Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Free and Bound Polyphenolic Fractions from RRTP
2.3. Purification of RRTP-FP and P-RRTP-BP by Macroporous Resins
2.3.1. Screening of Macroporous Resins
2.3.2. The Adsorption Kinetics of AB-8 Resin
2.3.3. Static Adsorption and Desorption Assay
2.3.4. Dynamic Adsorption and Resolution Assay
2.4. Determination of Total Phenolic Contents
2.5. Quantification of the Contents of Individual Phenolic Compounds
2.6. In Vitro Antioxidant Assay
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Measurement of Nitric Oxide (NO) Production
2.10. Measurement of Intracellular ROS
2.11. Determination of the Mitochondrial Membrane Potential
2.12. Quantitative Real-Time PCR (qRT-PCR)
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results and Discussion
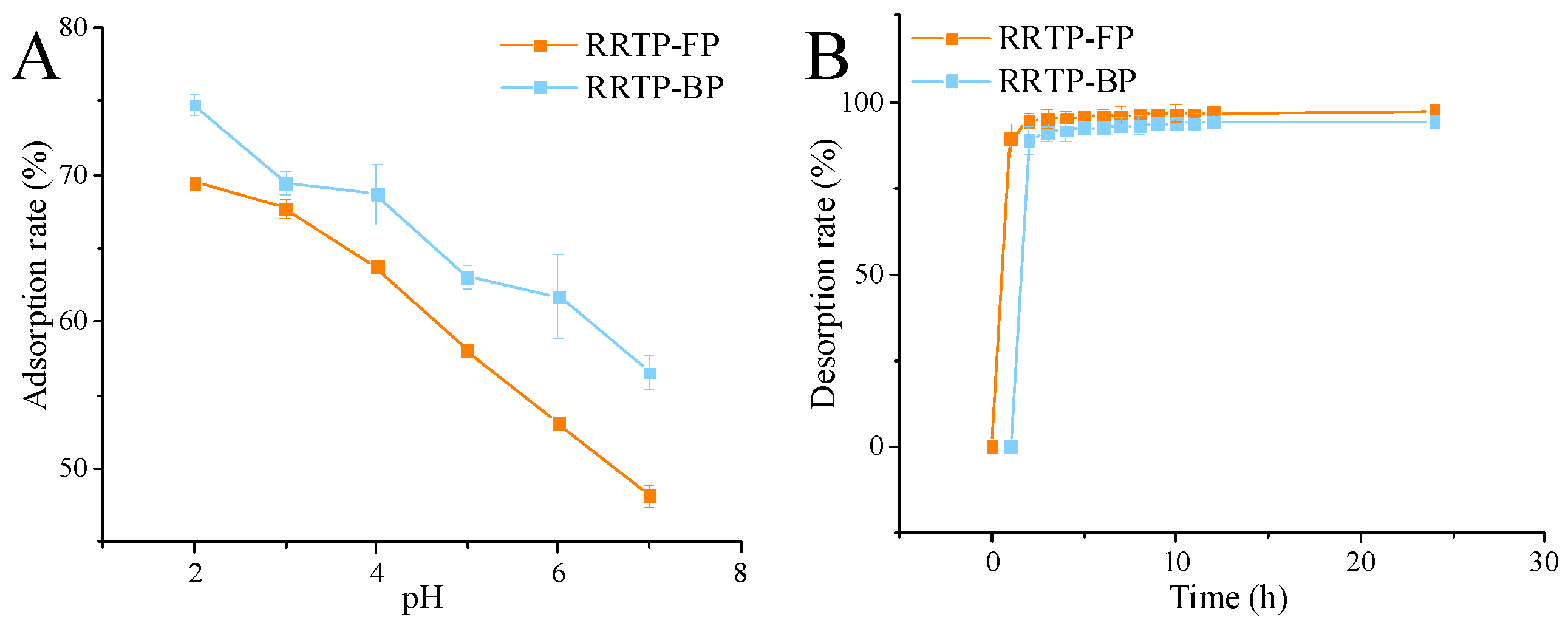
3.1. Static Adsorption and Desorption
3.1.1. Screening of Macroporous Resins
3.1.2. Adsorption Kinetics of RRTP-FP and RRTP-BP on AB-8 Resin
3.1.3. Static Adsorption and Desorption Assay
3.2. Dynamic Adsorption and Desorption of AB−8 Resin
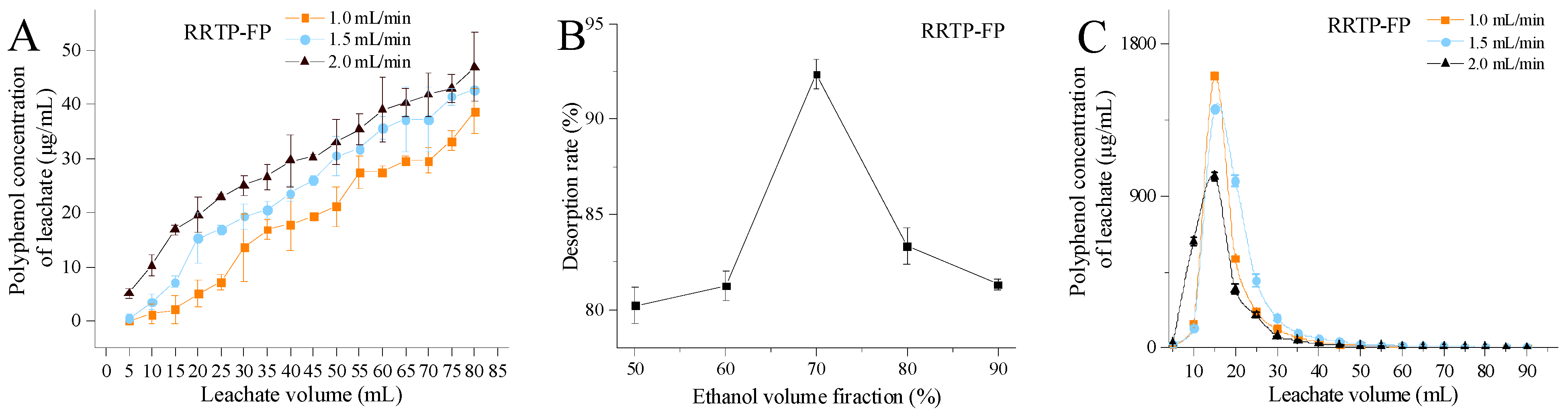
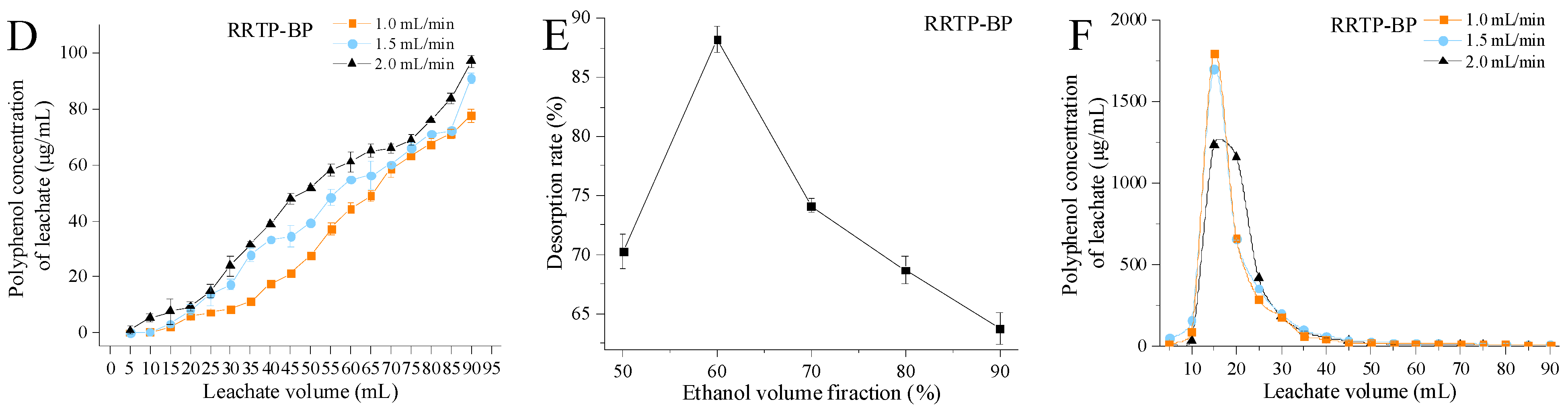
3.2.1. Effects of Loading Flow Rate and Loading Volume
3.2.2. Effect of Eluent Volume Fraction
3.2.3. Effects of Elution Flow Rate and Elution Volume
