Comparison of Aroma Profiles of Whiskeys Fermented from Different Grain Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Chemicals
2.3. Aroma Analysis
2.3.1. Free Volatile Detection
2.3.2. Bound Volatile Detection
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
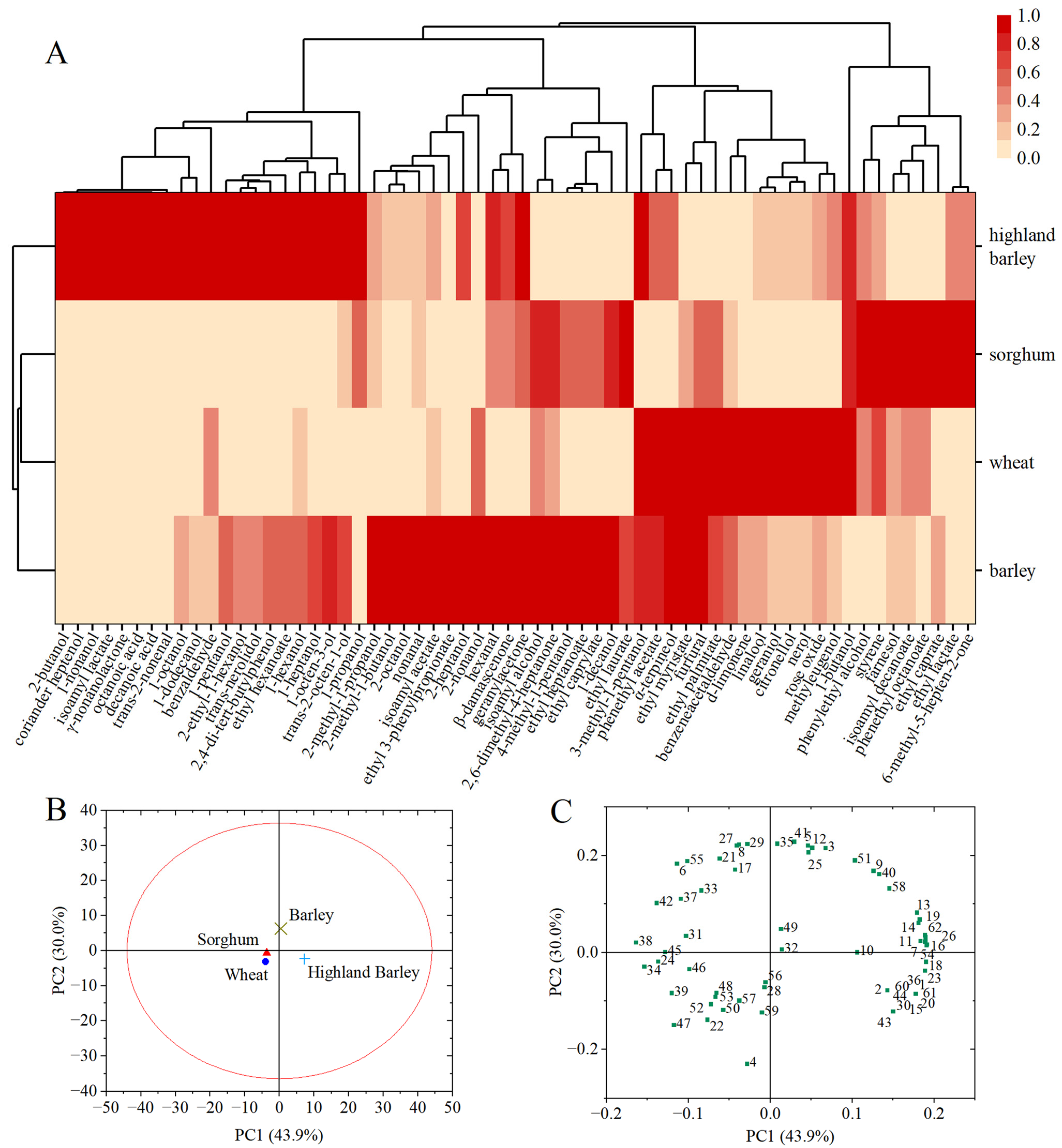
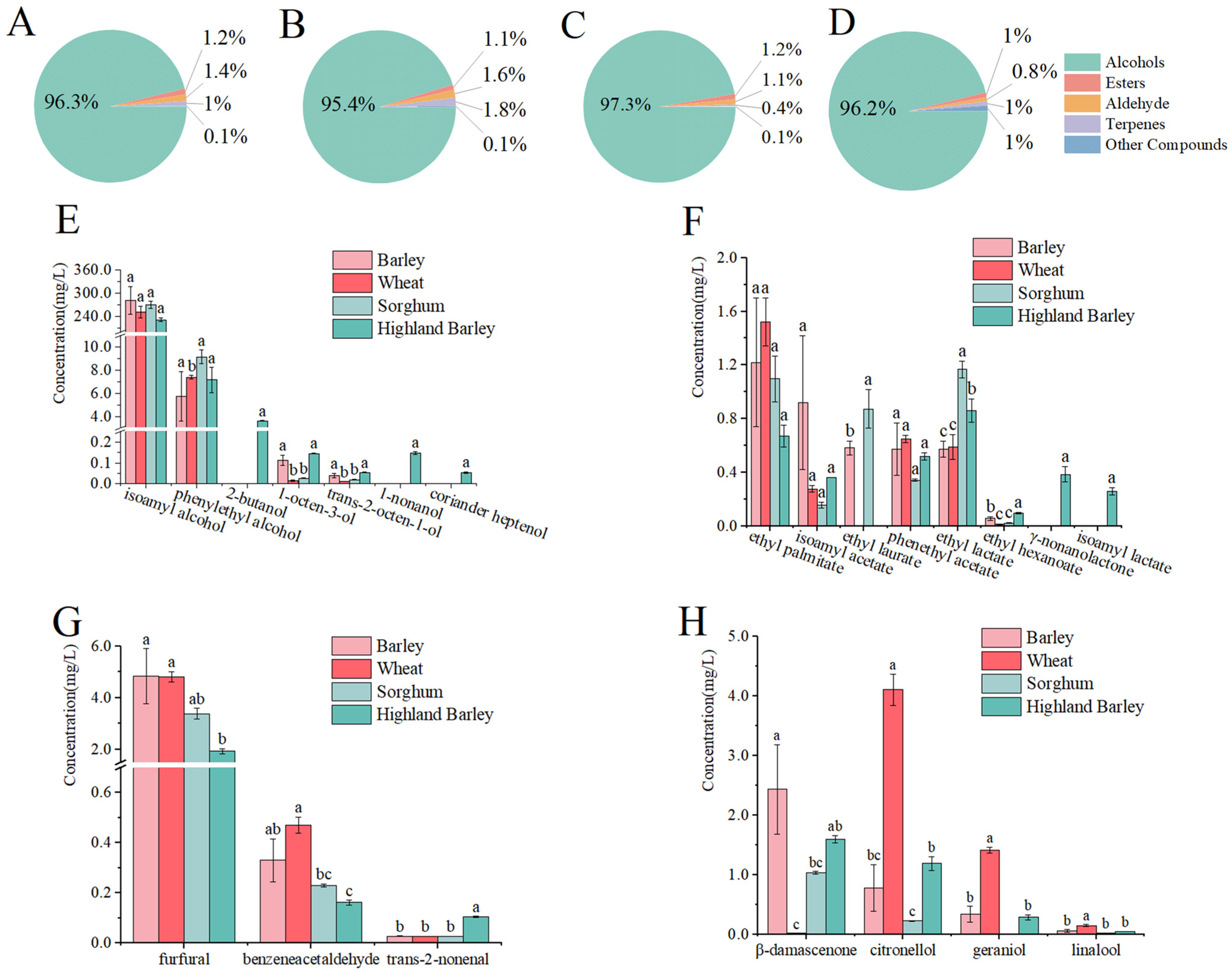
3.1. Analyses of Whiskey Aroma
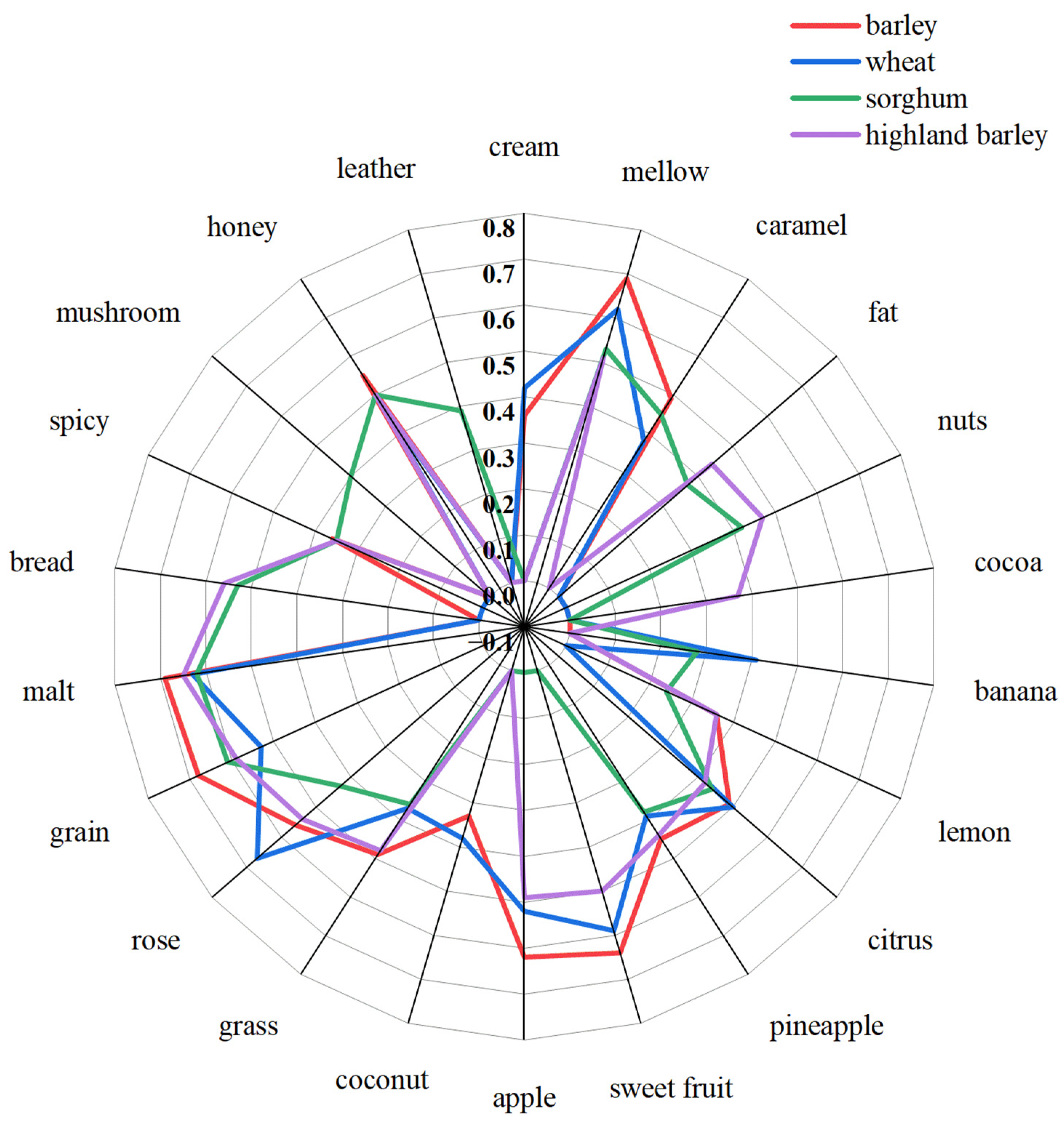
3.2. Analyses of Sensory Evaluation
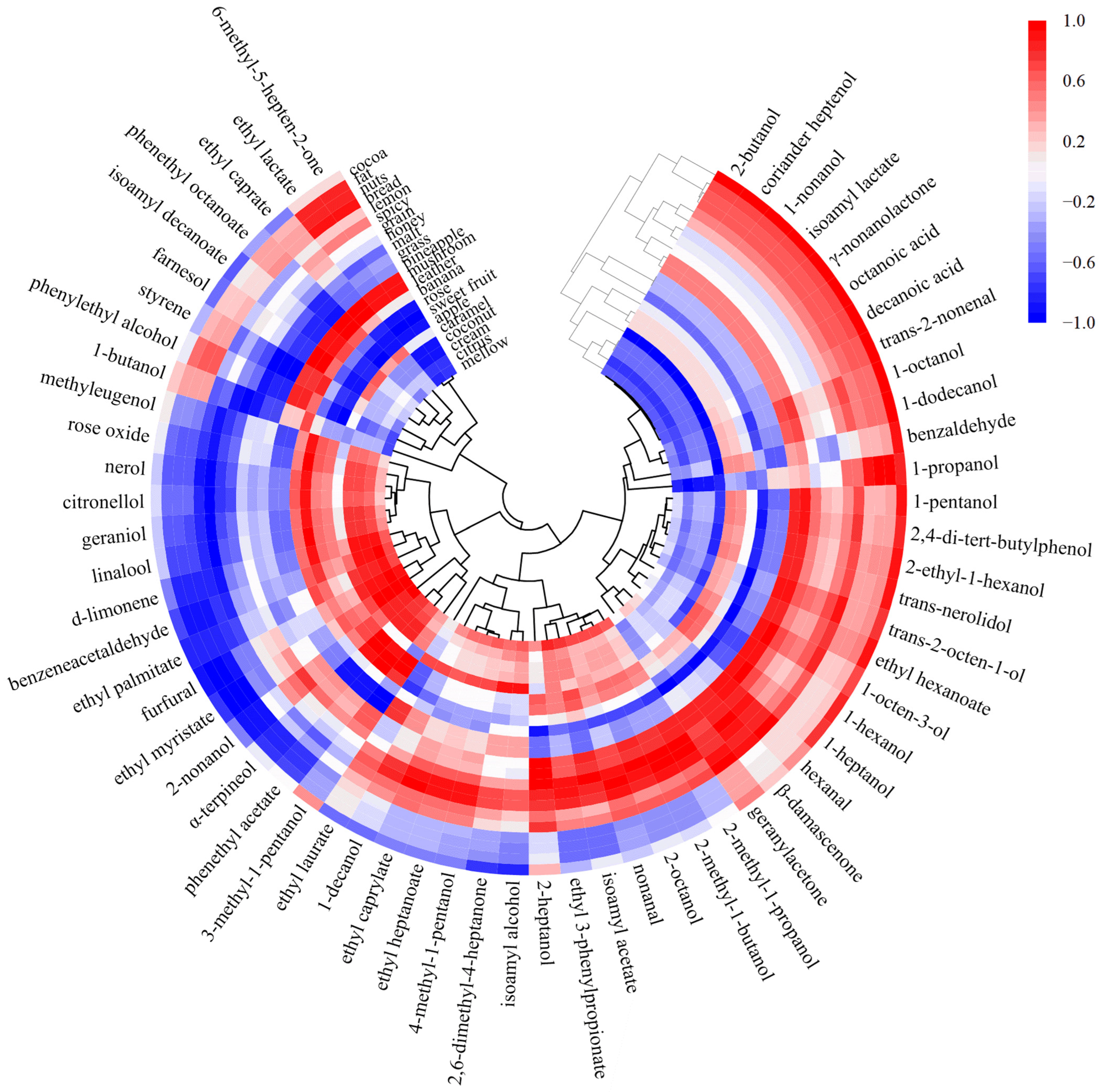
3.3. Correlation Analysis between Aroma Components and Sensory Evaluation
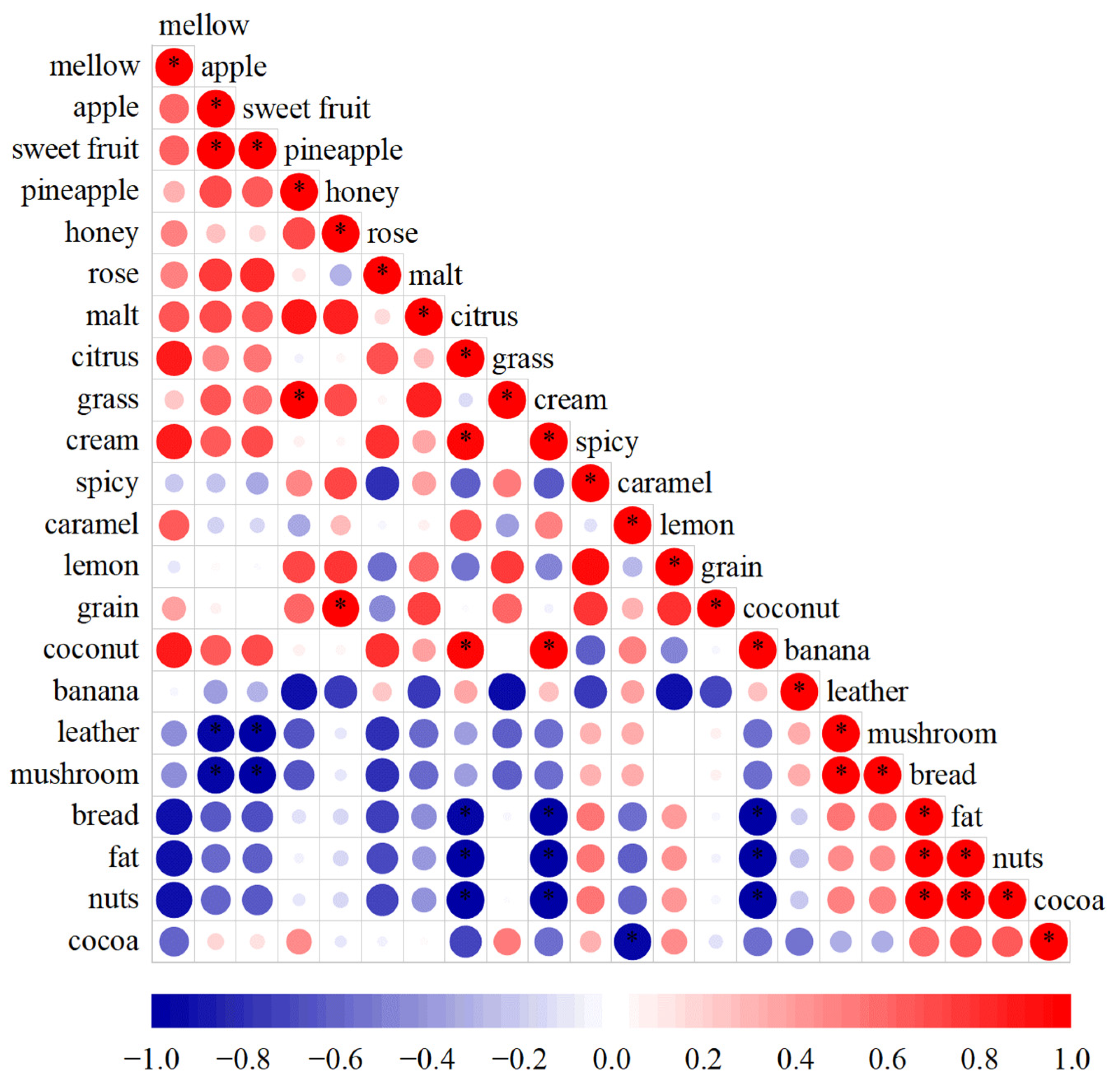
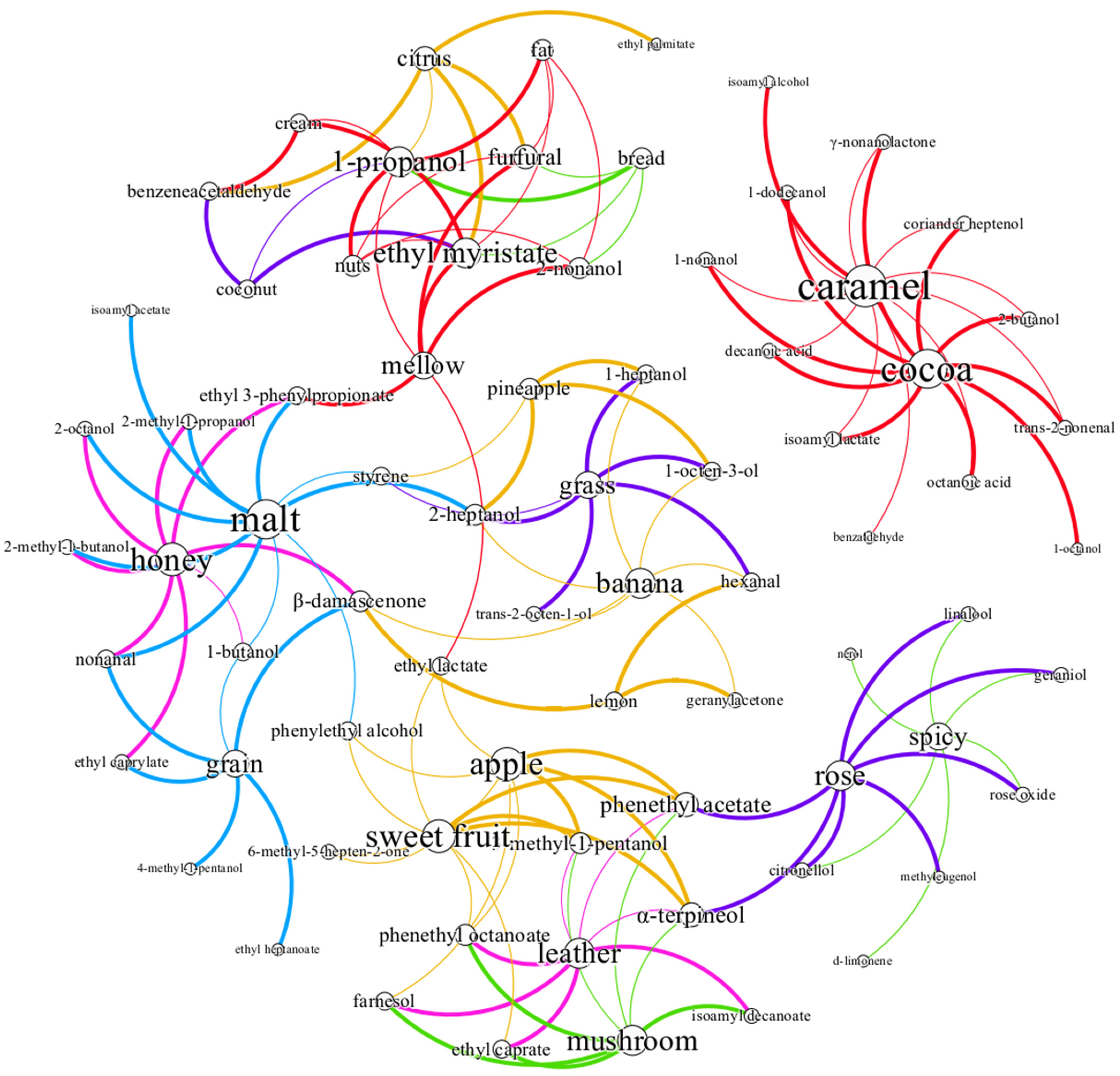
3.3.1. Potential Interaction of Aroma Sensory Attributes
3.3.2. Correlation Network Analysis between Volatile Substances and Sensory Evaluation
| No. | Compounds | RI | Concentration (mg/L) * | Odor Thresholds (mg/L) | Odour Activity Value (OAV) | Descriptor # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barley Whiskey | Wheat Whiskey | Sorghum Whiskey | Highland Barley Whiskey | Barley Whiskey | Wheat Whiskey | Sorghum Whiskey | Highland Barley Whiskey | |||||
| Alcohols | ||||||||||||
| 1 | 2-Butanol | 1038.4 | nf | nf | nf | 3.6 ± 0.034 a | 50 ζ | - | - | - | <1 | fruity, sweet, apricot |
| 2 | 1-Propanol | 1053.0 | 41.379 ± 4.75 a | 40.29 ± 9.833 a | 45.26 ± 1.818 a | 49.423 ± 0.997 a | 53.952 δ | <1 | <1 | <1 | <1 | sweet, fruity, apple |
| 3 | 2-Methyl-1-propanol | 1105.7 | 35.613 ± 9.764 a | 18.207 ± 3.855 a | 20.472 ± 0.605 a | 24.373 ± 0.254 a | 28.3 δ | 1.26 | <1 | <1 | <1 | ethereal, winey |
| 4 | 1-Butanol | 1155.1 | 0.45 ± 0.021 c | 1.081 ± 0.056 a | 0.957 ± 0.021 b | 0.96 ± 0.01 b | 2.73 γ | <1 | <1 | <1 | <1 | oily, sweet, balsamic |
| 5 | 2-Methyl-1-butanol | 1208.1 | 7.903 ± 0.61 a | 7.131 ± 0.469 a | 7.179 ± 0.208 a | 7.303 ± 0.149 a | 24 α | <1 | <1 | <1 | <1 | fatty, leathery, cocoa |
| 6 | Isoamyl alcohol | 1208.4 | 281.389 ± 35.864 a | 251.732 ± 15.31 a | 269.861 ± 8.853 a | 231.586 ± 4.885 a | 56.1 η | 5.02 | 4.49 | 4.81 | 4.13 | whiskey, fruity, banana |
| 7 | 1-Pentanol | 1247.4 | 0.31 ± 0.016 b | 0.202 ± 0.008 c | 0.18 ± 0.005 c | 0.432 ± 0.001 a | 64.43 ζ | <1 | <1 | <1 | <1 | oily, sweet, balsamic |
| 8 | 4-Methyl-1-pentanol | 1307.1 | 0.039 ± 0.002 a | nf | 0.019 ± 0.001 b | nf | nf | - | - | - | - | nutty |
