Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata)
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of the NADESs
2.2. Characterization of the NADESs
2.2.1. Fourier Transform Infra-Red (FTIR)
2.2.2. Raman Spectroscopy
2.2.3. pH Determination
2.2.4. Viscosity and Density
2.3. Determinations of the Characteristics of the Raw Material
2.4. Sample Preparation
2.5. Process of Extraction
2.5.1. Extraction by Using NADESs
Ultrasonic Bath-Assisted Extraction
NADES Ultrasound Tip Extraction
2.5.2. Conventional Extraction
2.5.3. Yield of the Process
2.6. Characterization of the Extract
2.6.1. Total Phenolic Compounds (TPC)
2.6.2. Antioxidant Activity
3. Results and Discussion
3.1. Characterization of the NADESs
3.1.1. Density and Viscosity
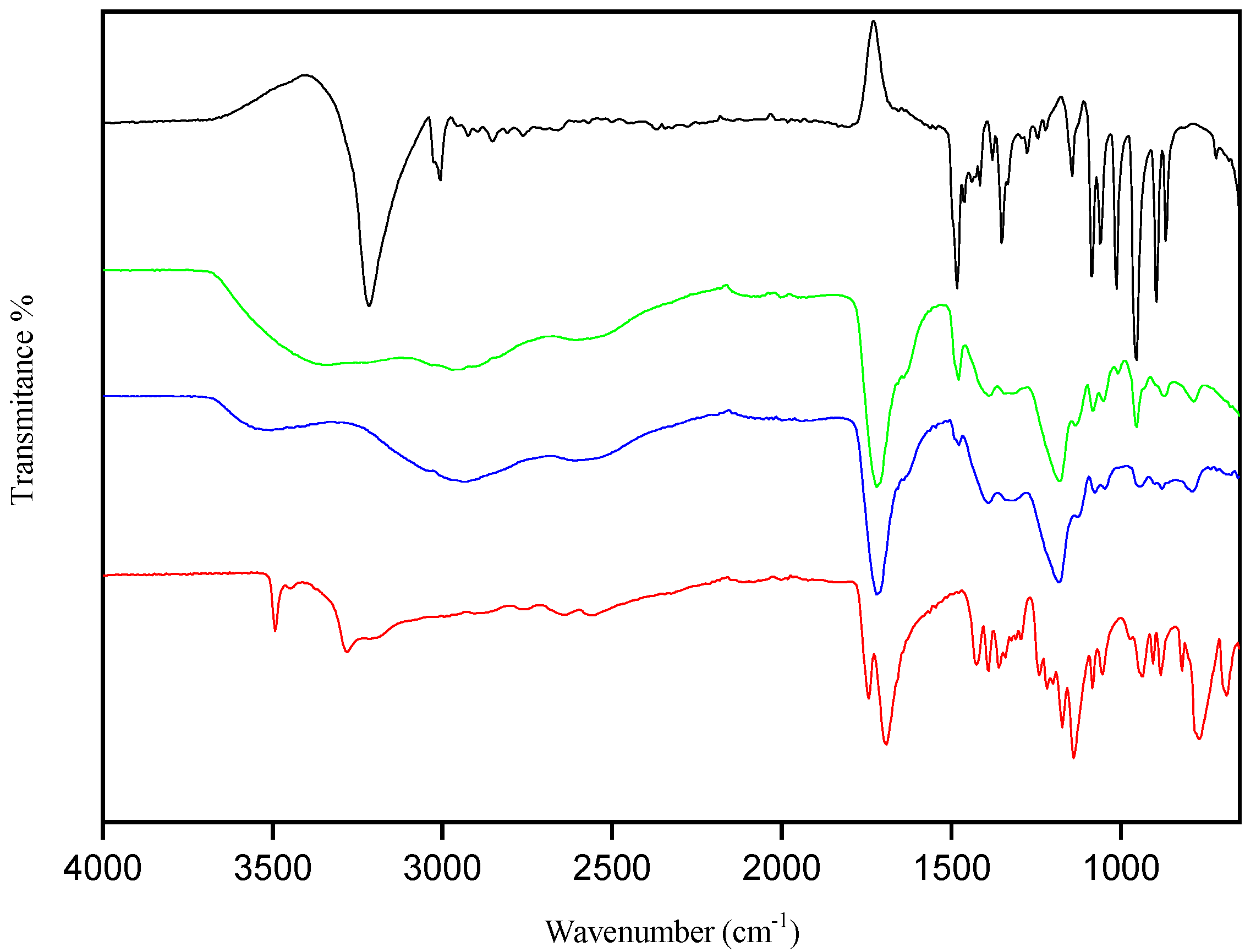
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
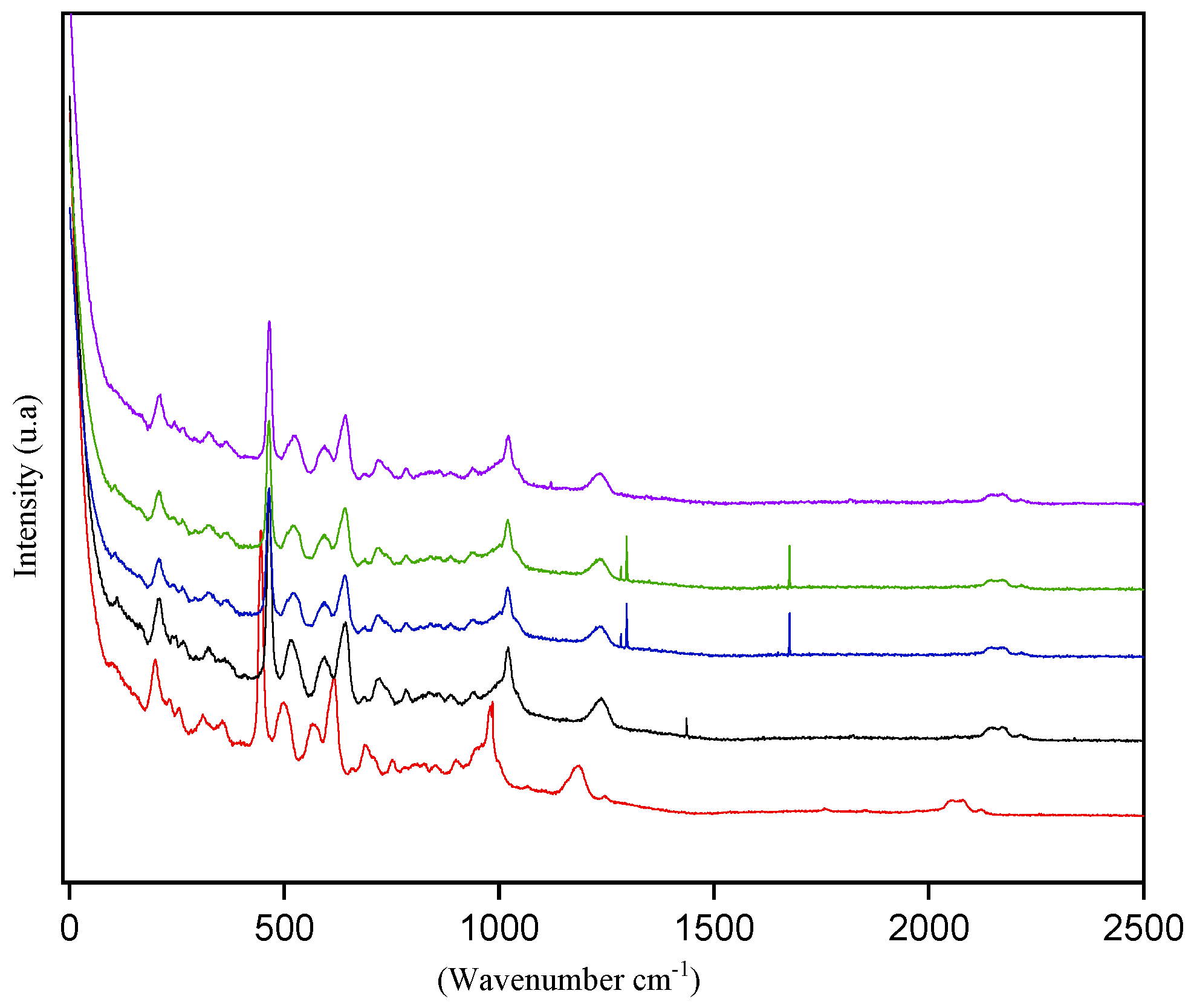
3.1.3. Raman Spectroscopy
3.1.4. pH Determination
3.2. Properties of the Raw Material
Total Phenolic Content and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Razborzec, M.I. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crops. Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.; Carvalho, J.E.; Ruiz, A.L.; Maróstica, M.R., Jr.; Pastore, G.M. Evaluation of the antioxidant, antiproliferative and antimutagenic potential of araçá-boi fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; Silva, W.P. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- Dupuy, N. Chimiometrie en Spectrometrie Infrarouge. Memoire Presenté à l’Université des Sciences et Technologie de Lille pour Obtenir l’habilitation a Diriger les Recherches; Université de Lille: Lille, France, 1997; 90p. [Google Scholar]
- Pertuzatti, P.B.; Barcia, M.T. Biotransformation in temperate climate fruit: A focus on berries. Am. J. Food Sci. Technol. 2015, 3, 12–17. [Google Scholar]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant, and antiinflammatory phenolic activities in extra virgin olive oil. Cur. Opin. Biotech. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Thakur, K.; Chen, G.-H.; Hu, F.; Zhang, J.-G.; Zhang, H.-B.; Wei, Z.-J. Metabolic effect of 1-Deoxynojirimycin from mulberry leaves on db/db diabetic mice using liquid chromatography–mass spectrometry-based metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef]