3.3. Total Phenolic Contents of RRTP−FP, P−RRTP−FP, RRTP−BP, and P−RRTP−BP
3.4. Quantitative Analysis of Free and Bound Phenols by HPLC
3.4.1. HPLC Analysis of RRTP−FP and P−RRTP−FP
3.4.2. HPLC Analysis of RRTP−BP and P−RRTP−BP
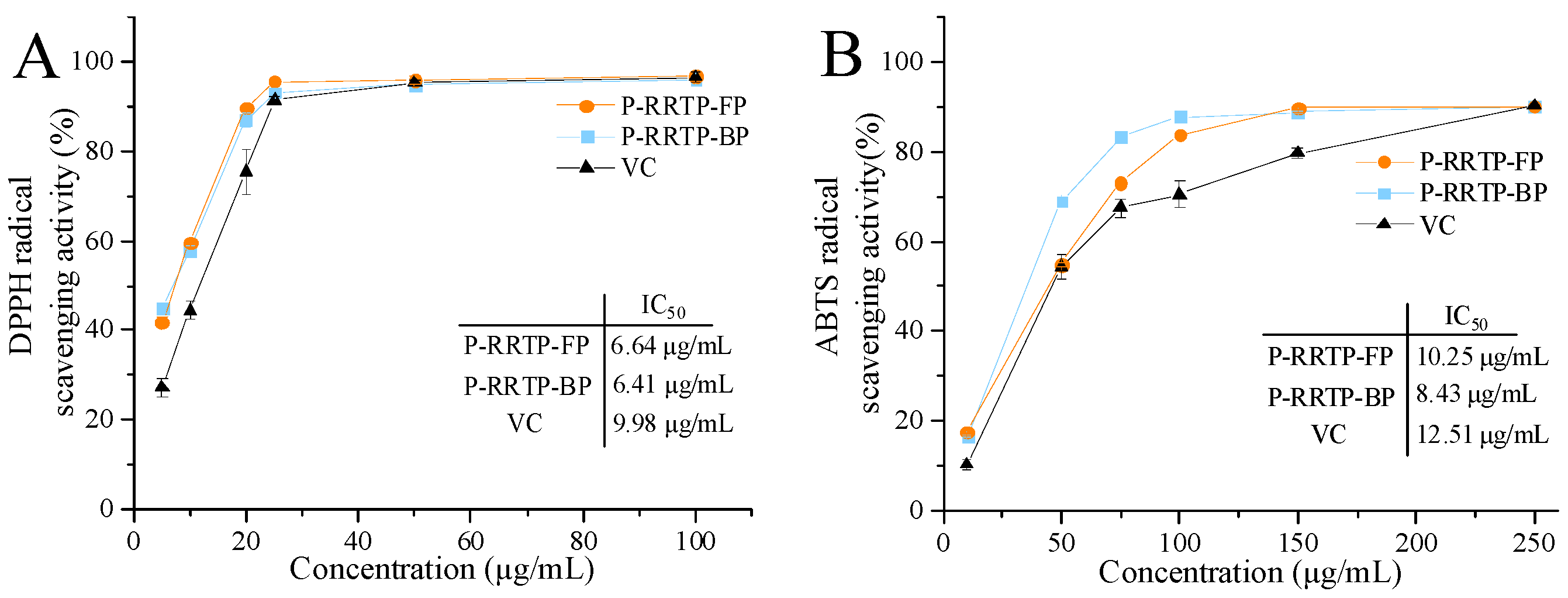
3.5. Antioxidant Activity of P−RRTP−FP and P−RRTP−BP
3.6. Anti−Inflammatory Activities of P−RRTP−FP and P−RRTP−BP
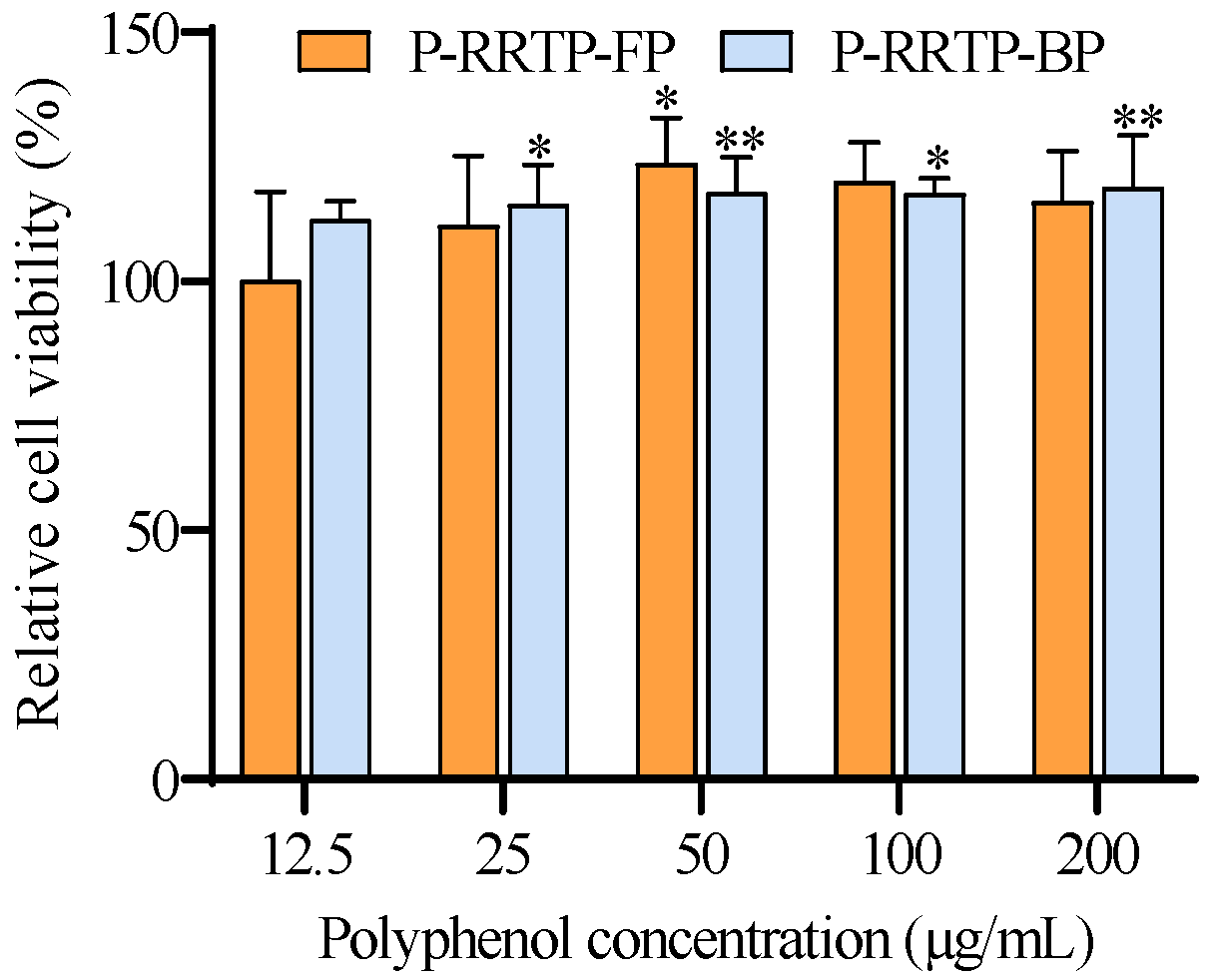
3.6.1. Effects of P−RRTP−FP and P−RRTP−BP on the Viability of RAW264.7 Cells
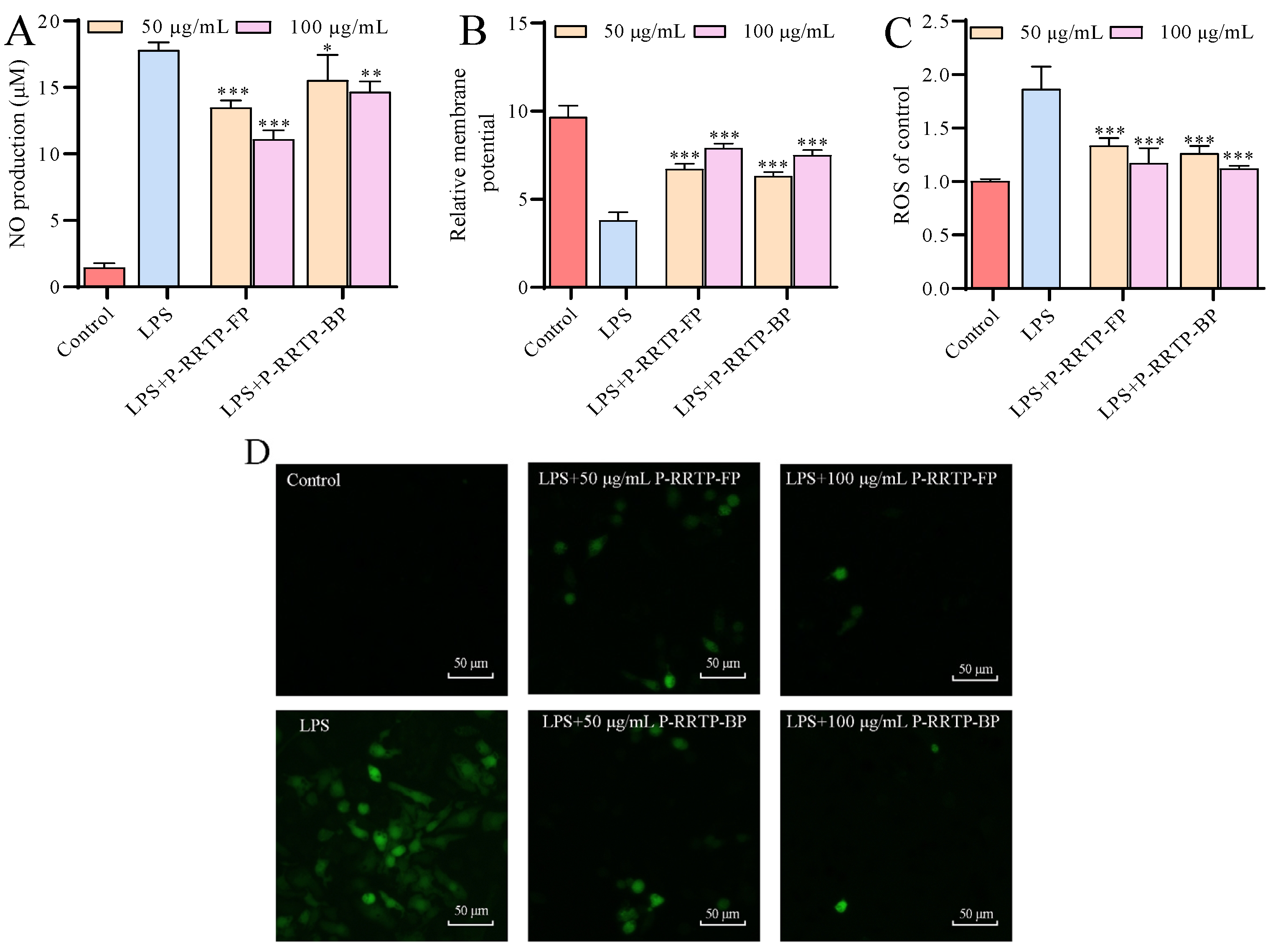
3.6.2. Effects of P−RRTP−FP and P−RRTP−BP on NO Production of LPS−Induced RAW264.7 Cells
3.6.3. Effects of P−RRTP−FP and P−RRTP−BP on Mitochondrial Dysfunction in LPS−Induced RAW264.7 Cells
3.6.4. Effects of P−RRTP−FP and P−RRTP−BP on Intracellular ROS Production of LPS−Induced RAW264.7 Cells
3.6.5. Effects of P−RRTP−FP and P−RRTP−BP on LPS−Induced mRNA Expression of Inflammatory Mediators
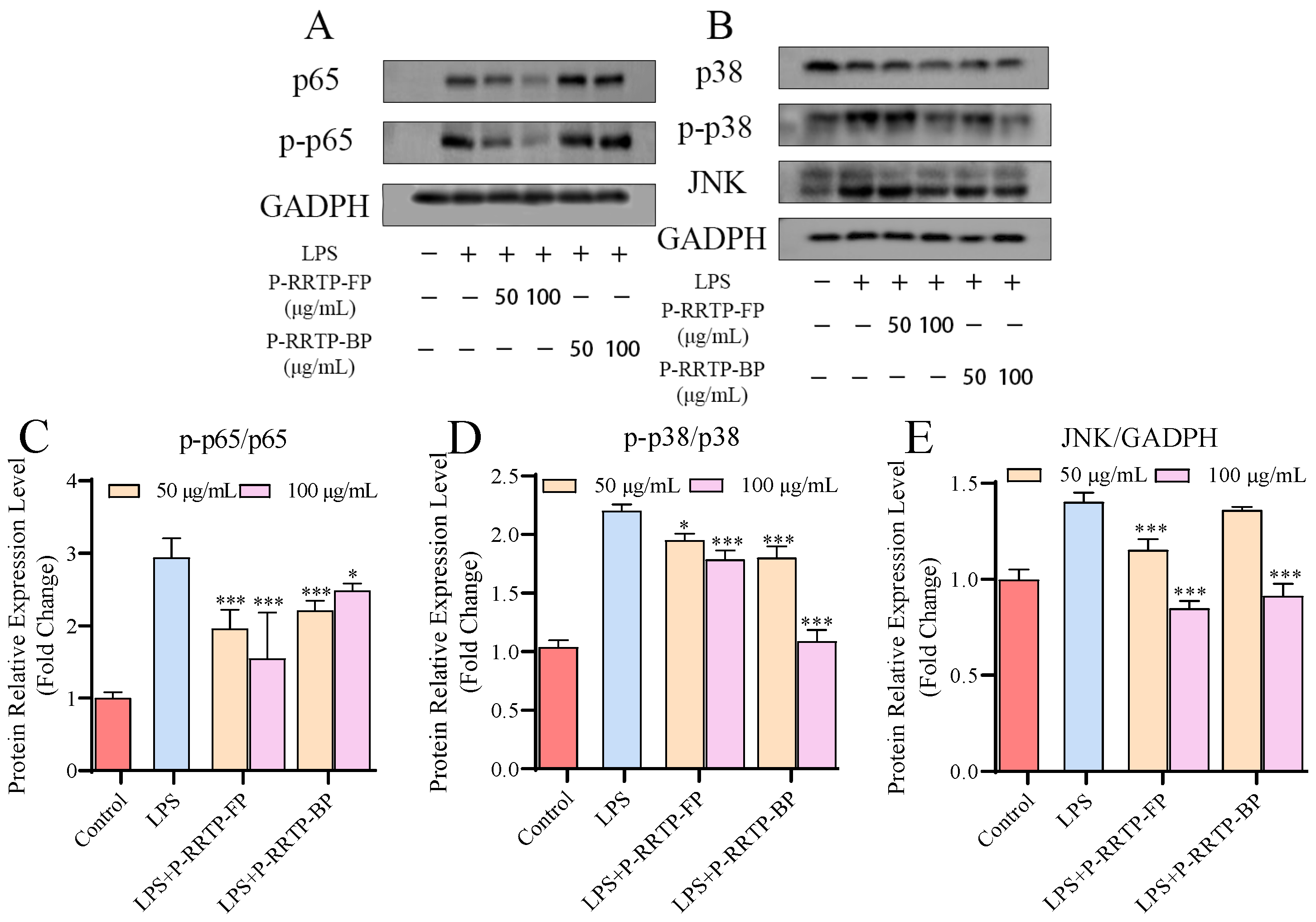
3.6.6. Effects of P−RRTP−FP and P−RRTP−BP on LPS−Induced NF−κB Activation in RAW264.7 Cells
3.7. Effects of P−RRTP−FP and P−RRTP−BP on LPS−Induced MAPK Activation in RAW264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, P.B.; Valentao, P. Insights into natural products in inflammation. Int. J. Mol. Sci. 2018, 19, 644. [Google Scholar] [CrossRef]