| 9 | 2-Heptanol | 1311.6 | 0.014 ± 0.002 a | nf | nf | 0.009 ± 0.00 b | 1.434 ε | <1 | - | - | <1 | mushroom, oily, fatty |
| 10 | 3-Methyl-1-pentanol | 1321.6 | 0.035 ± 0.006 a | 0.037 ± 0.001 a | nf | 0.041 ± 0.001 a | 1 ζ | <1 | <1 | - | <1 | winey, cocoa, green, fruity |
| 11 | 1-Hexanol | 1348.3 | 0.194 ± 0.026 b | 0.118 ± 0.003 c | 0.068 ± 0.003 d | 0.279 ± 0.00 a | 0.537 θ | <1 | <1 | <1 | <1 | oily, fruity, sweet, green |
| 12 | 2-Octanol | 1414.9 | 0.011 ± 0.002 a | nf | nf | 0.002 ± 0.00 b | 0.0715 ζ | <1 | - | - | <1 | spicy, green, woody |
| 13 | 1-Octen-3-ol | 1442.9 | 0.113 ± 0.025 a | 0.016 ± 0.004 b | 0.027 ± 0.001 b | 0.145 ± 0.002 a | 0.006 θ | 18.87 | 2.62 | 4.44 | 24.24 | earthy, green, oily |
| 14 | 1-Heptanol | 1447.7 | 0.079 ± 0.01 a | 0.057 ± 0.003 b | 0.053 ± 0.001 b | 0.09 ± 0.001 a | 26.6 δ | <1 | <1 | <1 | <1 | leafy, green, fruity, apple |
| 15 | Coriander heptenol | 1453.5 | nf | nf | nf | 0.053 ± 0.004 a | 2 ζ | - | - | - | <1 | sweet, oily, green |
| 16 | 2-Ethyl-1-hexanol | 1477.5 | 0.012 ± 0.003 b | nf | nf | 0.026 ± 0.001 a | 24.623 ε | <1 | - | - | <1 | citrus, floral, oily, sweet |
| 17 | 2-Nonanol | 1502.8 | 0.018 ± 0.004 a | 0.009 ± 0.001 b | nf | nf | 0.058 ζ | <1 | <1 | - | - | waxy, green, creamy |
| 18 | 1-Octanol | 1544.0 | 0.033 ± 0.007 b | 0.014 ± 0.001 c | 0.02 ± 0.001 c | 0.076 ± 0.002 a | 1.1 δ | <1 | <1 | <1 | <1 | waxy, green, citrus, floral |
| 19 | Trans-2-octen-1-ol | 1604.4 | 0.04 ± 0.011 a | 0.011 ± 0.00 b | 0.02 ± 0.002 b | 0.054 ± 0.001 a | 0.02 ζ | 1.99 | <1 | <1 | 2.69 | green, citrus, fatty |
| 20 | 1-Nonanol | 1648.7 | nf | nf | nf | 0.148 ± 0.006 a | 0.0455 ζ | - | - | - | 3.25 | fatty, floral, rose, orange |
| 21 | 1-Decanol | 1755.6 | 0.025 ± 0.007 a | nf | 0.021 ± 0.001 a | nf | 0.5 ζ | <1 | - | <1 | - | fatty, waxy, floral, orange |
| 22 | Phenylethyl alcohol | 1916.6 | 5.755 ± 2.134 a | 7.4 ± 0.16 a | 9.158 ± 0.603 a | 7.164 ± 1.098 a | 2.6 η | 2.21 | 2.85 | 3.52 | 2.76 | sweet, floral, rose |
| 23 | 1-Dodecanol | 1958.5 | 0.026 ± 0.009 b | 0.013 ± 0.003 b | 0.014 ± 0.002 b | 0.072 ± 0.008 a | 1.001 ζ | <1 | <1 | <1 | <1 | earthy, soapy, waxy, fatty |
| 24 | Farnesol | 2299.6 | 0.016 ± 0.008 b | 0.027 ± 0.003 b | 0.085 ± 0.021 a | nf | 5 ζ | <1 | <1 | <1 | - | fresh, sweet, floral |
| Esters | ||||||||||||