- Chang, Y.C.; Yang, M.Y.; Chen, S.C.; Wang, C.J. Mulberry leaf polyphenol extract improves obesity by inducing adipocyte apoptosis and inhibiting preadipocyte differentiation and hepatic lipogenesis. J. Funct. Foods 2016, 21, 249–262. [Google Scholar] [CrossRef]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthongngam, L.; Ratanachamnong, P.; Srisawat, S.; Pongrapeeporn, K.U.S. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam leaves. J. Ethnopharm. 2008, 116, 439–446. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson Neto, J.A.C.C. Antioxidant activities and antitumor screening of extracts from Cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidalvalverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid-state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 325, 113761. [Google Scholar] [CrossRef]
- Choi, Y.H.; Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chem. 2022, 370, 131042. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a natural deep eutectic solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, M.; Kashyap, H.K. Microstructure of deep eutectic solvents: Current understanding and challenges. J. Phys. Chem. B 2020, 124, 10601–10616. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Lubben, M.J.; Winnert, J.M.; Leiva, A.; Brennecke, J.F.; Canales, R.I. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol. J. Chem. Thermodyn. 2019, 133, 272–284. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromat. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, C.; Zhang, C.; Xue, Z.; Zheng, Y.; Guo, L. Green extraction of phenolic acids from Artemisia argyi leaves by tailor-made ternary deep eutectic solvents. Molecules 2019, 24, 2842. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef]
- Manu, A.; Cardano, F.; Fin, A.; Baldino, S.; Prandi, C. Choline chloride-based ternary deep band gap systems. J. Mol. Liq. 2021, 330, 115717. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.-L.; Li, P.; Liu, E.-H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Chaves, E.S.; Vialich, J.; Saueriv, E. Ultrasound-assisted extraction from chocolate powder samples for the determination of Fe, K and Na. Braz. J. Food Technol. 2014, 17, 236–242. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Hu, H.; Wan, J.; Li, P. Natural deep eutectic solvents for simultaneous extraction of multi-bioactive components from jinqi jiangtang preparations. Pharmaceutics 2019, 11, 18. [Google Scholar] [CrossRef]
