Evolution of Quality Parameters and Bioactivity of Actinidia chinensis cv. Sungold (Kiwifruit) Slices Subjected to Different Drying Conditions Storage for 4 Months
Abstract
1. Introduction
2. Materials and Methods
2.1. Dried Kiwifruit Preparation
2.2. Preparation of Extracts
2.3. Physico-Chemical and Colorimetric Analysis
2.4. Evolution of Organic Acids during Storage
2.5. Total Phenols and Flavonoids Content and Evaluation of Bioactive Compounds Evolution during Storage
2.6. Radical Scavenging Activity
2.7. Firmness and Texture Analysis
2.8. Sensorial Analysis
2.9. Statistical Analysis
3. Results and Discussion
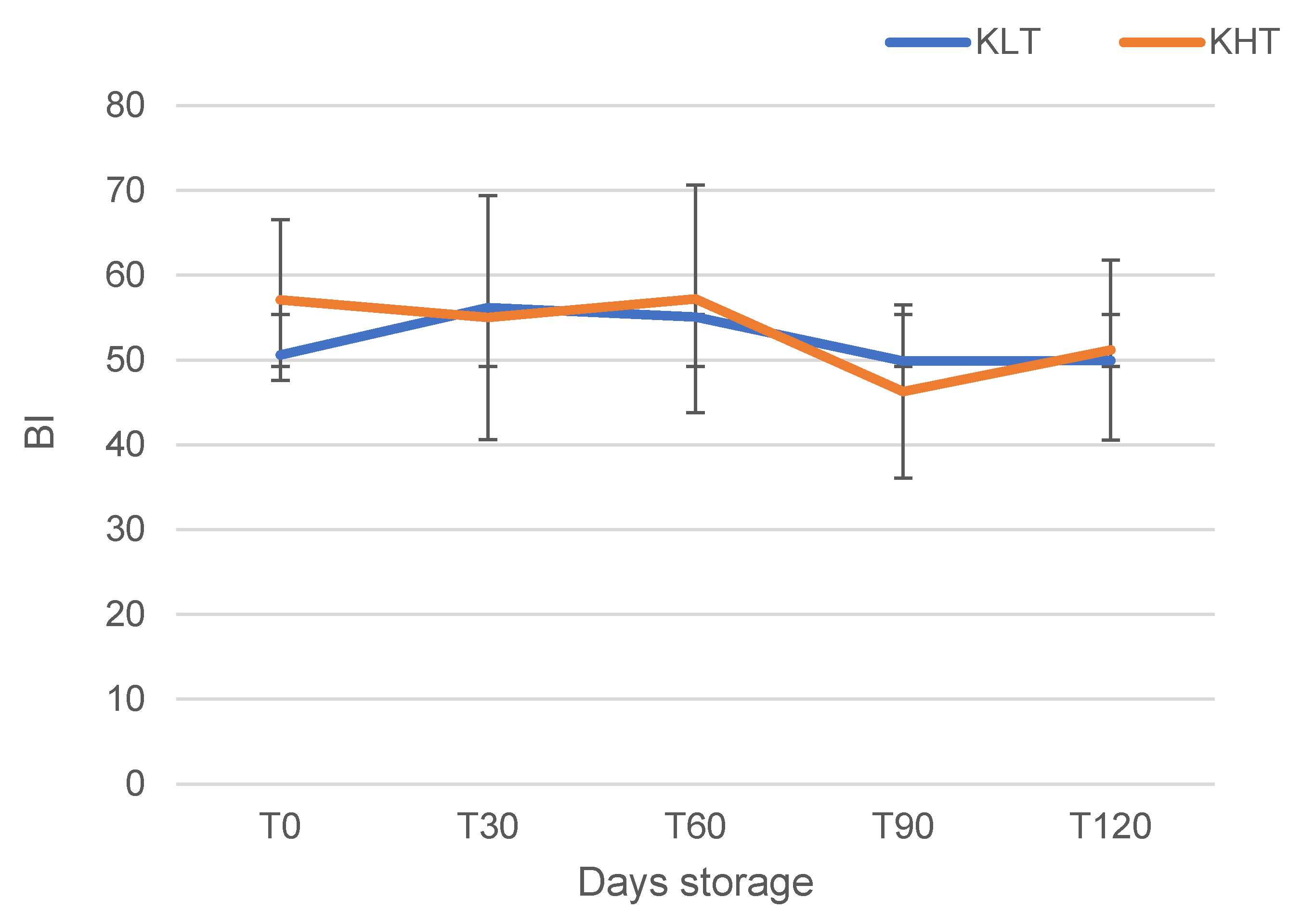
3.1. Quality Parameters
3.2. Organic Acid Evolution during Storage
3.3. Total Phenol and Flavonoid Content and Radical Scavenging Activity
3.4. Degradation Kinetic of TPC and TFC Content during Storage
3.5. Texture and Sensory Analysis
3.6. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Testolin, R.; Ferguson, A.R. Kiwifruit (Actinidia spp.) production and marketing in Italy. N. Z. Crop. Hort. Sci. 2009, 37, 1–32. [Google Scholar] [CrossRef]
- Singletary, K. Kiwifruit: Overview of the potential health benefits. Nutr. Today 2012, 47, 133–147. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Krupa, T.; Latocha, P.; Liwinska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hort. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Izli, N.; Izli, G.; Taskin, O. Drying kinetics, colour, total phenolic content and antioxidant capacity properties of kiwi dried by different methods. J. Food Meas. 2017, 11, 64–74. [Google Scholar] [CrossRef]
- Ma, T.; Lan, T.; Ju, Y.; Cheng, G.; Que, Z.; Geng, T.; Fang, Y.; Sun, X. Comparison of the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms of products: Towards making kiwifruit more nutritious and functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef]