| 25 | Isoamyl acetate | 1117.0 | 0.918 ± 0.498 a | 0.277 ± 0.026 a | 0.157 ± 0.02 a | 0.362 ± 0.001 a | 0.245 β | 3.75 | 1.13 | <1 | 1.48 | sweet, banana, fruity |
| 26 | Ethyl hexanoate | 1226.2 | 0.056 ± 0.012 b | 0.013 ± 0.001 c | 0.023 ± 0.002 c | 0.096 ± 0.005 a | 0.0553 θ | 1.02 | <1 | <1 | 1.74 | sweet, fruity, pineapple |
| 27 | Ethyl heptanoate | 1327.9 | 0.002 ± 0.00 a | nf | 0.001 ± 0.00 b | nf | 13.2 γ | <1 | - | <1 | - | fruity, pineapple, winey |
| 28 | Ethyl lactate | 1343.7 | 0.571 ± 0.06 c | 0.591 ± 0.092 c | 1.168 ± 0.062 a | 0.861 ± 0.088 b | 128.084 δ | <1 | <1 | <1 | <1 | sweet, fruity, acidic |
| 29 | Ethyl caprylate | 1431.0 | 0.102 ± 0.02 a | 0.058 ± 0.004 b | 0.081 ± 0.004 ab | 0.061 ± 0.003 b | 0.147 η | <1 | <1 | <1 | <1 | fruity, winey, waxy, sweet |
| 30 | Isoamyl lactate | 1562.4 | nf | nf | nf | 0.258 ± 0.026 a | nf | - | - | - | - | fruity, creamy, nutty |
| 31 | Ethyl caprate | 1630.0 | 0.062 ± 0.01 b | 0.053 ± 0.009 b | 0.093 ± 0.003 a | 0.05 ± 0.003 b | 1.12 γ | <1 | <1 | <1 | <1 | sweet, waxy, fruity, apple |
| 32 | Phenethyl acetate | 1824.8 | 0.575 ± 0.195 a | 0.647 ± 0.027 a | 0.342 ± 0.009 a | 0.518 ± 0.026 a | 0.908 δ | <1 | <1 | <1 | <1 | floral, rose, sweet, honey |
| 33 | Ethyl laurate | 1847.1 | 0.583 ± 0.052 b | nf | 0.874 ± 0.142 a | nf | 0.4 ε | 1.46 | - | 2.18 | - | sweet, waxy, floral, soapy |
| 34 | Isoamyl decanoate | 1861.5 | 0.018 ± 0.003 bc | 0.027 ± 0.003 b | 0.048 ± 0.009 a | 0.009 ± 0.001 c | >5 ζ | <1 | <1 | <1 | <1 | waxy, banana, fruity, sweet |
| 35 | Ethyl 3-phenylpropionate | 1893.4 | 0.002 ± 0.001 a | nf | nf | nf | 0.013 θ | <1 | - | - | - | rose, honey, fruity |
| 36 | γ-Nonanolactone | 2036.6 | nf | nf | nf | 0.385 ± 0.056 a | 0.0097 θ | - | - | - | 39.67 | coconut, creamy, waxy |
| 37 | Ethyl myristate | 2038.7 | 0.482 ± 0.09 a | 0.459 ± 0.064 a | 0.23 ± 0.041 b | 0.13 ± 0.021 b | 46.606 δ | <1 | <1 | <1 | <1 | sweet, waxy |
| 38 | Ethyl palmitate | 2218.3 | 1.221 ± 0.477 a | 1.522 ± 0.177 a | 1.097 ± 0.171 a | 0.669 ± 0.082 a | 39.299 δ | <1 | <1 | <1 | <1 | waxy, fruity, creamy |
| 39 | Phenethyl octanoate | 2332.8 | 0.04 ± 0.01 a | 0.052 ± 0.006 a | 0.07 ± 0.015 a | 0.043 ± 0.005 a | 10 ζ | <1 | <1 | <1 | <1 | sweet, waxy, green, cocoa |
| Aldehydes | ||||||||||||
| 40 | Hexanal | 1083.9 | 0.059 ± 0.001 a | nf | 0.024 ± 0.002 c | 0.049 ± 0.001 b | 0.0255 θ | 2.33 | - | <1 | 1.93 | green, fatty, fruity |