- Paniċ, M.; Radic Stojkovic, M.; Kraljic, K.; Skevin, D.; Radojcic Redovnikovic, I.; Gaurina Srcek, V.; Radosevic, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Ivanoviċ, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Wei, Z.F.; Wang, X.Q.; Peng, X.; Wang, W.; Zhao, C.J.; Zu, Y.G.; Fu, Y.J. Fast and green extraction and separation of main bioactive flavonoids from Radix scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH behaviour of seventeen deep eutectic solvents. BioResources 2019, 13, 5042–5051. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants means of Folin Ciocalteu reagent. Methods Enzymol. 2021, 299, 152–178. [Google Scholar]
- Kuskosvi, E.M.; Asuero, A.G.; García-Parilla, M.C.; Troncoso, A.M.; Fett, R. Actividad antioxidante de pigmentos antociánicos. Ciênc. Tecnol. Aliment. 2004, 24, 691–693. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Curko, N.; Tomasevic, M.; Kovacevic, K.; Ganic, K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 211, 159–166. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; Rodrigues, A.M.C.; Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules 2022, 27, 8310. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2016, 102, 195–203. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach Encyclopedia of Analytical Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Silva, D.T.; Pauletto, R.; Cavalheiro, S.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Comp. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Glucose and fructose hydrates in aqueous solution by IR spectroscopy. J. Phys. Chem. A 2007, 111, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D. Formation of type III deep eutectic solvents and effect of water on their intermolecular interactions. Fluid Phase Equilibria 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, Y.; Ji, X.; Zhu, W.; Lu, L.; Lu, X. Effect of water concentration on the microstructures of choline chloride/urea (1, 2)/water mixture. Fluid Phase Equilibria 2018, 470, 134–139. [Google Scholar] [CrossRef]
- Bichara, L.C.; Lanús, H.E.; Ferrer, E.G.; Gramajo, M.B.; Brandán, S.A. Vibrational Study and Force Field of the Citric Acid Dimer Based on the SQM Methodology. Adv. Phys. Chem. 2011, 2011, 347072. [Google Scholar] [CrossRef]
- Baranska, H.; Kuduk-Jaworska, J.; Szostak, R.; Romaniewska, A. Vibrational spectra of racemic and enantiomeric malic acids. J. Raman Spectrosc. 2003, 34, 68–76. [Google Scholar] [CrossRef]
- Ruggiero, L.; Sodo, A.; Bruni, F.; Ricci, M.A. Hydration of monosaccharides studied by Raman scattering. J. Raman Spectrosc. 2018, 49, 1066–1075. [Google Scholar] [CrossRef]