- Saeed, K.; You, L.; Chen, C.; Zhao, Z.; Fu, X.; Liu, R. Comparative assessment of phytochemical profiles and antioxidant and antiproliferative activities of kiwifruit (Actinidia deliciosa) cultivars. J. Food Biochem. 2019, 43, e13025. [Google Scholar] [CrossRef]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, A.; Lv, Z.; Gao, Z. Nondestructive measurement of kiwifruit firmness, soluble solid content (SSC), titratable acidity (TA), and sensory quality by vibration spectrum. Food Sci. Nutr. 2020, 8, 1058–1066. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Barba, F.J.; Kouba, M.; do Prado-Silva, L.; Anderson de Souza Sant’Ana, V.O. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Barba, F.J.; Lilian, R.B.; Neura Bragagnolo, M.; Mercadante, A.Z.; Barbosa-Canovas, G.V.; Vibeke Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Sablani, S.S. Drying of Fruits and Vegetables: Retention of Nutritional/Functional Quality. Dry. Technol. 2006, 24, 123–135. [Google Scholar] [CrossRef]
- Kaya, A.; Aydın, O.; Dincer, I. Experimental and numerical investigation of heat and mass transfer during drying of Hayward kiwi fruits (Actinidia deliciosa Planch). J. Food Eng. 2009, 88, 323–330. [Google Scholar] [CrossRef]
- Ozen, T.; Zenginbal, H.; Yazicioglu, E.; Gul, F.; Demirtas, I. A Comparison Investigation on Antioxidant Activities, Physicochemical Properties and Phytochemical Contents of Kiwifruit Genotypes and Cultivars. Int. J. Fruit Sci. 2019, 19, 115–135. [Google Scholar] [CrossRef]
- Available online: https://www.marketresearch.com/LP-Information-Inc-v4134/Global-DriedFruit-Snacks-Growth-33498637/ (accessed on 5 February 2024).
- Available online: https://www.industryarc.com/Research/Global-Dried-Kiwi-Fruit-Market-Research-513453 (accessed on 5 February 2024).