| 41 | Nonanal | 1395.7 | 0.014 ± 0.003 a | 0.004 ± 0.001 b | 0.006 ± 0.001 b | 0.006 ± 0.00 b | 0.122 δ | <1 | <1 | <1 | <1 | waxy, aldehydic, citrus |
| 42 | Furfural | 1466.2 | 4.833 ± 1.066 a | 4.816 ± 0.203 a | 3.382 ± 0.204 ab | 1.927 ± 0.103 b | 44.029 δ | <1 | <1 | <1 | <1 | sweet, woody, bread |
| 43 | Benzaldehyde | 1525.9 | 0.204 ± 0.05 bc | 0.301 ± 0.029 b | 0.161 ± 0.01 c | 0.455 ± 0.008 a | 4.203 δ | <1 | <1 | <1 | <1 | fruity, powdery, nutty |
| 44 | Trans-2-nonenal | 1531.2 | 0.027 ± 0.001 b | 0.028 ± 0.00 b | 0.027 ± 0.00 b | 0.105 ± 0.002 a | 0.0006 η | 45.80 | 46.28 | 44.22 | 175.20 | fatty, green, cucumber |
| 45 | Phenylacetaldehyde | 1649.0 | 0.33 ± 0.085 ab | 0.47 ± 0.032 a | 0.229 ± 0.006 bc | 0.162 ± 0.009 c | 0.111 β | 2.97 | 4.23 | 2.07 | 1.46 | green, sweet, floral, honey |
| Terpenes | ||||||||||||
| 46 | D-limonene | 1181.5 | 0.027 ± 0.003 b | 0.072 ± 0.015 a | nf | nf | nf | - | - | - | - | citrus, orange, sweet |
| 47 | Styrene | 1253.0 | 0.003 ± 0.002 a | 0.006 ± 0.00 a | 0.007 ± 0.001 a | 0.004 ± 0.00 a | 0.125 ζ | <1 | <1 | <1 | <1 | sweet, balsamic, floral |
| 48 | Linalool | 1534.0 | 0.059 ± 0.023 b | 0.148 ± 0.016 a | 0.024 ± 0.001 b | 0.05 ± 0.001 b | 0.023 β | 2.55 | 6.45 | 1.06 | 2.18 | citrus, floral, sweet, rose |
| 49 | α-terpineol | 1694.6 | 0.034 ± 0.003 a | 0.035 ± 0.001 a | 0.029 ± 0.00 b | 0.032 ± 0.00 ab | 1.96 δ | <1 | <1 | <1 | <1 | floral, terpenic |
| 50 | Citronellol | 1760.8 | 0.779 ± 0.391 bc | 4.103 ± 0.263 a | 0.224 ± 0.008 c | 1.187 ± 0.117 b | 0.1 ζ | 7.79 | 41.03 | 2.24 | 11.87 | floral, rosy, sweet, citrus |
| 51 | β-Damascenone | 1831.0 | 2.431 ± 0.752 a | 0.021 ± 0.00 c | 1.032 ± 0.02 bc | 1.592 ± 0.065 ab | 0.00012 θ | 20,260 | 171 | 8600 | 13,270 | apple, rose, honey, tobacco |
| 52 | Nerol | 1797.9 | 0.065 ± 0.021 b | 0.315 ± 0.011 a | 0.02 ± 0.001 c | 0.07 ± 0.007 b | 0.5 ζ | <1 | <1 | <1 | <1 | citrus, floral, green, sweet |
| 53 | Geraniol | 1843.3 | 0.339 ± 0.129 b | 1.416 ± 0.048 a | nf | 0.286 ± 0.044 b | 0.2 ζ | 1.69 | 7.08 | - | 1.43 | floral, sweet, rosey, fruity |
| 54 | Trans-nerolidol | 2028.4 | 0.059 ± 0.022 a | 0.044 ± 0.008 a | 0.043 ± 0.007 a | 0.078 ± 0.007 a | 1 ζ | <1 | <1 | <1 | <1 | floral, green, citrus, woody |
| Others | ||||||||||||
