Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABAA Receptors in Rodent Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fermentation of Whey Protein Hydrolysate (WPH)
2.3. Assay of Total Sugar
2.4. Analysis of Glu and GABA by High-Performance Liquid Chromatography (HPLC)
2.5. Experimental Animals
2.6. Evaluation of Sleep Latency and Duration in Pentobarbital-Treated Mice
2.7. EEG Analysis in SD Rats
2.8. GABAA Receptor Binding Test with Antagonists
2.9. Analysis of Gene Expression Levels of GABAergic and Serotonergic Receptors Using SYBR™ Green qRT-PCR
2.10. Analysis of Protein Levels of GABAergic and Serotonergic Receptors Using Western Blotting
2.11. Statistical Analysis
3. Results
3.1. GABA Production by Lactobacillus brevis M2
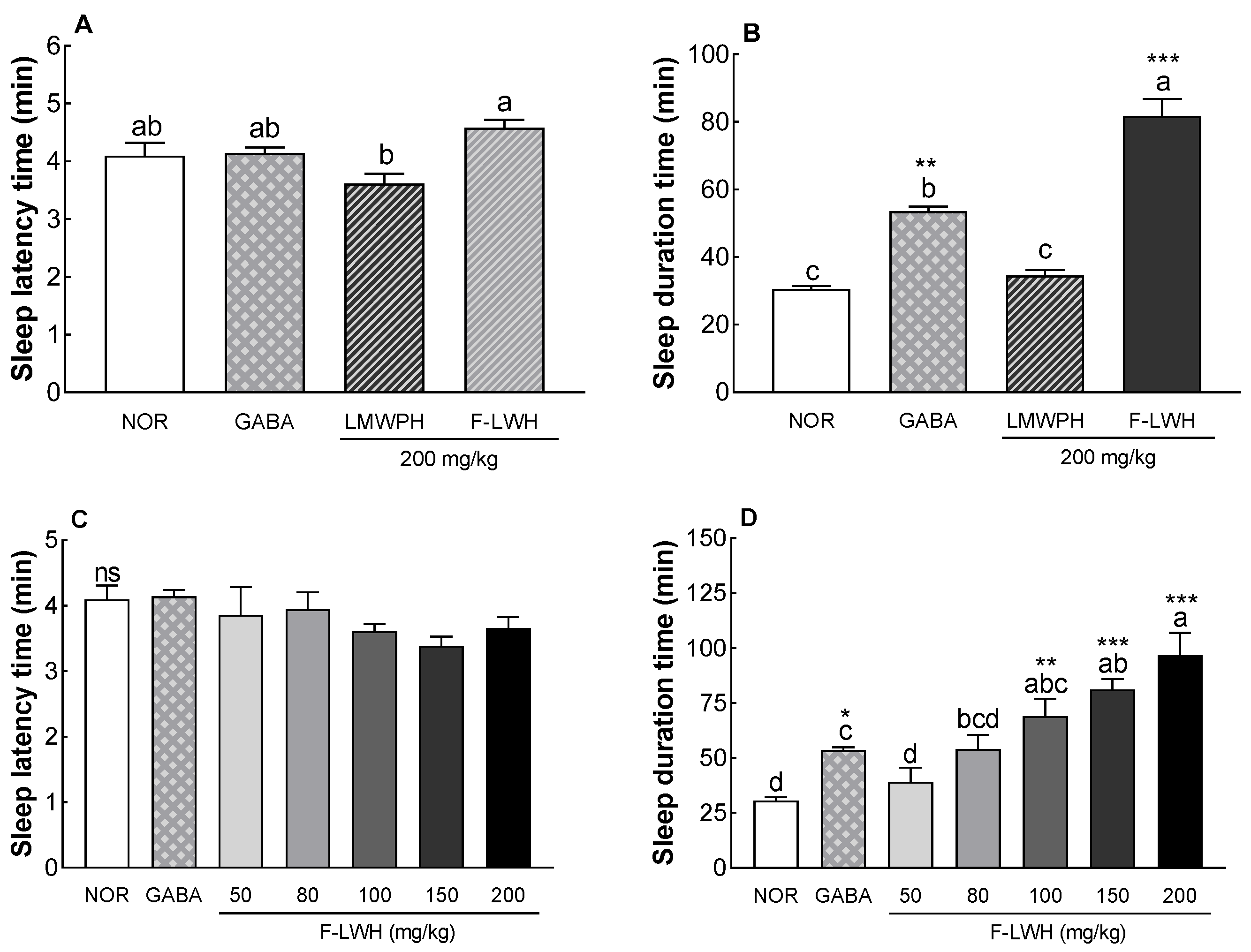
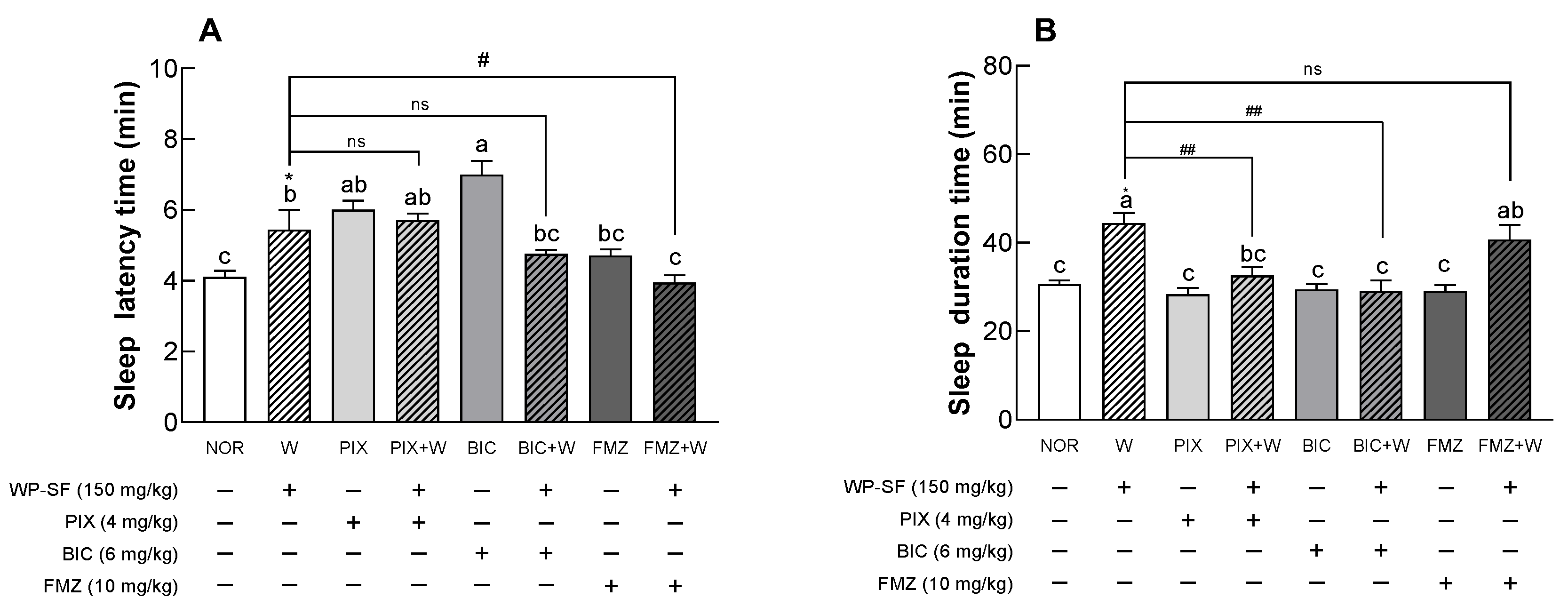
3.2. Sleep Latency and Duration in Mice Treated with Pentobarbital Following Administration of WP-SF
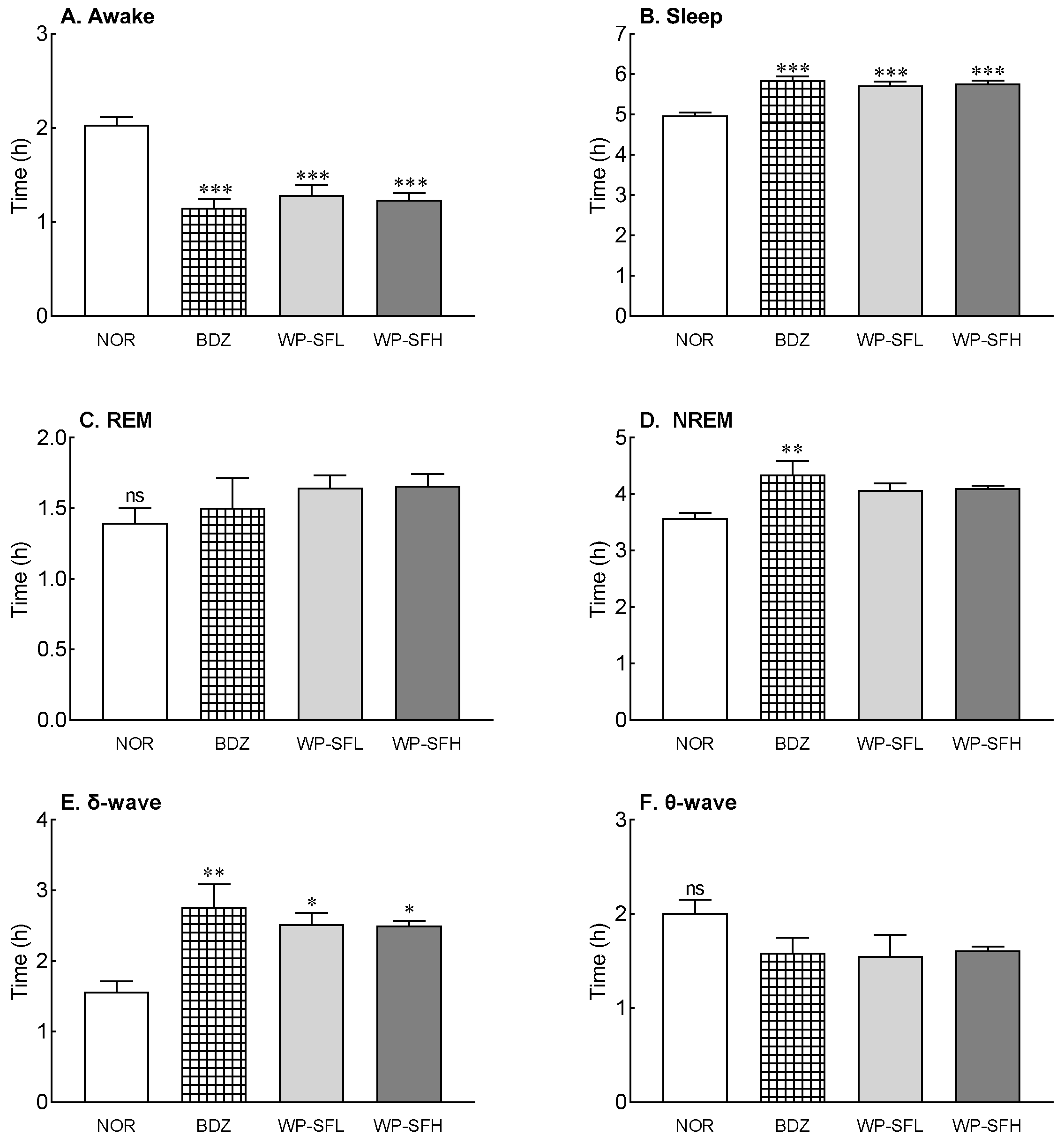
3.3. Changes in EEG during Sleep Owing to WP-SF Administration
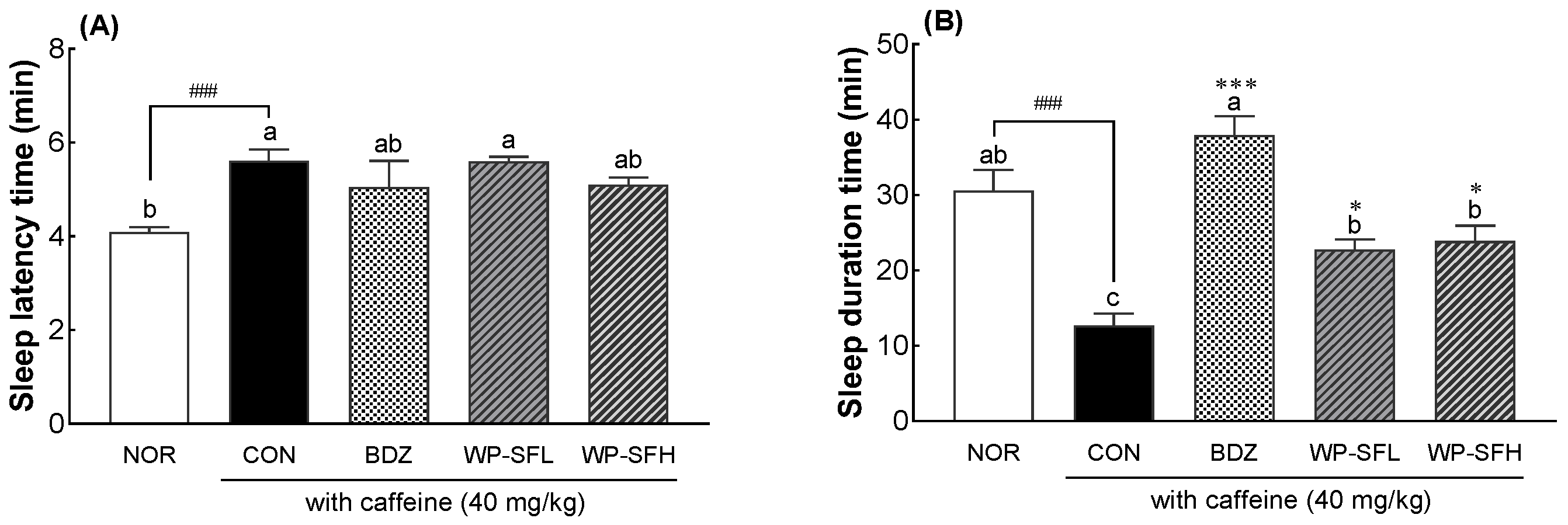
3.4. Insomnia-Alleviating Effects of WP-SF in a Caffeine-Induced Insomnia Mouse Model
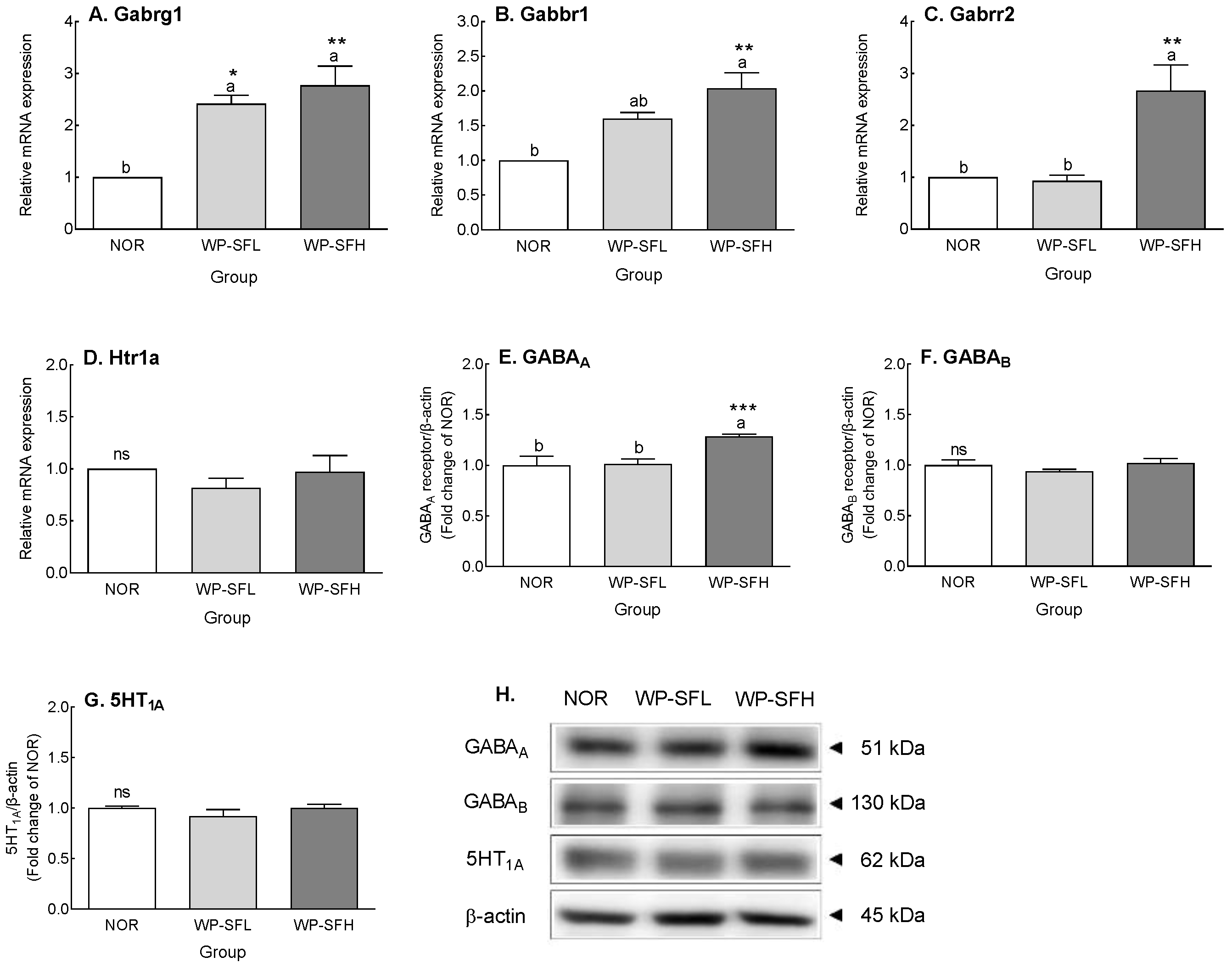
3.5. GABA Content and GABA Receptor Expression Levels in the Brain of Mice after 3 Weeks of Oral Administration of WP-SF
3.6. Binding Site of WP-SF in the GABAA Receptor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The global problem of insufficient sleep and its serious public health implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Sealy, E.A. Sleep, sleep deprivation, sleeplessness and their effect on society. Sch. J. App. Med. Sci. 2022, 10, 1647–1663. [Google Scholar] [CrossRef]