- Song, C.; Fan, W.-H.; Ding, L.; Chen, X.; Chen, Z.-Y.; Wang, K. Terahertz and infrared characteristic absorption spectra of aqueous glucose and fructose solutions. Sci. Rep. 2018, 8, 8964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Parikh, B.S.; Shah, E.; Liu, L.Z.; Cotta, M.A. Cellulosic ethanol production from green solvent-pretreated rice straw. Biocatal. Agric. Biotechnol. 2016, 7, 14–23. [Google Scholar] [CrossRef]
- Hu, S.Q.; Zhang, Z.F.; Zhou, Y.X.; Song, J.L.; Fan, H.L.; Han, B.X. Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids. Green Chem. 2009, 11, 873–877. [Google Scholar] [CrossRef]
- Tang, B.; Park, H.E.; Row, K.H. Simultaneous extraction of flavonoids from Chamaecyparis obtusa using deep eutectic solvents as additives of conventional extractions solvents. J. Chrom. Sci. 2015, 53, 836–840. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Kareem, M.A.K.; Mjalli, F.S.; Hashim, M.A.; Al Nashef, I.M. Phosphoniumbased ionic liquids analogues and their physical properties. J. Chem. Eng. Data 2010, 55, 4632–4637. [Google Scholar] [CrossRef]
- Canuto, G.A.B.; Xavier, A.A.O.; Neves, L.C.; Benassi, M.T. Caracterização físicoquímica de polpas de frutos da Amazônia e sua correlação com a atividade antiradical livre. Rev. Bras. Frutic. 2010, 32, 1196–1205. [Google Scholar] [CrossRef]
- Araújo, F.F.; Farias, D.P.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Souza, F.G.; Catharino, R.R.; Sacramento, C.K.; Pastore, G.M. Chemical characterization of Eugenia stipitata: A native fruit from the Amazon rich in nutrients and source of bioactive compounds. Food Res. Int. 2021, 129, 109904. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.P.; Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]




| Hydrogen Donor | Source | Bioactive Compound | Reference |
|---|---|---|---|
| MA | Carya cathayensis Sarg | Polyphenols | [19] |
| Fruc, MA | Cherry pomace | Polyphenols | [35] |
| CA | Grape and olive pomace | Polyphenols | [33] |
| Gluc, Fruc | Lippia citriodora | Polyphenols | [36] |
| CA, Gluc, MA | C. cajan leaves | Polyphenols | [37] |
| LA, MA, Gluc, Fruc | Helichrysum arenarium L. | Polyphenols | [1] |
| Hydrogen Bond Acceptor (HBA) | Hydrogen Bond Donor (HBD) | Molar Ratio | Water * (%) |
|---|---|---|---|
| Choline chloride | Fructose | 5:2 | 0–40 |
| Choline chloride | Glucose | 5:2 | 0–40 |
| Choline chloride | Malic acid | 1:1 | 0–40 |