- Movagharnejad, K.; Pouya, S. The Effect of the drying method on the quality of dried kiwi slices. Int. J. Health Med. 2017, 2, 1–5. [Google Scholar] [CrossRef][Green Version]
- Ozgen, F. Comparing the drying characteristics of apple and kiwi fruits. Therm. Sci. 2021, 25, S327–S331. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and Unconventional Dried Fruit Snacks as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, F.; Celik, N. Evaluation of Design Parameters on Drying of Kiwi Fruit. Appl. Sci. 2019, 9, 10. [Google Scholar] [CrossRef]
- Akar, G.; Mazı, I.B. Color change, ascorbic acid degradation kinetics, and rehydration behavior of kiwifruit as affected by different drying methods. J. Food Proc. Eng. 2019, 42, e13011. [Google Scholar] [CrossRef]
- Özcan, M.M.; Juhaimi, F.A.; Ahmed, I.A.M.; Nurhan, U.; Babiker, E.B.; Ghafoor, K. Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J. Food Sci. Technol. 2020, 57, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.K.; Siew, E.S.; Soon, W.L. Drying characteristics and quality evaluation of kiwi slices under hot air natural convective drying method. Int. Food Res. J. 2015, 22, 2188–2195. [Google Scholar]
- Correia, P.; Guiné, R.P.; Correia, A.C.; Gonçalves, F.; Brito, M.F.S.; Ribeiro, J.R.P. Physical, chemical and sensory properties of kiwi as influenced by drying conditions. J. Agric. Eng. Int. 2017, 19, 203–212. [Google Scholar]
- Simal, S.; Femenia, A.; Carcel, J.A.; Rosello, C. Mathematical modelling of the drying curves of kiwi fruits: Influence of the ripening stage. J. Sci. Food Agric. 2005, 85, 425–432. [Google Scholar] [CrossRef]
- Simal, S.; Femenia, A.; Garau, M.C.; Rosselló, C. Use of exponential, page’s and diffusional models to simulate the drying kinetics of kiwi fruit. J. Food Eng. 2005, 66, 323–328. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of Colour Change of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Diamante, L.; Durand, M.; Savage, G.; Vanhanen, L. Effect of temperature on the drying characteristics, colour and ascorbic acid content of green and gold kiwifruits. Int. Food Res. J. 2010, 17, 441–451. [Google Scholar]
- Kaya, A.; Aydın, O.; Kolaylı, S. Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food Bioprod. Proc. 2010, 88, 165–173. [Google Scholar] [CrossRef]
- Tepe, F.B.; Tepe, T.K.; Ekinci, A. Impact of air temperature on drying characteristics and some bioactive properties of kiwi fruit slices. Chem. Ind. Chem. Eng. Q. 2022, 28, 151–159. [Google Scholar] [CrossRef]