| 55 | 2,6-Dimethyl-4-heptanone | 1165.2 | 0.336 ± 0.054 a | 0.247 ± 0.025 a | 0.309 ± 0.035 a | 0.207 ± 0.145 a | nf | - | - | - | - | green, fruity, pineapple, banana |
| 56 | 6-Methyl-5-hepten-2-one | 1337.1 | 0.036 ± 0.004 b | 0.035 ± 0.006 b | 0.073 ± 0.004 b | 0.053 ± 0.004 a | 1.008 ζ | <1 | <1 | <1 | <1 | citrus, green, lemongrass, apple |
| 57 | Rose oxide | 1351.5 | 0.019 ± 0.005 bc | 0.061 ± 0.008 a | 0.003 ± 0.00 c | 0.024 ± 0.001 b | nf | - | - | - | - | green, rose, spicy |
| 58 | Geranylacetone | 1858.1 | 0.01 ± 0.004 a | 0.005 ± 0.001 a | 0.008 ± 0.001 a | 0.01 ± 0.001 a | nf | - | - | - | - | rose, leafy, floral, green |
| 59 | Methyleugenol | 2010.4 | 0.025 ± 0.01 bc | 0.083 ± 0.006 a | 0.007 ± 0.001 c | 0.044 ± 0.005 b | 10 ζ | <1 | <1 | <1 | <1 | spicy, cinnamon, clove |
| 60 | Octanoic acid | 2046.6 | nf | nf | nf | 1.815 ± 0.144 a | 2.7 γ | - | - | - | <1 | fatty, waxy, oily |
| 61 | Decanoic acid | 2233.8 | nf | nf | nf | 1.101 ± 0.126 a | 2.8 α | - | - | - | <1 | sour, fatty, citrus |
| 62 | 2,4-Di-tert-butylphenol | 2262.5 | 0.01 ± 0.004 b | nf | nf | 0.018 ± 0.001 a | 0.373 ε | <1 | - | - | <1 | nf |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanikawa, A. Flavors in Malt Whisky: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 260–278. [Google Scholar] [CrossRef]
- Ashmore, P.L.; DuBois, A.; Tomasino, E.; Harbertson, J.F.; Collins, T.S. Impact of Dilution on Whisky Aroma: A Sensory and Volatile Composition Analysis. Foods 2023, 12, 1276. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.J.; Ochoa, A.; Kerth, C.R.; Miller, R.K.; Murray, S.C. Assessing the impact of corn variety and Texas terroir on flavor and alcohol yield in new-make bourbon whiskey. PLoS ONE 2019, 14, e0220787. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Stern, D.J.; Ling, L.C. Studies on Flavor Volatiles of Some Sweet Corn Products. J. Agric. Food Chem. 1994, 42, 791–795. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Stern, D.J. Studies on Popcorn Aroma and Flavor Volatiles. J. Agric. Food Chem. 1997, 45, 837–843. [Google Scholar] [CrossRef]
- Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K.N. The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods 2021, 10, 443. [Google Scholar] [CrossRef]
- Mehfooz, T.; Ali, T.M.; Hasnain, A. Effect of Cross-Linking on Characteristics of Succinylated and Oxidized Barley Starch. J. Food Meas. Charact. 2019, 13, 1058–1069. [Google Scholar] [CrossRef]