| Choline chloride | Citric acid | 1:1 | 0–40 |
| NADESs | ρ (g mL−1) | µ Pa s |
|---|---|---|
| ChCl:Fruc | 1.277 ± 0.054 | 0.24777 |
| ChCl:Gluc | 1.234 ± 0.002 | 0.2723 |
| ChCl:MA | 1.281 ± 0.003 | 0.09991 |
| ChCl:CA | 1.375 ± 0.020 | 0.46921 |
| * Ethanol | 0.783 | 0.0008 |
| NADESs * | pH |
|---|---|
| ChCl:Fruc | 7.10 |
| ChCl:Gluc | 7.38 |
| ChCl:MA | 2.45 |
| ChCl:CA | 2.34 |
| NADES | Ultrasound Bath | Ultrasound Tip | ||||
|---|---|---|---|---|---|---|
| TPC | ABTS | DPPH | TPC | ABTS | DPPH | |
| NADESs 20% | ||||||
| ChCl:Fruc | 161.99 ± 3.06 | 10.2 ± 0.20 | 73.8 ± 8.18 | ND | ND | ND |
| ChCl:Gluc | 88.51 ± 3.48 | 10.8 ± 0.26 | 78.9 ± 5.56 | ND | ND | ND |
| ChCl:MA | 177.43 ± 1.07 | 45.93 ± 0.75 | 148.9 ± 1.49 | ND | ND | ND |
| ChCl:CA | 173.15 ± 2.73 | 29.37 ± 0.73 | 96.9 ± 2.0 | ND | ND | ND |
| NADESs 40% | ||||||
| ChCl:Fruc | 48.50 ± 0.83 | 13.85 ± 1.4 | 1.18 ± 0.15 | 49.61 ± 0.49 | 157.06 ± 8.18 | 25.19 ± 0.40 |
| ChCl:Gluc | 17.40 ± 3.66 | 14.18 ± 2.1 | 1.23 ± 0.12 | 23.96 ± 4.83 | 160.0 ± 5.56 | 15.66 ± 0.58 |
| ChCl:MA | 206.81 ± 1.83 | 29.14 ± 1.8 | 8.72 ± 0.34 | 183.76 ± 7.73 | 173.56 ± 9.68 | 29.39 ± 0.43 |
| ChCl:CA | 225.26 ± 2.75 | 61.10 ± 0.9 | 14.20 ± 0.20 | 273.40 ± 13.09 | 204.9 ± 12.89 | 31.55 ± 0.43 |
| Conventional | ||||||
| Ethanol:water (70:30) | 325.19 ± 7.38 | 291.31 ± 8.4 | 12.00 ± 0.4 | |||
| Freeze-dried pulp | ||||||
| Araza pulp | 484.25 ± 10.99 | 72.78 ± 1.69 | 1.18 ± 0.13 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, Y.C.N.; Pires, I.V.; Ferreira, N.R.; Moreira, S.G.C.; Silva, L.H.M.d.; Rodrigues, A.M.d.C. Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata). Foods 2024, 13, 1983. https://doi.org/10.3390/foods13131983
Sakurai YCN, Pires IV, Ferreira NR, Moreira SGC, Silva LHMd, Rodrigues AMdC. Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata). Foods. 2024; 13(13):1983. https://doi.org/10.3390/foods13131983
Chicago/Turabian StyleSakurai, Yasmin Caroline Nóvoa, Ianê Valente Pires, Nelson Rosa Ferreira, Sanclayton Geraldo Carneiro Moreira, Luiza Helena Meller da Silva, and Antonio Manoel da Cruz Rodrigues. 2024. "Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata)" Foods 13, no. 13: 1983. https://doi.org/10.3390/foods13131983
APA StyleSakurai, Y. C. N., Pires, I. V., Ferreira, N. R., Moreira, S. G. C., Silva, L. H. M. d., & Rodrigues, A. M. d. C. (2024). Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata). Foods, 13(13), 1983. https://doi.org/10.3390/foods13131983