- Gümüşay, Ö.A.; Yalçın, M.Y. Effects of freeze-drying process on antioxidant and some physical properties of cherry Laurel and Kiwi fruits. Akad. GIDA 2019, 17, 9–15. [Google Scholar] [CrossRef]
- Tashooq, A.B.; Syed, Z.H.; Sajad, M.W.; Rather, M.A.; Reshi, M.; Naseer, B.; Qadri, T.; Khalil, A. The impact of different drying methods on antioxidant activity, polyphenols, vitamin C and rehydration characteristics of Kiwifruit. Food Biosci. 2022, 48, 101821. [Google Scholar] [CrossRef]
- Doymaz, I. Impact of citric acid on the drying characteristics of kiwifruit slices. Acta Sci. Technol. 2020, 42, e40570. [Google Scholar] [CrossRef]
- Sicari, V.; Romeo, R.; Leporini, M.; Pellicanò, T.M.; Tundis, R.; Loizzo, M.R. Comparison of traditional hot water and vacuum assisted blanching methods on the physico-chemical quality parameters and antioxidant activity of zucchini (Cucurbita pepo L.) slices. Food Meas. 2022, 16, 281–294. [Google Scholar] [CrossRef]
- AOAC. Acidity of Fruit Products, Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Gutiérrez-López, G.F.; Alamilla-Beltrán, L.; del Pilar Buera, M.; Welti-Chanes, J.; Parada-Arias, E.; Barbosa-Cánovas, G.V. Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Caballero-Ceron, C.; Guerrero-Beltran, J.A.; Mujica-Paz, H.; Torres, J.A.; Welti-Chanes, J. Moisture Sorption Isotherms of Foods: Experimental Methodology, Mathematical Analysis, and Practical Applications. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Ferreira, D.M.S.; Barroca, M.J.; Gonçalves, F.M. Study of the drying kinetics of solar-dried pears. Biosyst. Eng. 2007, 98, 422–429. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, W.; Wang, A.; Gu, A.; Lv, Z. Kinetic models applied to quality change and shelf life prediction of kiwifruits. LWT 2021, 138, 110610. [Google Scholar] [CrossRef]
- MacRae, E.A.; Lallu, N.; Searle, A.; Bowen, J. Changes in the softening and composition of kiwifruit (Actinidia deliciosa) affected by maturity at harvest and postharvest treatments. J. Sci. Food Agric. 1989, 49, 413–430. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Understanding consumer acceptance of early harvested ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2001, 22, 205–213. [Google Scholar] [CrossRef]