- Naha, S.; Sivaraman, M.; Sahota, P. Insomnia: A current review. Mo. Med. 2024, 121, 44–51. [Google Scholar]
- de Mendonça, F.M.R.; de Mendonça, G.P.R.R.; Souza, L.C.; Galvão, L.P.; Paiva, H.S.; de Azevedo Marques Périco, C.; Torales, J.; Ventriglio, A.; Castaldelli-Maia, J.M.; Sousa Martins Silva, A. Benzodiazepines and sleep architecture: A systematic review. CNS Neurol. Disord. Drug Targets 2023, 22, 172–179. [Google Scholar] [CrossRef]
- Madari, S.; Golebiowski, R.; Mansukhani, M.P.; Kolla, B.P. Pharmacological management of insomnia. Neurotherapeutics 2021, 18, 44–52. [Google Scholar] [CrossRef]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal remedies and their possible effect on the GABAergic system and sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A Review. Front. Nutr. 2022, 20, 815640. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of synthetic peptides in cosmetics for sensitive skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Sinha, R.; Radha, C.; Prakash, J.; Kaul, P. Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007, 101, 1484–1491. [Google Scholar] [CrossRef]
- Li, J.; Zhu, F. Whey protein hydrolysates and infant formulas: Effects on physicochemical and biological properties. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13337. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Jin, C.-M.; Choi, H.-S.; Suh, H.J.; Chang, Y.B. Improvement of sleep duration and quality through GABAA receptor by whey protein hydrolysate containing DIQK as active main compound. J. Dairy Sci. 2024. [Google Scholar] [CrossRef]