- Gibson, B.R.; Boulton, C.A.; Box, W.G.; Graham, N.S.; Lawrence, S.J.; Linforth, R.S.; Smart, K.A. Carbohydrate Utilization and The Lager Yeast Transcriptome During Brewery Fermentation. Yeast 2008, 25, 549–562. [Google Scholar] [CrossRef]
- Smith, B.C.; Sester, C.; Ballester, J.; Deroy, O. The Perceptual Categorisation of Blended and Single Malt Scotch Whiskies. Flavour 2017, 6, 5. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Al-Karaki, G.N.; Hammouri, M.K. Variability of Some Physico-Chemical Characteristics of Wheat Cultivars Grown under Arid and Semiarid Mediterranean Conditions. Int. J. Food Prop. 2001, 4, 91–101. [Google Scholar] [CrossRef]
- Morris, S.; Byrne, J.L.; Murphy, B.; Whelan, S.J.; Carroll, J.P.; Ryan, D. The Optimisation of Cooking Parameters for Spirt Whiskey Production from Native Irish Wheat: A Response Surface Method Approach. Foods 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, P.; Wardencki, W. Volatile Composition of Raw Spirits of Different Botanical Origin. J. Inst. Brewing 2012, 118, 393–400. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Feng, S.; Ma, X.; Wang, S.; Zhang, Y. Correlation between Microbe, Physicochemical Properties of Jiuqu in Different Plateau Areas and Volatile Flavor Compounds of Highland Barley Alcoholic Drink. Food Biosci. 2023, 51, 102276. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, Y.; Xu, Q.; Mascher, M.; Guo, G.; Li, S.; Mao, L.; Liu, Q.; Xia, Z.; Zhou, J.; et al. Origin and Evolution of Qingke Barley in Tibet. Nat. Commun. 2018, 9, 5433. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.L.; An, Y.; Chen, S.; Qian, M.C. Characterization of Qingke Liquor Aroma from Tibet. J. Agric. Food Chem. 2019, 67, 13870–13881. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-L.; Wang, D.-L.; Zhang, W.-J.; Jia, S.-R. The Production of The Chinese Baijiu from Sorghum and Other Cereals. J. Inst. Brewing 2017, 123, 600–604. [Google Scholar] [CrossRef]
- Konfo, C.T.R.; Chabi, N.W.; Dahouenon-Ahoussi, E.; Cakpo-Chichi, M.; Soumanou, M.M.; Sohounhloue, D.C.K. Improvement of African Traditional Sorghum Beers Quality and Potential Applications of Plants Extracts for Their Stabilization: A Review. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 190–196. [Google Scholar] [CrossRef]
