Chemical Characterisation of New Oils Extracted from Cañihua and Tarwi Seeds with Different Organic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds Sampling
2.2. Chemicals
2.3. Oils Extraction
2.4. Antioxidant Capacity
2.5. Total Phenolic Compounds
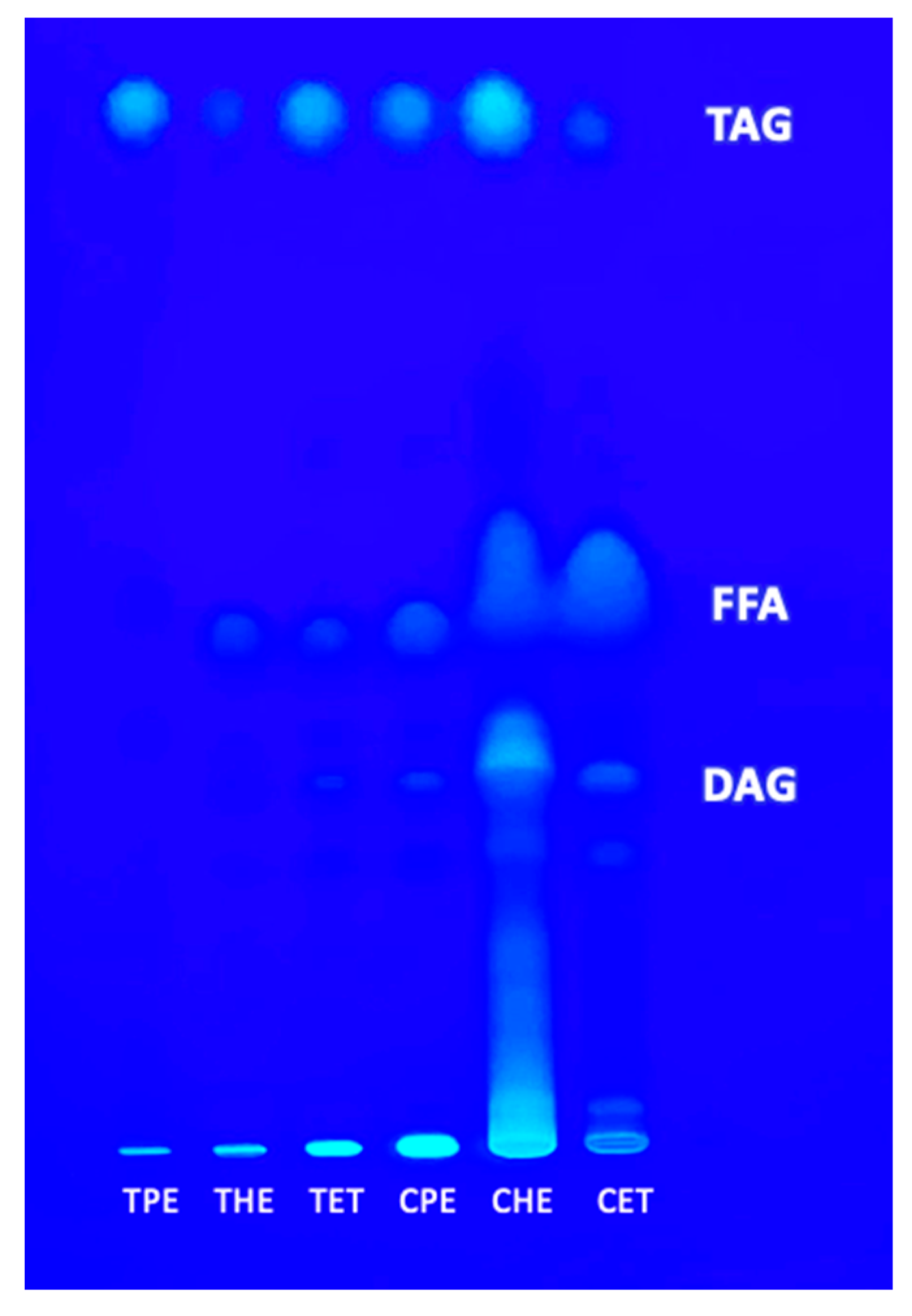
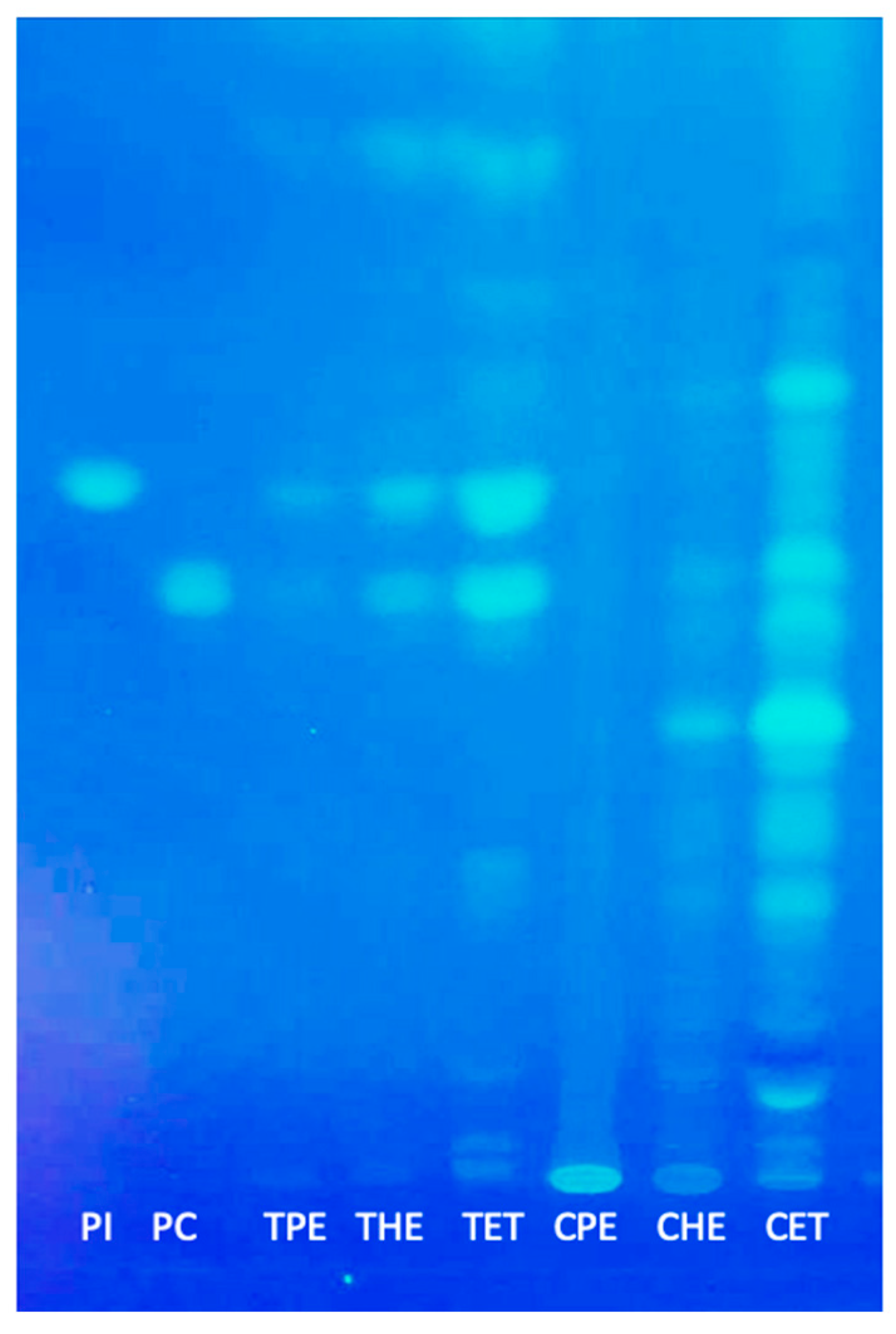
2.6. High-Performance Thin-Layer Chromatography (HPTLC)
2.7. Fatty Acid Profile Analysis
2.8. Determination of Tocopherols
2.8.1. Saponification of Extracted Oils
2.8.2. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Oils Extraction
| Sample | Content of Oil (%) | Solvent | Reference |
|---|---|---|---|
| Tarwi—BO | 18.31 ± 0.44 | Hexane | Present work |
| Tarwi—PE | 19.38 ± 0.32 | Petroleum ether | [30] |
| Tarwi—EC | 18.3 ± 2.1 | Hexane | [31] |
| Cañihua—BO | 6.73 ± 0.29 | Hexane | Present work |
| Cañihua—AM | 6.15 ± 0.76 | Petroleum ether | [28] |
| Cañihua—PE | 8.50 ± 0.36 | Hexane | [27] |
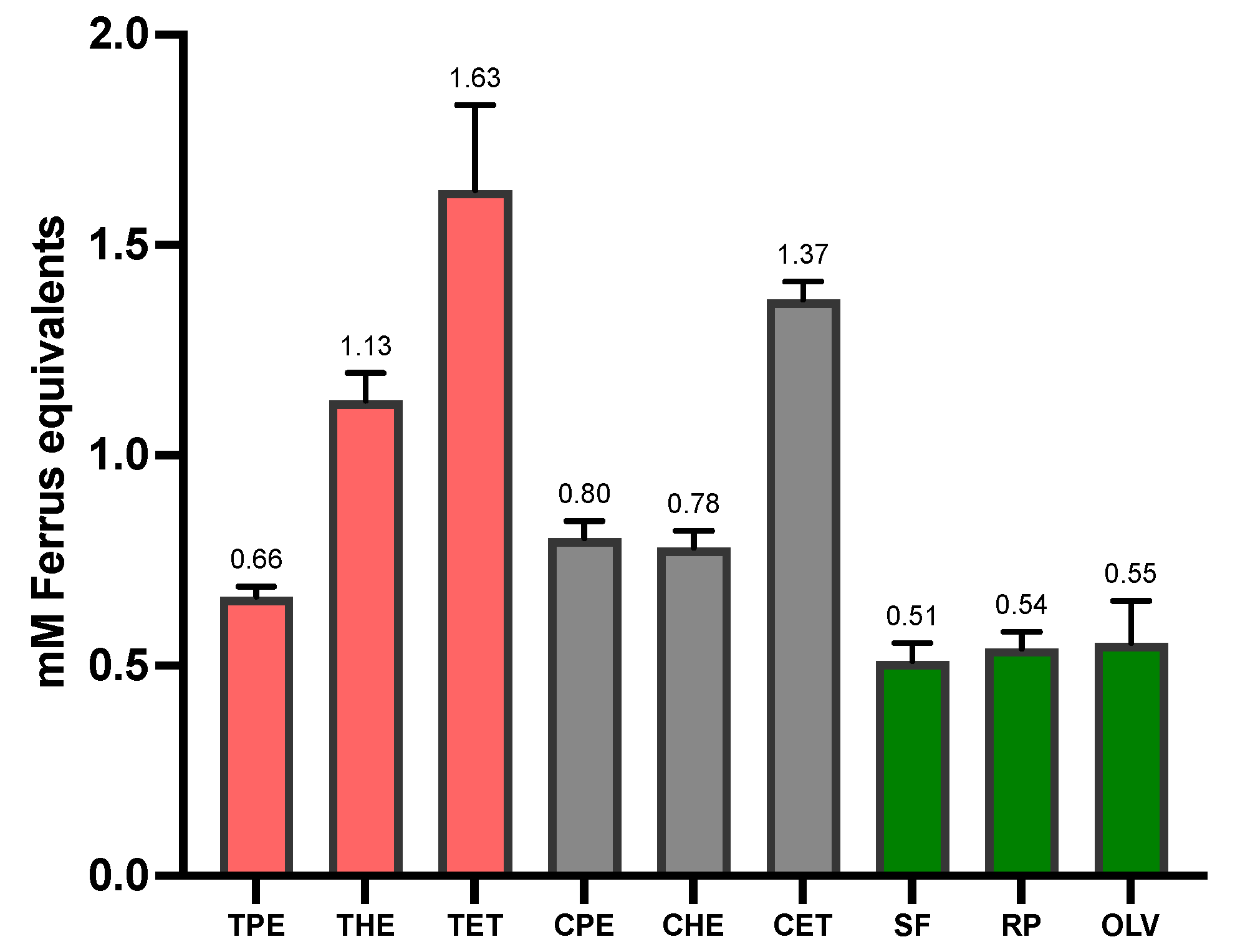
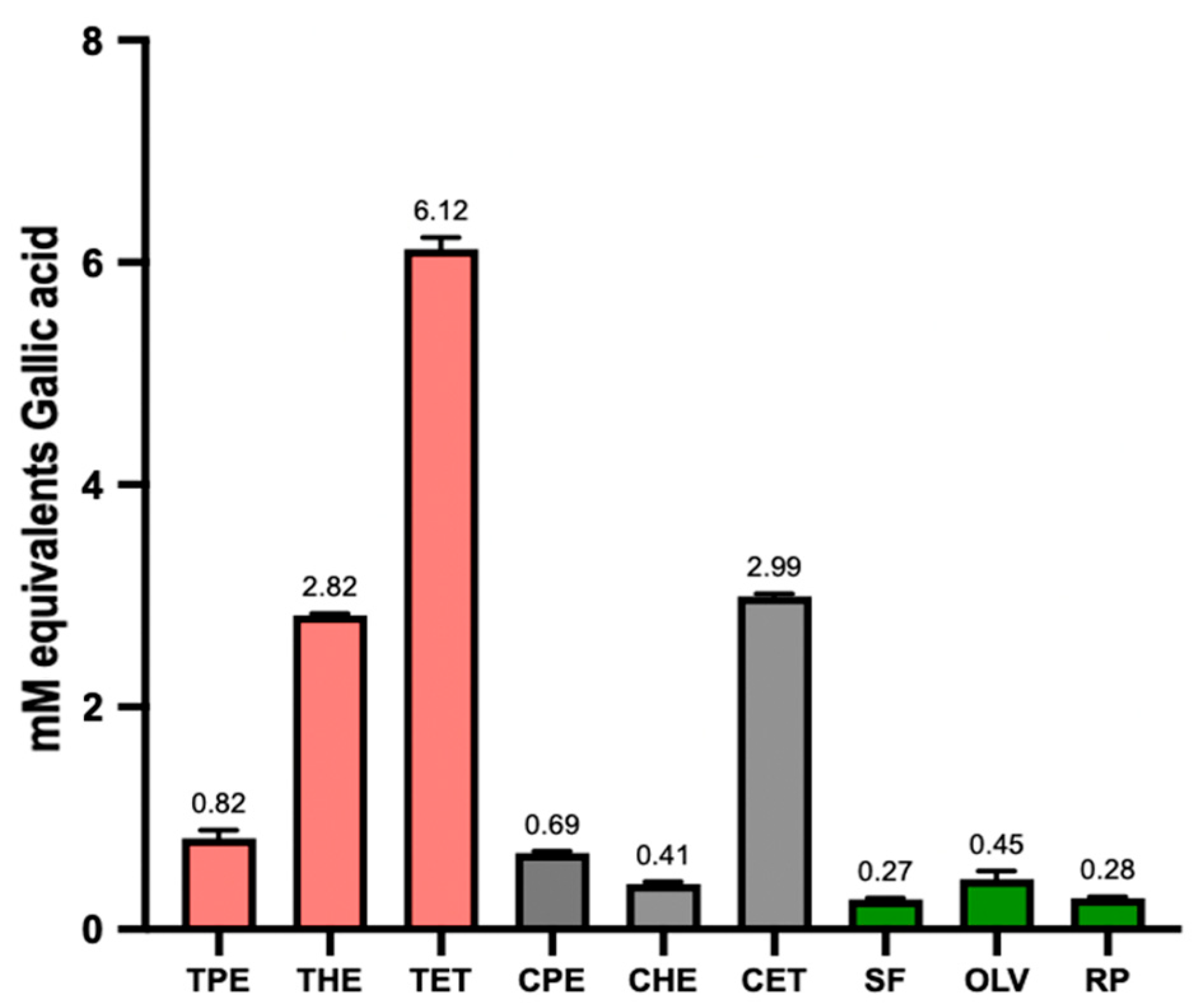
3.2. Total Antioxidant Capacity (TAC) and Total Phenolic Content (TPC)
3.3. Polar and Neutral Lipids
3.4. Fatty Acid Profile
3.4.1. Fatty Acid Composition of Tarwi Oils
3.4.2. Fatty Acid Composition of Cañihua Olis
3.4.3. Comparison of the Fatty Acid Compositions of Tarwi and Cañihua Oils with Commercial Oils
3.5. Tocopherols
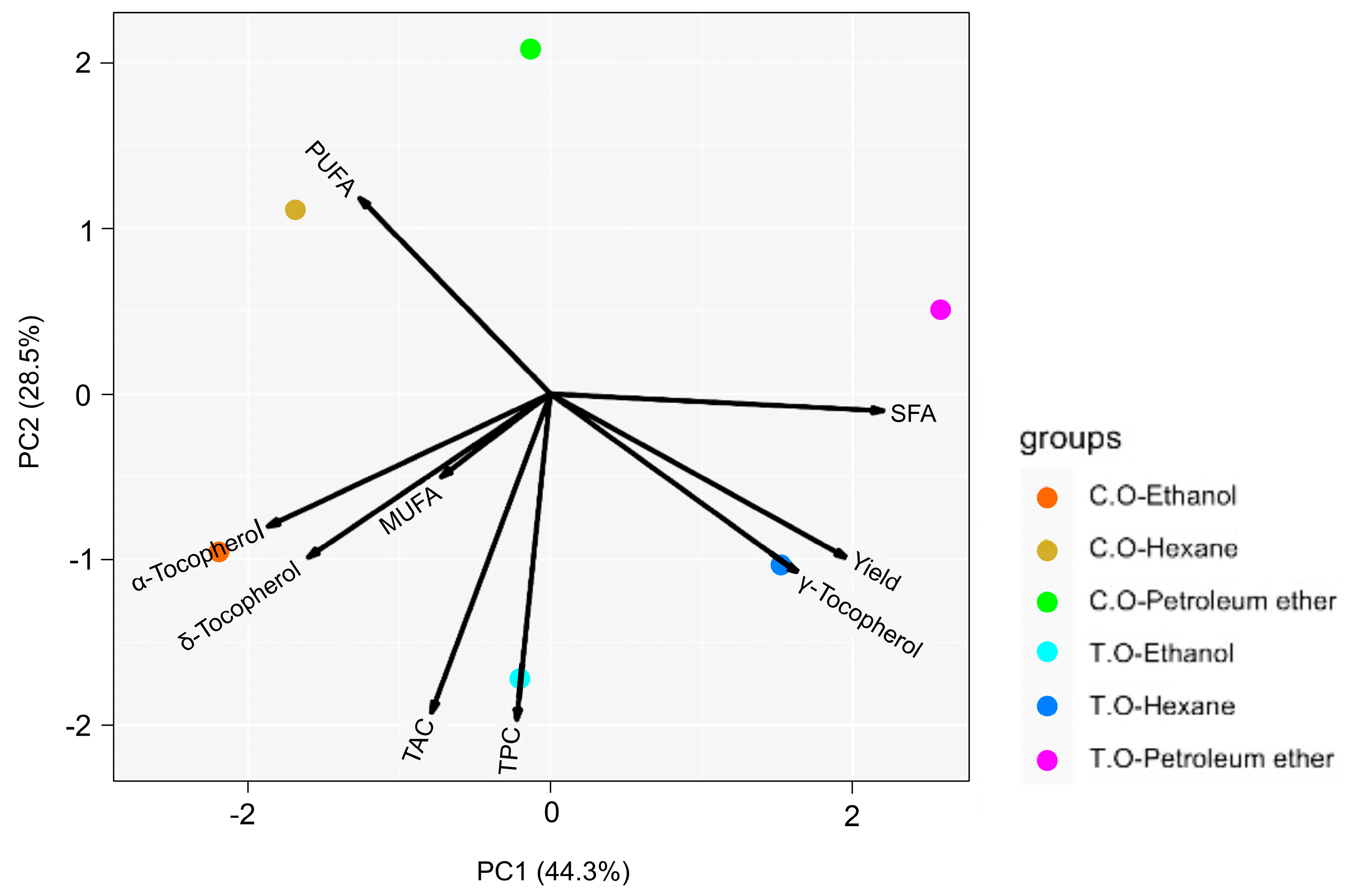
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Cordova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Compos. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Gunstone, F. Vegetable Oils in Food Technology: Composition, Properties and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jones, P.J.H.; Papamandjaris, A.A. Lipids: Cellular Metabolism. In Present Knowledge in Nutrition; Wiley and Blackway: Hoboken, NJ, USA, 2012; pp. 132–148. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R. Nutritional Value and Bioactive Compounds in Andean Ancient Grains. Proceedings 2020, 53, 1. [Google Scholar] [CrossRef]