- Miclo, L.; Perrin, E.; Driou, A.; Papadopoulos, V.; Boujrad, N.; Vanderesse, R.; Boudier, J.F.; Desor, D.; Londen, G.; Gaillard, J.L. Characterization of alpha-casozepine, a tryptic peptide from bovine alpha(s1)-casein with benzodiazepine-like activity. FASEB J. 2001, 15, 1780–1782. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, L.; Su, G.; Huang, M.; Luo, D.; Zhao, M. Identification and screening of potential bioactive peptides with sleep-enhancing effects in bovine milk casein hydrolysate. J. Agric. Food Chem. 2021, 69, 11246–11258. [Google Scholar] [CrossRef]
- Guimarães, A.P.; Seidel, H.; Pires, L.V.d.M.; Trindade, C.O.; Baleeiro, R.D.S.; Souza, P.M.d.; Silva, F.G.D.E.; Coelho, D.B.; Becker, L.K.; Oliveira, E.C.d. GABA supplementation, increased heart-rate variability, emotional response, sleep efficiency and reduced depression in sedentary overweight women undergoing physical exercise: Placebo-controlled, randomized clinical trial. J. Diet. Suppl. 2024, 21, 1–15. [Google Scholar] [CrossRef]
- Hosseini, A.; Mobasheri, L.; Rakhshandeh, H.; Rahimi, V.B.; Najafi, Z.; Askari, V.R. Edible herbal medicines as an alternative to common medication for sleep disorders: A review article. Curr. Neuropharmacol. 2024, 22, 1205–1232. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Kim, S.B.; Lim, J.W. Calcium-binding peptides derived from tryptic hydrolysates of cheese whey protein. Asian-Australas. J. Anim. Sci. 2004, 17, 1459–1464. [Google Scholar] [CrossRef]