- Harker, F.R.; Carr, B.T.; Lenjo, M.; MacRae, E.A.; Wismer, W.V.; Marsh, K.B.; Williams, M.; White, A.; Lund, C.M.; Walker, S.B.; et al. Consumer liking for kiwifruit flavour: A meta-analysis of five studies on fruit quality. Food Qual. Prefer. 2009, 20, 30–41. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics antioxidant capacity total phenolic content colour texture and microstructure of apple (var Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.S.; Silva, M.A. Retention of Vitamin C in Drying Processes of Fruits and Vegetables—A Review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.C.; Lago, M.G.; Castelo-Branco, V.N.; Oliveira, F.R.; Torres, A.G.; Perrone, D.; Monteiro, M. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 2016, 197, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Herna´ndez, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, A.A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef]
- Degirmencioglu, N.; Gürbüz, O.; Karatepe, G.E.; Irkin, R. Influence of hot air drying on phenolic compounds and antioxidant capacity of blueberry (Vaccinium myrtillus) fruit and leaf. J. Appl. Bot. Food Qual. 2017, 90, 115–127. [Google Scholar] [CrossRef]
- Wang, S.Y.; Stretch, A.W. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem. 2001, 49, 969–974. [Google Scholar] [CrossRef]
- Pal, R.S.; Kumar, V.A.; Arora, S.; Sharma, A.K.; Kumar, V.; Agrawal, S. Physi-cochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015, 58, 262–271. [Google Scholar] [CrossRef]
- Santos, S.C.; Guiné, R.P.; Barros, A. Influence of drying on the properties of pears of the Rocha variety (Pyrus communis L.). Int. J. Food Eng. 2013, 9, 197–207. [Google Scholar] [CrossRef]
- Kaur, P.; Abha, C.; Bikram, S.G. An efficient microwave assisted extraction of phenolic compounds and antioxidant potential of Ginkgo biloba. Nat. Prod. Comun. 2012, 7, 203–206. [Google Scholar] [CrossRef]
- Mahjoorian, A.; Mokhtarian, M.; Fayyaz, N.; Rahmati, F.; Sayyadi, S.; Ariaii, P. Modeling of drying kiwi slices and its sensory evaluation. Food Sci. Nutr. 2017, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]


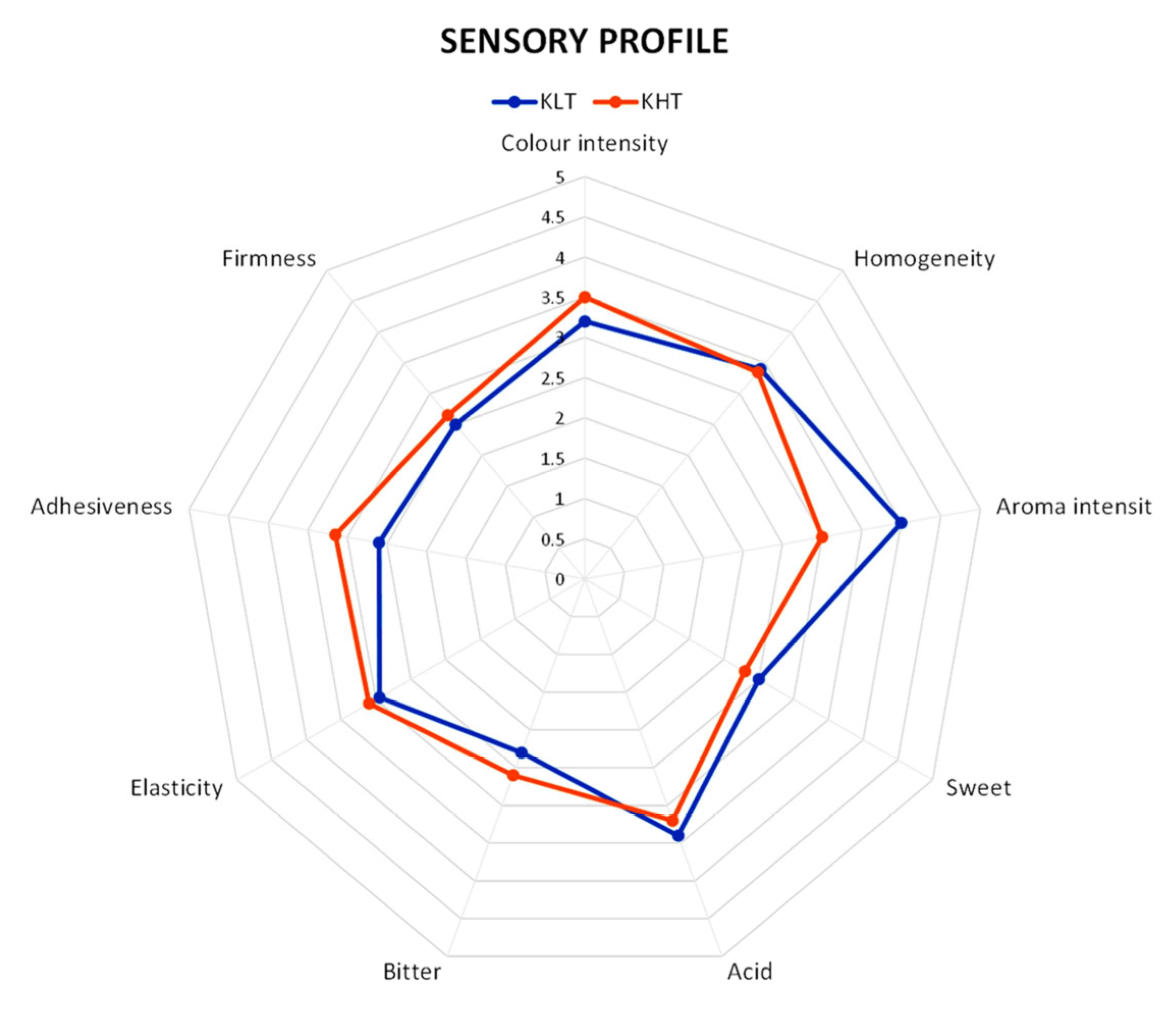
| Category | Descriptor | Definition |
|---|---|---|
| Appearance | Color intensity | Overall product color intensity |
| Homogeneity | Product shape perception | |
| Olfactory | Aroma intensity | Overall product typical flavor intensity |
| Taste | Sweet | Overall product sweetness intensity |
| Acid | Overall product acidity intensity | |
| Bitter | Overall product bitterness intensity | |
| Texture | Elasticity | Springiness feeling intensity |
| Adhesiveness | Mouth adhesivity feeling intensity | |
| Firmness | Structural firmness feeling intensity |
| Days Storage | Moisture (wb) | aw | pH | TSS (°Brix) | TA (g Citric Acid/100 g dw) | BI |