- Bustos, M.C.; Ramos, M.I.; Pérez, G.T.; León, A.E. Utilization of Kañawa (“Chenopodium pallidicaule”Aellen) Flour in Pasta Making. J. Chem. 2019, 2019, 4385045. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Upadhyaya, K.C. Vegetable Oil: Nutritional and Industrial Perspective. Curr. Genomics 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. New insights into chemical compositions and health promoting effects of edible oils from new resources. Food Chem. 2021, 364, 130363. [Google Scholar] [CrossRef]
- Prins, U.; van Haren, R. Andean Lupin: (Lupinus mutabilis): Cropping and Its Opportunities for Europe; Hanzehogeschool Groningen: Groningen, The Netherlands, 2019. [Google Scholar]
- Ferchichi, N.; Toukabri, W.; Vrhovsek, U.; Nouairi, I.; Angeli, A.; Masuero, D.; Mhamdi, R.; Trabelsi, D. Proximate composition, lipid and phenolic profiles, and antioxidant activity of different ecotypes of Lupinus albus, Lupinus luteus and lupinus angustifolius. J. Food Meas. Charact. 2021, 15, 1241–1257. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Vidaurre-Ruiz, J.M. Biology and Biotechnology of Quinoa; Springer: Singapore, 2021; pp. 243–264. [Google Scholar]
- Luque de Castro, M.D.; García Ayuso, L.E. Enviromental Applications | Soxhlet Extraction. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 2701–2709. [Google Scholar]
- Cerretani, L.; Bendini, A. Chapter 67—Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 625–635. [Google Scholar]
- Tejeda, L.; Vasquez Iriarte, P.E.; Ortiz, J.V.; Aliaga-Rossel, E.; Mollinedo, P.; Peñarrieta, J.M. Characterization of Bolivian chili peppers; antioxidant capacity, total phenolic compounds, capsaicin and β-carotene concentration. Nutr. Food Sci. 2022, 52, 1314–1327. [Google Scholar] [CrossRef]