- Munezero, E.; Imathiu, S.; Bikoro, M. Effects of Varying Grilled Sorghum Content on the Quality Parameters of Urwagwa, a Traditional Rwandese Banana-based Alcoholic Beverage. J. Food Res. 2018, 7, 6. [Google Scholar] [CrossRef]
- Boudries, N.; Belhaneche, N.; Nadjemi, B.; Deroanne, C.; Mathlouthi, M.; Roger, B.; Sindic, M. Physicochemical and functional Properties of Starches from Sorghum Cultivated in The Sahara of Algeria. Carbohyd. Polym. 2009, 78, 475–480. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Genisheva, Z.; Nunes, J.A.R.; Duarte, W.F. Production and Characterization of a New Sweet Sorghum Distilled Beverage. Sugar Tech. 2019, 21, 966–975. [Google Scholar] [CrossRef]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- GB/T 10220-2012; Sensory Analysis—Methodology—General Guidance. Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Camara, J.S.; Marques, J.C.; Perestrelo, R.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative Study of The Whisky Aroma Profile Based on Headspace Solid Phase Microextraction Using Different Fibre Coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Schieberle, P. Characterization of the Most Odor-Active Compounds in An American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zheng, X.P.; Song, P.; Sun, Z.L.; Tian, T.T. Characterization of Volatiles in the Six Most Well-Known Distilled Spirits. J. Am. Soc. Brew. Chem. 2018, 71, 161–169. [Google Scholar] [CrossRef]
- Buglass, A.J. Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects; John Wiley & Sons: Chichester, UK, 2011; Volume 1, pp. 455–627. [Google Scholar]
- Boothroyd, E.; Linforth, R.S.T.; Jack, F.; Cook, D.J. Origins of the Perceived Nutty Character of New-Make Malt Whisky Spirit. J. Inst. Brewing 2014, 120, 16–22. [Google Scholar] [CrossRef]
- Sherman, E.; Harbertson, J.F.; Greenwood, D.R.; Villas-Bôas, S.G.; Fiehn, O.; Heymann, H. Reference Samples Guide Variable Selection for Correlation of Wine Sensory and Volatile Profiling Data. Food Chem. 2018, 267, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between Volatile Composition and Sensory Properties in Spanish Albariño Wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Li, T.; Zhao, M.; Yang, J.; Chuai, Q.; Raza, A.; Yang, P.; Yu, M.; Hu, D.; Zou, T.; Song, H. Characterization of Key Aroma-Active Compounds in Bobaizhi (Angelica dahurica) before and after Boiling by Sensomics Approach. J. Food Compos. Anal. 2022, 105, 104247. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Commercial Rums by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Bartlett Pear Brandies by Means of the Sensomics Concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Liu, M.; Shen, C.; Sun, X.; Zhao, M.; Sun, J.; Li, H.; Zheng, F.; Huang, M.; et al. Characterization of Key Odorants Causing the Roasted and Mud-Like Aromas in Strong-Aroma Types of Base Baijiu. Food Res. Int. 2019, 125, 108546. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Luo, S.; Zhang, J.; Huang, M.; Chen, F.; Zheng, F.; Sun, X.; Li, H. Characterization of Key Aroma Compounds in Meilanchun Sesame Flavor Style Baijiu by Application of Aroma Extract Dilution Analysis, Quantitative Measurements, Aroma Recombination, and Omission/Addition Experiments. RSC Adv. 2018, 8, 23757–23767. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Y.; Wu, J.; Yu, B.; Zhao, H.; Huang, M.; Zheng, F. Sensory-Directed Decoding of Key Aroma Compounds from Jiugui-Series Baijiu, the Representative of Fuyu-Flavor-Type Baijiu (FFTB). J. Food Compos. Anal. 2022, 114, 104799. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Odour Thresholds_Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Poisson, L.; Schieberle, P. Characterization of the Key Aroma Compounds in an American Bourbon Whisky by Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2008, 56, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Wang, D.; Li, Y.; Li, J.; Du, J. Comparison of Aroma Profiles of Whiskeys Fermented from Different Grain Ingredients. Foods 2024, 13, 2031. https://doi.org/10.3390/foods13132031
Guo S, Wang D, Li Y, Li J, Du J. Comparison of Aroma Profiles of Whiskeys Fermented from Different Grain Ingredients. Foods. 2024; 13(13):2031. https://doi.org/10.3390/foods13132031
Chicago/Turabian StyleGuo, Siqian, Dan Wang, Yanting Li, Jingming Li, and Jinkun Du. 2024. "Comparison of Aroma Profiles of Whiskeys Fermented from Different Grain Ingredients" Foods 13, no. 13: 2031. https://doi.org/10.3390/foods13132031
APA StyleGuo, S., Wang, D., Li, Y., Li, J., & Du, J. (2024). Comparison of Aroma Profiles of Whiskeys Fermented from Different Grain Ingredients. Foods, 13(13), 2031. https://doi.org/10.3390/foods13132031