|---|---|---|---|---|---|---|
| FKF | ||||||
| T0 | 81.35 ± 2.32 | 0.99 ± 0.00 | 3.19 ± 0.04 | 14.50 ± 0.08 | 1.51 ± 0.00 | |
| KLT | ||||||
| T0 | 22.12 ± 0.23 | 0.45 ± 0.01 c | 3.56 ± 0.01 b | 2.43 ± 0.06 b | 0.74 ± 0.01 e | 50.57 ± 9.48 |
| T30 | 22.09 ± 1.02 | 0.40 ± 0.00 d | 3.56 ± 0.01 b | 4.87 ± 0.12 a | 0.75 ± 0.01 a | 56.20 ± 14.38 |
| T60 | 22.15 ± 1.25 | 0.47 ± 0.00 b | 3.51 ± 0.00 b | 4.37 ± 0.23 a | 0.68 ± 0.00 b | 55.07 ± 13.40 |
| T90 | 22.21 ± 0.98 | 0.47 ± 0.00 b | 3.56 ± 0.02 b | 4.80 ± 0.00 a | 0.63 ± 0.03 c | 49.88 ± 10.20 |
| T120 | 23.02 ± 2.06 | 0.52 ± 0.00 a | 3.70 ± 0.06 a | 4.27 ± 0.46 a | 0.58 ± 0.00 d | 49.92 ± 10.62 |
| Sign. | ns | ** | ** | ** | ** | ns |
| KHT | ||||||
| T0 | 19.21 ± 2.34 | 0.50 ± 0.00 ab | 3.52 ± 0.01 ab | 5.38 ± 0.03 a | 0.76 ± 0.07 b | 57.09 ± 8.11 |
| T30 | 19.16 ± 1.98 | 0.44 ± 0.00 c | 3.54 ± 0.01 a | 3.5 ± 0.00 e | 0.69 ± 0.00 b | 55.00 ± 10.54 |
| T60 | 19.13 ± 0.78 | 0.44 ± 0.00 c | 3.37 ± 0.02 c | 5.17 ± 0.06 b | 0.93 ± 0.04 a | 57.22 ± 10.21 |
| T90 | 19.27 ± 2.32 | 0.51 ± 0.00 a | 3.44 ± 0.00 cd | 4.03 ± 0.05 d | 0.73 ± 0.04 b | 46.29 ± 8.91 |
| T120 | 19.87 ± 1.36 | 0.49 ± 0.00 b | 3.46 ± 0.05 c | 4.30 ± 0.00 c | 0.72 ± 0.00 b | 51.19 ± 10.91 |
| Sign. | ns | ** | ** | ** | ** | ns |
| Days Storage | FKF | KLT | KHT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | a* | b* | L | a* | b* | L | a* | b* | |
| T0 | 58.57 ± 4.37 | 1.19 ± 0.78 | 15.34 ± 3.07 | 55.19 ± 2.85 | 7.45 ± 1.71 b | 18.74 ± 3.82 | 59.46 ± 3.74 ab | 7.09 ± 1.86 | 23.11 ± 3.60 |
| T30 | 57.07 ± 4.29 | 8.61 ± 2.28 ab | 20.88 ± 5.11 | 57.19 ± 3.88 b | 6.54 ± 1.21 | 21.69 ± 4.92 | |||
| T60 | 56.65 ± 3.78 | 9.04 ± 2.53 a | 20.29 ± 5.26 | 60.18 ± 3.85 a | 7.14 ± 1.24 | 23.54 ± 4.95 | |||
| T90 | 55.60 ± 4.23 | 7.94 ± 1.86 ab | 18.28 ± 3.76 | 58.75 ± 3.58 ab | 7.39 ± 0.97 | 22.41 ± 4.25 | |||
| T120 | 56.06 ± 4.12 | 7.99 ± 1.82 ab | 18.41 ± 3.85 | 57.87 ± 4.56 ab | 6.89 ± 1.50 | 20.39 ± 5.13 | |||
| Sign. | ns | * | ns | * | ns | ns | |||
| Days Storage | Ascorbic Acid (mg/100 g dw) | Citric Acid (mg/100 g dw) | Malic Acid (mg/100 g dw) | Oxalic Acid (mg/100 g dw) | Tartaric Acid (mg/100 g dw) |
|---|---|---|---|---|---|
| FKF | |||||
| T0 | 957.22 ± 5.70 | 2215.47 ± 4.02 | 638.53 ± 5.06 | 62.96 ± 0.82 | 674.98 ± 2.38 |
| KLT | |||||
| T0 | 1009.74 ± 5.11 a | 4664.67 ± 5.13 d | 390.45 ± 0.59 a | 27.49 ± 0.59 a | 508.54 ± 0.85 a |
| T30 | 924.98 ± 3.93 b | 3621.49 ± 3.97 e | 385.64 ± 1.23 b | 27.14 ± 2.14 a | 441.33 ± 1.58 b |