- Norlander, S.; Dahlgren, L.; Sardari, R.R.R.; Marmon, S.; Tullberg, C.; Nordberg Karlsson, E.; Grey, C. Effect of kilning on the macronutrient composition profile of three Swedish oat varieties. Cereal Chem. 2024, 101, 382–396. [Google Scholar] [CrossRef]
- Lindberg Yilmaz, J.; Adlercreutz, P.; Tullberg, C. Polar Lipids Reduce In Vitro Duodenal Lipolysis Rate of Oat Oil and Liquid Oat Base Products. Eur. J. Lipid Sci. Technol. 2021, 123, 2000317. [Google Scholar] [CrossRef]
- Gimeno, E.; Calero, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; de la Torre, M.C.; López-Sabater, M.C. Simultaneous determination of α-tocopherol and β-carotene in olive oil by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 881, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-tocopherol) levels in plant oils. Flavour Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Li, Y.; Fine, F.; Fabiano-Tixier, A.-S.; Abert-Vian, M.; Carre, P.; Pages, X.; Chemat, F. Evaluation of alternative solvents for improvement of oil extraction from rapeseeds. Comptes Rendus Chim. 2014, 17, 242–251. [Google Scholar] [CrossRef]
- Loredo-Dávila, S.; Espinosa-Hernández, V.; Goytia-Jiménez, M.; Diaz-Ballote, L.; Soto-Hernández, R. Fatty acid methyl ester profile from lupinus in the identification of sweet and bitter species from this gender with oil of Lupinus uncinatus Schlecht seeds. J. Nutr. Food Sci. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Huamaní, F.; Tapia, M.; Portales, R.; Doroteo, V.; Ruiz, C.; Rojas, R. Proximate analysis, phenolics, betalains, and antioxidant activities of three ecotypes of kañiwa (Chenopodium pallidicaule aellen) from Perú. Pharmacologyonline 2020, 1, 229–236. [Google Scholar]
- Bruin, A. Investigation of the Food Value of Quinua and Cañihua Seed. J. Food Sci. 1964, 29, 872–876. [Google Scholar] [CrossRef]