- Pello Lazaro, A.M.; Blanco-Colio, L.M.; Franco Pelaez, J.A.; Tunon, J. Anti-inflammatory drugs in patients with ischemic heart disease. J. Clin. Med. 2021, 10, 2835. [Google Scholar] [CrossRef]
- Li, Y.; Deng, W.; Wu, L.; Chen, S.; Zheng, Z.; Song, H. Anti-Inflammatory effects of polyphenols from plum (Prunus Salicina Lindl) on RAW264. 7 macrophages induced by monosodium urate and potential mechanisms. Foods 2023, 12, 254. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Yang, C.; Yang, W.; Wang, Y.; Du, Y.; Zhao, T.; Shao, H.; Ren, D.; Yang, X. Nonextractable polyphenols from fu brick tea alleviates ulcerative colitis by controlling colon microbiota-targeted release to inhibit intestinal inflammation in mice. J. Agric. Food. Chem. 2024, 72, 7397–7410. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, L.; He, X.; Ma, Y.; Cao, J.; Jiang, W. Potential beneficial effects of functional components of edible plants on COVID-19: Based on their anti-inflammatory and inhibitory effect on SARS-CoV-2. Food Innov. Adv. 2023, 2, 44–59. [Google Scholar] [CrossRef]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent advances on main active ingredients, pharmacological activities of Rosa roxbughii and its development and utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Huang, Q.; Liu, G.; Li, C. Phytochemical profile, bioactivity and prebiotic potential of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber during in vitro digestion and fermentation. Food Funct. 2022, 13, 8880–8891. [Google Scholar] [CrossRef]
- Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical profile and bioactivity of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber by solid-state fermentation with Aspergillus niger. Molecules 2024, 29, 1689. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. 2022, 377, 131922. [Google Scholar] [CrossRef]
- Guo, C.; Qiao, J.; Zhang, S.; Ren, X.; Li, M. Purification of polyphenols from kiwi fruit peel extracts using macroporous resins and high-performance liquid chromatography analysis. Int. J. Food Sci. Technol. 2018, 53, 1486–1493. [Google Scholar] [CrossRef]
- Sun, N.; Xie, J.; Xie, J.; Chen, Y.; Hu, X.; Yu, Q. Purification, composition and activity of bound polyphenols from mung bean coat dietary fiber. Food Res. Int. 2022, 162, 111997. [Google Scholar] [CrossRef]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Y.; Chen, Q.; Fu, X.; Huang, Q.; Zhang, B.; Dong, H.; Li, C. Exploring the synergistic benefits of insoluble dietary fiber and bound phenolics: Unveiling the role of bound phenolics in enhancing bioactivities of insoluble dietary fiber. Trends Food Sci. Tech. 2024, 149, 104554. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, Y.; Lin, Z.; Han, X.; Liao, W. Macroporous resin purification and characterization of flavonoids from Platycladus orientalis (L.) Franco and their effects on macrophage inflammatory response. Food Funct. 2017, 8, 86–95. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.-C.; Ho, L.-H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945, 68–74. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, L. Adsorption/desorption characteristics and chromatographic purification of polyphenols from Vernonia patula (Dryand.) Merr. using macroporous adsorption resin. Ind. Crops Prod. 2021, 170, 113729. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.-L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; De Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009, 115, 469–475. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, X.; Chen, G.-L.; Yu, J.; Yang, L.-Q.; Gao, Y.-Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.-I.; Kühl, A.A.; Erben, U.; May, C.; Schulzke, J.-D.; Siegmund, B. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Liu, C.; Wang, F.; Zhou, C.; Zhu, M.; Sun-Waterhouse, D.; Wang, Z. Phenolic compounds from Chaenomeles speciosa alleviate inflammation in lipopolysaccharide-treated RAW264. 7 macrophages via the NF-κB and MAPK pathways. Food Sci. Hum. Wellness 2023, 12, 1071–1080. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Chen, Q.; Luo, L.; Ma, M.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol. Nutr. Food Res. 2020, 64, 1900943. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.A.; Dolara, P.; Espín, J.C. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, S.; Yu, L.; Li, Y.; Liu, Y.; Ye, X.; Zheng, X. Apios americana Medikus tuber polysaccharide exerts anti-inflammatory effects by activating autophagy. Int. J. Biol. Macromol. 2019, 130, 892–902. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Xu, J.; Feng, J.; He, X. Propacin, a coumarinolignoid isolated from durian, inhibits the lipopolysaccharide-induced inflammatory response in macrophages through the MAPK and NF-κB pathways. Food Funct. 2020, 11, 596–605. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63, 3392. [Google Scholar] [CrossRef]
- Zhou, J.-P.; Yang, X.-N.; Song, Y.; Zhou, F.; Liu, J.-J.; Hu, Y.-Q.; Chen, L.-G. Rosiglitazone alleviates lipopolysaccharide-induced inflammation in RAW264. 7 cells via inhibition of NF-κB and in a PPARγ-dependent manner. Exp. Ther. Med. 2021, 22, 743. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. 2020, 60, 2790–2800. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Yoo, A.; Seo, H.D.; Hahm, J.-H.; Jung, C.H.; Ahn, J.; Ha, T.Y. Dark tea extract attenuates age-related muscle loss by suppressing oxidative stress and inflammation in skeletal muscle of mice. J. Funct. Foods 2024, 112, 105980. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Rosillo, M.; Sanchez-Hidalgo, M.; Cárdeno, A.; De La Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef]
- Yang, D.J.; Liu, S.C.; Chen, Y.C.; Hsu, S.H.; Chang, Y.P.; Lin, J.T. Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage RAW 264.7 Cells. J. Food Biochem. 2015, 39, 334–343. [Google Scholar] [CrossRef]
- Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-inflammatory effects of ellagic acid on keratinocytes via MAPK and STAT pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef]

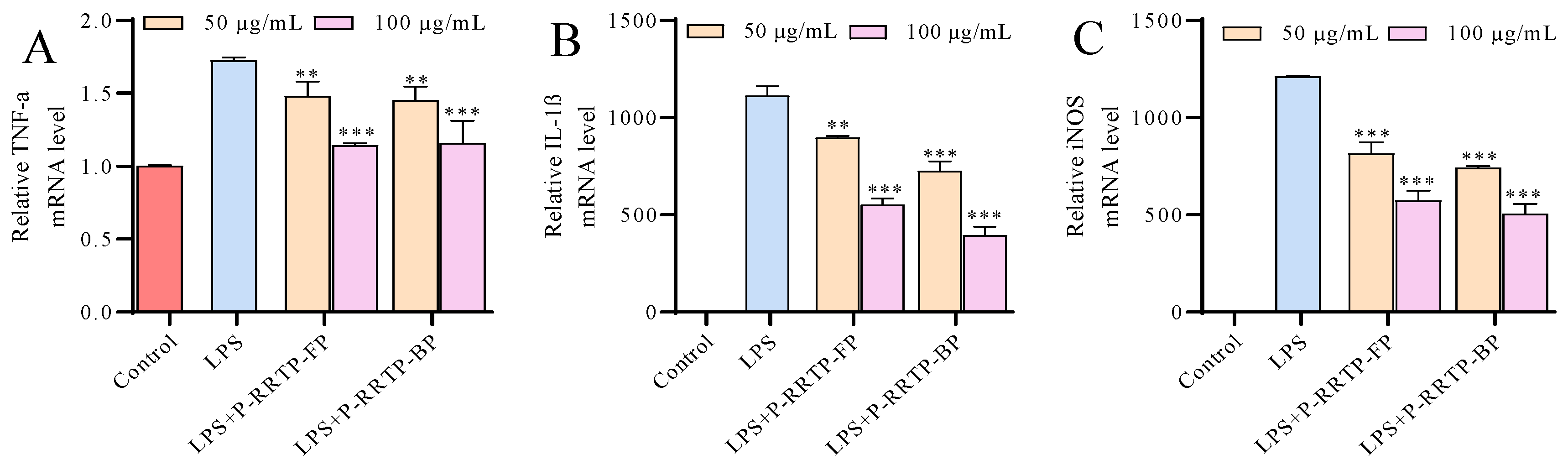
| Resin Types | Adsorption Capacity (mg/g) | Adsorption Rate (%) | Desorption Rate (%) |
|---|---|---|---|
| AB-8 | 2.33 ± 0.10 a | 69.18 ± 2.76 a | 94.13 ± 1.92 a |
| DM130 | 1.90 ± 0.08 b | 57.12 ± 2.11 b | 89.19 ± 2.74 b |
| D101 | 2.20 ± 0.06 c | 59.69 ± 1.18 b | 84.03 ± 0.68 c |
| Resin Types | Adsorption Capacity (mg/g) | Adsorption Rate (%) | Desorption Rate (%) |
|---|---|---|---|
| AB-8 | 6.67 ± 0.13 a | 74.25 ± 1.30 a | 97.19 ± 2.62 a |
| DM130 | 4.90 ± 0.22 b | 62.69 ± 2.46 b | 97.37 ± 2.85 a |
| D101 | 6.08 ± 0.22 c | 47.27 ± 1.04 c | 96.04 ± 4.43 a |
