, Y.; Ngadi, M.O. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef]
- Hao, L.L.; Sun, J.W.; Pei, M.Q.; Zhang, G.F.; Li, C.; Li, C.M.; Ma, X.K.; He, S.X.; Liu, L.B. Impact of non-covalent bound polyphenols on conformational, functional properties and in vitro digestibility of pea protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Microb. Drug Resist. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Song, G.S.; Zhou, L.K.; Zhao, L.W.; Wang, D.L.; Yuan, T.L.; Li, L.; Gong, J.Y. Analysis of non-covalent interaction between β−lactoglobulin and hyaluronic acid under ultrasound−assisted treatment: Conformational structures and interfacial properties. Int. J. Biol. Macromol. 2024, 256, 128529. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, N.; Lu, X.L.; Li, W.J.; Wang, R.Y.; Chang, J.B. Comparative study of the binding between chlorogenic acid and four proteins by isothermal titration calorimetry, spectroscopy and docking methods. Pharmacol. Rep. 2022, 74, 523–538. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.X.; Mao, L.K.; McClements, D.J.; Gao, Y.X. Development of protein−polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Wang, W.Y.; Liu, F.G.; Gao, Y.X. Quercetagetin loaded in soy protein isolate-κ-carrageenan complex: Fabrication mechanism and protective effect. Food Res. Int. 2016, 83, 31–40. [Google Scholar] [CrossRef]
- Thongkaew, C.; Gibis, M.; Hinrichs, J.; Weiss, J. Polyphenol interactions with whey protein isolate and whey protein isolate-pectin coacervates. Food Hydrocoll. 2014, 41, 103–112. [Google Scholar] [CrossRef]
- Al−Kassas, R.; Wen, J.Y.; Cheng, A.E.M.; Kim, A.M.J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- von Staszewski, M.; Jara, F.L.; Ruiz, A.L.T.G.; Jagus, R.J.; Carvalho, J.E.; Pilosof, A.M.R. Nanocomplex formation between β−lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Food. 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sheng, Y.N.; Feng, Y.C.; Diao, J.J.; Wang, C.Y.; Zhang, D.J. Changes in structural and functional properties of globulin-polyphenol complexes in mung beans: Exploration under different interaction ratios and heat treatment conditions. Int. J. Food Sci. Technol. 2022, 57, 1920–1935. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae−leaw, T.; Balange, A.K.; Maqsood, S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Ghobadi, M.; Varidi, M.J.; Koocheki, A.; Varidi, M. Effect of heat treatment on the structure and stability of Grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum nanoparticles. Int. J. Biol. Macromol. 2021, 182, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kristo, E.; Hazizaj, A.; Corredig, M. Structural Changes Imposed on Whey Proteins by UV Irradiation in a Continuous UV Light Reactor. J. Agric. Food. Chem. 2012, 60, 6204–6209. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Rivelli, D.P.; Filho, C.A.H.; Almeida, R.L.; Ropke, C.D.; Sawada, T.C.H.; Barros, S.B.M. Chlorogenic Acid UVA-UVB Photostability. Photochem. Photobiol. 2010, 86, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.P.; Zhao, J.; Liu, Y.F. The effect of non−covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Yi, X.Z.; Ding, B.M.; Sun, W.Q.; Yan, F.W.; Wei, S.D.; Li, Z.S. Interactions and emulsifying properties of ovalbumin with tannic acid. LWT Food Sci. Technol. 2018, 95, 282–288. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W. Stability of emulsion containing skipjack roe protein hydrolysate modified by oxidised tannic acid. Food Hydrocoll. 2014, 41, 146–155. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, C.; Liu, H.T.; Liu, Q.; Kong, B.H. Enhanced physical and oxidative stability of porcine plasma protein hydrolysates based oil-in-water emulsions by adding oxidized chlorogenic acid. Colloid Surf. A—Physicochem. Eng. Asp. 2018, 558, 330–337. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin−Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.H.; Wang, Z.Y.; Ren, X. The Effect of Chlorogenic Acid on Bacillus subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef] [PubMed]