- Atchison, G.W.; Nevado, B.; Eastwood, R.J.; Contreras-Ortiz, N.; Reynel, C.; Madriñán, S.; Filatov, D.A.; Hughes, C.E. Lost crops of the Incas: Origins of domestication of the Andean pulse crop tarwi, Lupinus mutabilis. Am. J. Bot. 2016, 103, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Méndez, A.; Pascual-Chagman, G.; Santa-Cruz-Olivos, J.; Norabuena Meza, E.; Calizaya-Milla, Y.E.; Huaringa-Joaquín, A.; Vargas Tapia, E.; Saintila, J. Fatty Acid Profile and Chemical Composition of Oil from Six Varieties of Lupine (Lupinus mutabilis) Consumed in Peru. J. Food Qual. 2023, 2023, 3531839. [Google Scholar] [CrossRef]
- Berti, P.R.; Villacrés, E.; Segovia, G.; Mazon, N.; Peralta, E. Lupinus mutabilis sweet, a traditional Ecuadorian grain: Fatty acid composition, use in the Ecuadorian food system, and potential for reducing malnutrition. J. Agric. Sci. 2013, 2, 153–159. [Google Scholar]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Adaramola, B.; Onigbinde, A.O. Influence of extraction technique on the mineral content and antioxidant capacity of edible oil extracted from ginger rhizome. Chem. Int. 2017, 3, 1–7. [Google Scholar]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, V.; Torres, A. Generalized linear model describes determinants of total antioxidant capacity of refined vegetable oils. Eur. J. Lipid Sci. Technol. 2012, 114, 332–342. [Google Scholar] [CrossRef]
- Özyurt, H. The comparison of the quality properties of some commercial cold pressed seed oils. J. Turkish Chem. Soc. 2019, 6, 149–156. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell. Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef]
- Kaimainen, M.; Ahvenainen, S.; Kaariste, M.; Järvenpää, E.; Kaasalainen, M.; Salomäki, M.; Salonen, J.; Huopalahti, R. Polar lipid fraction from oat (Avena sativa): Characterization and use as an o/w emulsifier. Eur. Food Res. Technol. 2012, 235, 507–515. [Google Scholar] [CrossRef]