| Compounds | Content (mg/g E) | |||
|---|---|---|---|---|
| RRTP−FP | P−RRTP−FP | RRTP−BP | P−RRTP−BP | |
| Gallic acid | 1.42 ± 0.03 d | 3.8 ± 0.04 c | 17.9 ± 0.43 b | 31.63 ± 1.04 a |
| Protocatechuic acid | 2.4 ± 0.03 b | 15 ± 0.82 a | 1.89 ± 0.05 b | 14.8 ± 0.71 a |
| Ellagic acid | 0.92 ± 0.03 d | 27.86 ± 0.45 b | 23.38 ± 0.44 c | 86 ± 0.43 a |
| Epicatechin | 0.77 ± 0.02 d | 31.96 ± 0.99 b | 7.45 ± 0.11 c | 63.27 ± 3.03 a |
| Epigallocatechin | 11.9 ± 0.51 b | 160.91 ± 5.35 a | 4.36 ± 0.15 d | 8.2 ± 0.33 c |
| Quercetin | 1.37 ± 0.06 b | 1.57 ± 0.16 b | 1.35 ± 0.03 b | 14.03 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, H.; Fu, X.; Huang, Q.; Li, Y. Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace. Foods 2024, 13, 2044. https://doi.org/10.3390/foods13132044
Li C, Li H, Fu X, Huang Q, Li Y. Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace. Foods. 2024; 13(13):2044. https://doi.org/10.3390/foods13132044
Chicago/Turabian StyleLi, Chao, Hengyi Li, Xiong Fu, Qiang Huang, and Yinghua Li. 2024. "Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace" Foods 13, no. 13: 2044. https://doi.org/10.3390/foods13132044
APA StyleLi, C., Li, H., Fu, X., Huang, Q., & Li, Y. (2024). Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace. Foods, 13(13), 2044. https://doi.org/10.3390/foods13132044