| T60 | 363.54 ± 2.11 c | 1217.32 ± 5.49 b | 230.60 ± 0.91 c | 1.23 ± 0.11 b | 302.42 ± 0.51 c |
| T90 | 352.06 ± 2.72 cd | 845.98 ± 1.86 c | 40.22 ± 1.12 d | nd | 32.59 ± 2.71 d |
| T120 | 339.94 ± 1.03 e | 886.74 ± 5.52 a | 28.87 ± 0.53 d | nd | nd |
| Sign. | ** | ** | ** | ** | ** |
| KHT | |||||
| T0 | 444.95 ± 3.52 a | 2008.12 ± 5.65 a | 341.21 ± 7.37 a | 99.67 ± 0.80 a | 526.99 ± 2.46 a |
| T30 | 427.30 ± 7.51 a | 1967.13 ± 10.30 b | 327.39 ± 1.99 b | 28.37 ± 0.84 b | 472.39 ± 2.64 bc |
| T60 | 428.88 ± 1.97 b | 1335.82 ± 4.23 c | 274.16 ± 3.00 c | 31.79 ± 3.75 b | 355.87 ± 1.01 abc |
| T90 | 419.78 ± 2.96 a | 124.35 ± 0.38 d | 30.56 ± 2.31 d | 32.77 ± 1.98 b | 118.98 ± 1.59 c |
| T120 | 384.29 ± 6.56 c | 90.88 ± 1.89 e | 20.58 ± 0.47 d | 1.99 ± 0.19 c | 49.64 ± 3.37 bc |
| Sign. | ** | ** | ** | ** | ** |
| Days Storage | TPC (mg GAE/100 g dw) | TFC (mg CTE/100 g dw) | DPPH (mmol Trolox/100 g dw) | ABTS (mmol Trolox/100 g dw) |
|---|---|---|---|---|
| FKF | ||||
| T0 | 941.79 ± 4.49 | 260.19 ± 6.22 | 1195.87 ± 15.19 | 56.05 ± 3.06 |
| KLT | ||||
| T0 | 979.42 ± 2.40 a | 281.84 ± 2.17 a | 1657.62 ± 0.92 a | 64.68 ± 0.34 a |
| T30 | 650.54 ± 2.32 b | 273.84 ± 2.04 b | 1318.95 ± 6.62 b | 61.49 ± 2.95 ab |
| T60 | 586.64 ± 2.05 c | 243.64 ± 3.70 c | 1241.47 ± 1.39 c | 58.00 ± 0.82 c |
| T90 | 495.14 ± 5.43 d | 180.05 ± 0.99 d | 1024.68 ± 4.37 d | 55.93 ± 3.76 c |
| T120 | 395.34 ± 0.85 e | 113.93 ± 1.12 d | 996.79 ± 2.63 e | 42.29 ± 1.61 d |
| Sign. | ** | ** | ** | ** |
| KHT | ||||
| T0 | 526.04 ± 2.40 a | 169.07 ± 5.27 a | 926.15 ± 2.75 a | 67.59 ± 1.68 a |
| T30 | 472.27 ± 4.80 b | 166.37 ± 3.50 a | 891.13 ± 5.13 b | 52.70 ± 2.37 b |
| T60 | 456.82 ± 3.18 c | 165.33 ± 2.78 a | 889.00 ± 2.95 b | 45.96 ± 0.24 c |
| T90 | 453.15 ± 2.64 c | 116.60 ± 2.90 b | 859.32 ± 1.49 c | 39.54 ± 1.39 d |
| T120 | 408.25 ± 2.11 d | 102.78 ± 1.61 c | 854.35 ± 1.30 c | 36.71 ± 0.52 d |
| Sign. | ** | ** | ** | ** |
| Bioactives | Drying | Reaction Order | K-Value (min−1) | R2 | Half-Life (t1/2) (Days) |
|---|---|---|---|---|---|
| TPC | KLT | 1 | 0.0018 | 0.9074 | 385.080 |
| KHT | 1 | 0.007 | 0.9527 | 99.021 | |
| TFC | KLT | 0 | 0.6078 | 0.8243 | 1.1404 |
| KHT | 0 | 1.432 | 0.9173 | 0.4840 |
| Days Storage | Hardness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience |
|---|---|---|---|---|---|---|
| KLT | ||||||
| T0 | 11,782.33 ± 8252.49 b | 0.835 ± 0.07 a | 0.656 ± 0.09 a | 7327.57 ± 5156.92 b | 5967.77 ± 4106.79 b | 0.289 ± 0.06 |