- Kumar, A.; Gowda, L.R. Food Additives: Liquid Chromatography. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Borek, S.; Pukacka, S.; Michalski, K.; Ratajczak, L. Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupinus albus L., and Lupinus mutabilis Sweet. J. Exp. Bot. 2009, 60, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.D.; Bianco, M.; Ventura, G.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Analysis of Phospholipids, Lysophospholipids, and Their Linked Fatty Acyl Chains in Yellow Lupin Seeds (Lupinus luteus L.) by Liquid Chromatography and Tandem Mass Spectrometry. Molecules 2020, 25, 805. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter Enteral Nutr. 2015, 39, 18s–32s. [Google Scholar] [CrossRef] [PubMed]
- Al-Amrousi, E.F.; Badr, A.N.; Abdel-Razek, A.G.; Gromadzka, K.; Drzewiecka, K.; Hassanein, M.M.M. A Comprehensive Study of Lupin Seed Oils and the Roasting Effect on Their Chemical and Biological Activity. Plants 2022, 11, 2301. [Google Scholar] [CrossRef]
- Rodríguez, G.; Aguirre, E.; Córdova-Chang, A.; Muñoz-Saenz, J.C.; Baquerizo, M.; Brandolini, A.; Villanueva, E.; Hidalgo, A. Modification of the nutritional quality and oxidative stability of lupin (Lupinus mutabilis Sweet) and sacha inchi (Plukenetia volubilis L.) oil blends. Molecules 2022, 27, 7315. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef]
- Galanty, A.; Grudzińska, M.; Paździora, W.; Paśko, P. Erucic Acid-Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; Pacheco, Y.M.; Ortega, A.; Varela, L.M.; Abia, R.; Muriana, F.J.G. Chapter 154—Oleic Acid: The Main Component of Olive Oil on Postprandial Metabolic Processes. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 1385–1393. [Google Scholar]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini. Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Carpio-Jiménez, C.d.; Molleda-Gutierrez, R.S.; Tapia-Delgado, P. Evaluation of Properties of “Chenopodium pallidicaule” (Cañihua) Oil for Possible Use in Cosmetic Formulations. J. Oleo Sci. 2023, 72, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am. J. Clin. Nutr. 1994, 60, 986S–990S. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: Intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.B.; Oakley, F.R.; Cooper, J.A.; Miller, G.J. Influence of a stearic acid–rich structured triacylglycerol on postprandial lipemia, factor VII concentrations, and fibrinolytic activity in healthy subjects123. Am. J. Clin. Nutr. 2001, 73, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.; Hecker, K.A.I.; Taylor, D.S.; Zhao, G.; Coval, S.; Binkoski, A.M.Y. CHAPTER 18—Dietary Macronutrients and Cardiovascular Risk. In Nutrition in the Prevention and Treatment of Disease; Coulston, A.M., Rock, C.L., Monsen, E.R., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 279–290. [Google Scholar]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Xie, D.; Jackson, E.N.; Zhu, Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: From fundamental research to commercial production. Appl. Microbiol. Biotechnol. 2015, 99, 1599–1610. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Haque, R.; Khan, S.A. Chapter 30—Docosahexaenoic Acid (DHA): A Dietary Supplement with Promising Anticancer Potential. In The Molecular Nutrition of Fats; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 389–400. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.O.; Soomro, H.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3, -6 and -9 fatty acids in animals. Anim. Biotechnol. 2022, 33, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Sbihi, H.M.; Nehdi, I.A.; Tan, C.P.; Al-Resayes, S.I. Bitter and sweet lupin (Lupinus albus L.) seeds and seed oils: A comparison study of their compositions and physicochemical properties. Ind. Crop. Prod. 2013, 49, 573–579. [Google Scholar] [CrossRef]