- Morato, P.N.; Lollo, P.C.; Moura, C.S.; Batista, T.M.; Carneiro, E.M.; Amaya-Farfan, J. A dipeptide and an amino acid present in whey protein hydrolysate increase translocation of GLUT-4 to the plasma membrane in Wistar rats. Food Chem. 2013, 139, 853–859. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for γ-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Yogeswara, I.; Kittibunchakul, S.; Rahayu, E.; Domig, K.; Haltrich, D.; Nguyen, T. Microbial production and enzymatic biosynthesis of γ-aminobutyric acid (GABA) using Lactobacillus plantarum FNCC 260 isolated from Indonesian fermented foods. Processes 2020, 9, 22. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Chang, Y.B.; Kim, H.; Lee, S.K.; Kim, H.-J.; Jeong, A.-H.; Suh, H.J.; Ahn, Y. Characteristics and absorption rate of whey protein hydrolysates prepared using Flavourzyme after treatment with Alcalase and Protamex. Molecules 2023, 28, 7969. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Brewer, C.F.; Miller, G.L. Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydr. Res. 1994, 254, 157–167. [Google Scholar] [CrossRef]
- Min, B.; Ahn, Y.; Cho, H.J.; Kwak, W.K.; Jo, K.; Suh, H.J. Chemical compositions and sleep-promoting activities of hop (Humulus lupulus L.) varieties. J. Food Sci. 2023, 88, 2217–2228. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, H.H.; Kim, B.-H.; Park, S.J.; Kim, Y.S.; Suh, H.J.; Jo, K. Heukharang lettuce (Lactuca sativa L.) leaf extract displays sleep-promoting effects through GABAA receptor. J. Ethnopharmacol. 2023, 314, 116602. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of gamma-aminobutyric acid in foods: From conventional methods to innovative technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shah, N.P. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H. Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biologica activities of Maillard reaction products (MRPs) in sugar-amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villegas, J.M.; Savoy de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef]