| T30 | 28,983.86 ± 7512.27 a | 0.712 ± 0.06 b | 0.579 ± 0.04 b | 16,881.93 ± 4660.77 a | 12,051.43 ± 3611.15 a | 0.332 ± 0.05 |
| T60 | 27,160.28 ± 7642.55 a | 0.798 ± 0.07 ab | 0.599 ± 0.04 ab | 16,403.81 ± 5150.93 a | 13,191.35 ± 4674.64 a | 0.349 ± 0.06 |
| T90 | 30,177.77 ± 7607.16 a | 0.727 ± 0.11 b | 0.552 ± 0.06 b | 16,858.77 ± 5026.39 a | 12,243.89 ± 3473.21 a | 0.323 ± 0.06 |
| T120 | 29,158.98 ± 8077.83 a | 0.772 ± 0.08 ab | 0.573 ± 0.03 b | 16,755.93 ± 4782.70 a | 12,784.67 ± 3317.61 a | 0.339 ± 0.04 |
| Sign. | ** | ** | ** | ** | ** | ns |
| KHT | ||||||
| T0 | 5038.91 ± 4020.93 b | 1.088 ± 0.39 a | 0.722 ± 0.07 a | 3403.76 ± 2676.40 b | 3313.20 ± 2266.48 b | 0.251 ± 0.09 |
| T30 | 15,228.75 ± 7873.64 a | 0.93 ± 0.22 ab | 0.661 ± 0.06 ab | 9923.41 ± 5039.80 a | 8553.76 ± 4357.73 a | 0.335 ± 0.06 |
| T60 | 14,507.64 ± 5317.43 a | 0.85 ± 0.05 ab | 0.625 ± 0.06 b | 9104.83 ± 3585.54 a | 7755.64 ± 3225.34 a | 0.271 ± 0.08 |
| T90 | 14,543.67 ± 5551.77 a | 0.818 ± 0.07 b | 0.647 ± 0.05 b | 9260.87 ± 3153.48 a | 7468.94 ± 2225.94 a | 0.302 ± 0.06 |
| T120 | 16,346.33 ± 4903.62 a | 0.855 ± 0.07 ab | 0.628 ± 0.05 b | 10,296.85 ± 3215.58 a | 8772.78 ± 2658.17 a | 0.321 ± 0.06 |
| Sign. | ** | * | ** | ** | ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzo, S.; Antonio, M.; Rosa, R.; Roberta, P.; Filomena, C.; Rosa, L.M. Evolution of Quality Parameters and Bioactivity of Actinidia chinensis cv. Sungold (Kiwifruit) Slices Subjected to Different Drying Conditions Storage for 4 Months. Foods 2024, 13, 2100. https://doi.org/10.3390/foods13132100
Vincenzo S, Antonio M, Rosa R, Roberta P, Filomena C, Rosa LM. Evolution of Quality Parameters and Bioactivity of Actinidia chinensis cv. Sungold (Kiwifruit) Slices Subjected to Different Drying Conditions Storage for 4 Months. Foods. 2024; 13(13):2100. https://doi.org/10.3390/foods13132100
Chicago/Turabian StyleVincenzo, Sicari, Mincione Antonio, Romeo Rosa, Pino Roberta, Conforti Filomena, and Loizzo Monica Rosa. 2024. "Evolution of Quality Parameters and Bioactivity of Actinidia chinensis cv. Sungold (Kiwifruit) Slices Subjected to Different Drying Conditions Storage for 4 Months" Foods 13, no. 13: 2100. https://doi.org/10.3390/foods13132100
APA StyleVincenzo, S., Antonio, M., Rosa, R., Roberta, P., Filomena, C., & Rosa, L. M. (2024). Evolution of Quality Parameters and Bioactivity of Actinidia chinensis cv. Sungold (Kiwifruit) Slices Subjected to Different Drying Conditions Storage for 4 Months. Foods, 13(13), 2100. https://doi.org/10.3390/foods13132100