- Frankel, E.N. The antioxidant and nutritional effects of tocopherols, ascorbic acid and beta-carotene in relation to processing of edible oils. Bibl. Nutr. Dieta. 1989, 43, 297–312. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a Source of Bioactive Antioxidant Compounds for Food Products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef]
- Przygoda, K.; Wejnerowska, G. Extraction of tocopherol-enriched oils from Quinoa seeds by supercritical fluid extraction. Ind. Crop. Prod. 2015, 63, 41–47. [Google Scholar] [CrossRef]

| Petroleum Ether | Hexane | Ethanol | |
|---|---|---|---|
| Tarwi Oil (%) | 16.57 ± 0.33 | 18.31 ± 0.44 | 13.49 ± 1.69 |
| Cañihua Oil (%) | 6.02 ± 0.42 * | 6.73 ± 0.29 * | 5.84 ± 0.44 * |
| Antioxidant Capacity (FRAP) (mM Ferrus Equivalents) | Total Phenolic Compounds (TPC) (mM Equivalents of Gallic Acid) | |||||
|---|---|---|---|---|---|---|
| Samples | Petroleum Ether | Hexane | Ethanol | Petroleum Ether | Hexane | Ethanol |
| Tarwi Oil | 0.66 ± 0.03 | 1.13 ± 0.07 | 1.63 ± 0.20 * | 0.82 ± 0.08 | 2.82 ± 0.01 | 6.12 ± 0.01 ** |
| Cañihua Oil | 0.80 ± 0.14 | 0.78 ± 0.04 | 1.37 ± 0.45 * | 0.69 ± 0.02 | 0.39 ± 0.01 | 2.99 ± 0.03 ** |
| Composition in % of Total Fatty Acids | |||
|---|---|---|---|
| Fatty Acid | Petroleum Ether | Hexene | Ethanol |
| C14:0 (myristic acid) | 0.13 ± 0.04 | 0.12 ± 0.01 | 0.13 ± 0.03 |
| C16:0 (palmitic acid) | 8.43 ± 0.11 | 8.69 ± 0.68 | 8.57 ± 0.52 |
| C16:1 (palmitoleic acid) | 0.20 ± 0.03 | 0.21 ± 0.02 | 0.21 ± 0.01 |
| C18:0 (stearic acid) | 5.17 ± 0.11 | 5.59 ± 0.37 | 4.75 ± 0.11 |
| C18:1-n9 (oleic acid) | 62.49 ± 0.80 | 55.02 ± 1.31 * | 62.03 ± 1.60 |