- Le, P.H.; Parmentier, N.; Le, T.T.; Raes, K. Evaluation of using a combination of enzymatic hydrolysis and lactic acid fermentation for γ-aminobutyric acid production from soymilk. LWT Food Sci. Technol. 2021, 142, 111044. [Google Scholar] [CrossRef]
- Zareian, M.; Ebrahimpour, A.; Sabo Mohamed, A.K.; Saari, N. Modeling of glutamic acid production by Lactobacillus plantarum MNZ. Electron. J. Biotechnol. 2013, 16, 12. [Google Scholar] [CrossRef]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, H.J.; Choung, S.Y. Treatment of GABA from fermented rice germ ameliorates caffeine-induced sleep disturbance in mice. Biomol. Ther. 2015, 23, 268–274. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef]
- Hong, K.; Park, Y.; Suh, H.J. Sleep-promoting effects of the GABA/5-HTP mixture in vertebrate models. Behav. Brain Res. 2016, 310, 36–41. [Google Scholar] [CrossRef]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Townshend, A.; Halson, S.L. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 69, 101764. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Deboer, T.; Landolt, H.P. Adenosine, caffeine, and sleep–wake regulation: State of the science and perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Oh, K.-W.; Cho, H.-K.; Eun, J.-S. Effects of the combined-preparation of germinated brown rice, cultured mountain ginseng and longanae arillus on pentobarbital-induced sleeping time. J Physiol. Pathol. Korean Med. 2010, 24, 598–601. [Google Scholar]