- Domith, I.; Duarte-Silva, A.T.; Garcia, C.G.; Calaza, K.D.; Paes-de-Carvalho, R.; Cossenza, M. Chlorogenic acids inhibit glutamate dehydrogenase and decrease intracellular ATP levels in cultures of chick embryo retina cells. Biochem. Pharmacol. 2018, 155, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kadyan, S.; Ukhanov, V.; Cheng, J.J.; Nagpal, R.; Cui, L.Q. Recent advances in the health benefits of pea protein bioactive peptides and the interaction with the gut microbiome. Curr. Opin. Food Sci. 2022, 48, 100944. [Google Scholar] [CrossRef]
- Mohan, N.M.; Zorgani, A.; Earley, L.; Chauhan, S.; Trajkovic, S.; Savage, J.; Adelfio, A.; Khaldi, N.; Martins, M. Preservatives from food−For food: Pea protein hydrolysate as a novel bio-preservative against O157:H7 on a lettuce leaf. Food Sci. Nutr. 2021, 9, 5946–5958. [Google Scholar] [CrossRef]







| Docking Parents | ΔG | Eunbound | EVHD | Eelec | Etotal | Etorsional | Hydrogren Bonds Formed |
|---|---|---|---|---|---|---|---|
| Legumin + HA | −4.85 | −5.04 | −4.35 | −3.78 | −5.04 | 3.28 | Legumin:E:ASN325:HN: HA::UNK0:O |
| Legumin:E:ARG323:HH12: HA::UNK0:O | |||||||
| Legumin:E:LYS321:HZ1: HA::UNK0:O | |||||||
| Legumin:E:ARG323:HH22: HA::UNK0:O | |||||||
| Legumin:E:ARG115:HH11: HA::UNK0:O | |||||||
| Vicilin + HA | −3.56 | −6.19 | −5.26 | −1.57 | −6.19 | 3.28 | Vicilin:C:LYS231:HN: HA::UNK0:O |
| HA::UNK0:H: Vicilin:C:PRO205:O | |||||||
| Vicilin:C:SER203:HN1: HA::UNK0:O | |||||||
| Vicilin:C:GLY204:HN: HA::UNK0:O | |||||||
| Legumin + CA | −5.39 | −4.62 | −7.14 | −1.54 | −4.62 | 3.28 | CA::UNL1:H: Legumin:F:VAL317:O |
| Legumin:D:ASN438:HD21: CA::UNL1:O | |||||||
| CA::UNL1:H: Legumin:F:ASN150:OD1 | |||||||
| Legumin:F:ASN150:HD21: CA::UNL1:O | |||||||
| Vicilin + CA | −5.55 | −4.44 | −7.48 | −1.36 | −4.44 | 3.28 | Vicilin:C:HIS230:HD1: CA::UNL1:O |
| Vicilin:C:LYS231:HN: CA::UNL1:O | |||||||
| CA::UNL1:H: Vicilin:C:HIS230:O | |||||||
| Vicilin:C:SER203:HN2: CA::UNL1:O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Liu, F.; Zhang, R.; Zhao, R.; He, Y.; Wang, C. Physicochemical Properties, Stability, and Functionality of Non-Covalent Ternary Complexes Fabricated with Pea Protein, Hyaluronic Acid and Chlorogenic Acid. Foods 2024, 13, 2054. https://doi.org/10.3390/foods13132054
Fu W, Liu F, Zhang R, Zhao R, He Y, Wang C. Physicochemical Properties, Stability, and Functionality of Non-Covalent Ternary Complexes Fabricated with Pea Protein, Hyaluronic Acid and Chlorogenic Acid. Foods. 2024; 13(13):2054. https://doi.org/10.3390/foods13132054
Chicago/Turabian StyleFu, Wenfei, Fujun Liu, Ronglei Zhang, Ru Zhao, Yuxin He, and Cuina Wang. 2024. "Physicochemical Properties, Stability, and Functionality of Non-Covalent Ternary Complexes Fabricated with Pea Protein, Hyaluronic Acid and Chlorogenic Acid" Foods 13, no. 13: 2054. https://doi.org/10.3390/foods13132054
APA StyleFu, W., Liu, F., Zhang, R., Zhao, R., He, Y., & Wang, C. (2024). Physicochemical Properties, Stability, and Functionality of Non-Covalent Ternary Complexes Fabricated with Pea Protein, Hyaluronic Acid and Chlorogenic Acid. Foods, 13(13), 2054. https://doi.org/10.3390/foods13132054