- Rial, R.V.; Akaârir, M.; Canellas, F.; Barceló, P.; Rubiño, J.A.; Martín-Reina, A.; Gamundí, A.; Nicolau, M.C. Mammalian NREM and REM sleep: Why, when and how. Neurosci. Biobehav. Rev. 2023, 146, 105041. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.-B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef]
- Bateson, A.N. Further potential of the GABA receptor in the treatment of insomnia. Sleep Med. 2006, 7, S3–S9. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Farrant, M.; Kaila, K. The cellular, molecular and ionic basis of GABAA receptor signaling. Prog. Brain Res. 2007, 160, 59–87. [Google Scholar] [CrossRef]
- Shin, D.J.; Germann, A.L.; Covey, D.F.; Steinbach, J.H.; Akk, G. Analysis of GABAA receptor activation by combinations of agonists acting at the same or distinct binding sites. Mol. Pharmacol. 2019, 95, 70–81. [Google Scholar] [CrossRef]
- Sieghart, W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv. Pharmacol. 2015, 72, 53–96. [Google Scholar] [CrossRef]
- Gielen, M.; Thomas, P.; Smart, T.G. The desensitization gate of inhibitory Cys-loop receptors. Nat. Commun. 2015, 6, 6829. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.R. Advantages of an antagonist: Bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013, 169, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hulse, G.; Kelty, E.; Hood, S.; Norman, A.; Basso, M.R.; Reece, A.S.A. Novel indications for benzodiazepine antagonist flumazenil in GABA mediated pathological conditions of the central nervous system. Curr. Pharm. Des. 2015, 21, 3325–3342. [Google Scholar] [CrossRef]

| Time (h) | pH | Absorbance (600 nm) | Total Sugar (mg/mL) | Glu (mg/mL) | GABA (mg/mL) |
|---|---|---|---|---|---|
| 0 | 6.79 | 1.051 0.037 | 17.45 0.23 a | 19.33 0.30 a | 0.14 0.05 d |
| 12 | 6.46 | 1.060 0.002 | 16.71 0.03 b,*** | - | - |
| 24 | 6.09 | 1.093 0.005 | 15.21 c,*** | - | - |
| 48 | 4.87 | 1.245 0.020 | 6.76 0.14 d,*** | 16.26 0.19 b,*** | 1.68 0.09 c,*** |
| 72 | 4.64 | 1.261 0.006 | 4.85 0.08 e,*** | 8.55 0.09 c,*** | 2.18 0.003 b,*** |
| 96 | 4.63 | 1.281 0.013 | 4.29 0.04 f,*** | 14.87 0.07 b,*** | 3.15 0.21 a,*** |
| Sample | GABA (μg/mg of Sample) |
|---|---|
| NOR | 17.50 ± 0.26 b |
| WP-SFL | 16.22 ± 0.81 b |
| WP-SFH | 21.16 ± 0.48 a* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, H.; Chang, Y.B.; Han, K.; Choi, H.-S.; Han, S.H.; Suh, H.J. Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABAA Receptors in Rodent Models. Foods 2024, 13, 2049. https://doi.org/10.3390/foods13132049
Lee H, Kim H, Chang YB, Han K, Choi H-S, Han SH, Suh HJ. Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABAA Receptors in Rodent Models. Foods. 2024; 13(13):2049. https://doi.org/10.3390/foods13132049
Chicago/Turabian StyleLee, Hyowon, Hyeongyeong Kim, Yeok Boo Chang, Kisoo Han, Hyeon-Son Choi, Sung Hee Han, and Hyung Joo Suh. 2024. "Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABAA Receptors in Rodent Models" Foods 13, no. 13: 2049. https://doi.org/10.3390/foods13132049
APA StyleLee, H., Kim, H., Chang, Y. B., Han, K., Choi, H.-S., Han, S. H., & Suh, H. J. (2024). Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABAA Receptors in Rodent Models. Foods, 13(13), 2049. https://doi.org/10.3390/foods13132049