| C18:2-n6 (linoleic acid) | 21.57 ± 0.35 | 28.15 ± 1.64 * | 21.79 ± 1.33 |
| C18:3-n3 (α-linolenic acid) | 1.04 ± 0.07 | 1.11 ± 0.08 | 1.42± 0.18 |
| C21:0 (henicosanoic acid) | 0.96 ± 0.03 | 0.79 ± 0.17 | 0.89 ± 0.05 |
| C20:1-n9 (cis-11-eicosenoic acid) | ND | 0.10 ± 0.80 | 0.11 ± 0.01 |
| C23:0 (tricosanoic acid) | ND | 0.22 ± 0.70 | ND |
| C22:1-n9 (erucic acid) | ND | ND | 0.11 ± 0.01 |
| Total number of fatty acids extracted | 8 | 10 | 10 |
| SFAs | 14.7 | 15.4 | 14.3 |
| MUFAs | 62.7 * | 55.3 | 62.3 * |
| PUFAs | 22.6 | 29.3 * | 23.3 |
| Composition in % of Total Fatty Acids | |||
|---|---|---|---|
| Fatty Acids | Petroleum Ether | Hexane | Ethanol |
| C14:0 (myristic acid) | 0.15 ± 0.01 | 0.22 ± 0.00 | 0.16 ± 0.01 |
| C16:0 (palmitic acid) | 9.49 ± 0.08 | 9.30 ± 1.30 | 9.60 ± 0.35 |
| C17:1 (cis-10-heptadecanoic acid) | 0.25 ± 0.01 | 0.30 ± 0.03 | 0.29 ± 0.01 |
| C18:0 (stearic acid) | 1.43 ± 0.09 | 1.75 ± 0.09 | 1.54 ± 0.04 |
| C18:1-n9T (elaidic acid) | ND | 0.91 ± 0.00 | ND |
| C18:1-n9 (oleic acid) | 37.85 ± 0.15 * | 38.12 ± 1.54 * | 40.07 ± 1.04 * |
| C18:2-n6 (linoleic acid) | 43.2 ± 0.31 * | 41.94 ± 0.88 * | 43.39 ± 1.09 * |
| C18:3-n6 (γ-linoleinic acid) | 0.20 ± 0.02 | 0.27 ± 0.00 | 0.16 ± 0.01 |
| C18:3-n3 (α-linolenic acid) | 2.40 ± 0.05 | 2.31 ± 0.40 | 2.60 ± 0.08 |
| C20:1-n9 (cis-11-eicosenoic acid) | 1.14 ± 0.01 | 1.16 ± 0.16 | 0.11 ± 0.01 |
| C20:3-n6 (cis-8.11.14-eicosatrienoic acid) | ND | 0.21 ± 0.00 | ND |
| C20:3-n3 (cis-11.14.17-eicosatrienoic acid) | 0.34 ± 0.01 | 0.37 ± 0,04 | ND |
| C21:0 (henicosanoic acid) | 1.00 ± 0.01 | 1.19 ± 0.11 | 1.05 ± 0.04 |
| C22:0 (behenic acid) | 0.53 ± 0.01 | 0.52 ± 0.66 | 0.55 ± 0.01 |
| C22:1-n9 (erucic acid) | 0.47 ± 0.02 | 0.68 ± 0.19 | 0.47 ± 0.01 |
| C22:6-n3 (cis-4.7.10.13.16.19- docosahexaenoic acid) | 0.63 ± 0.07 | ND | ND |
| C23:0 (tricosanoic acid) | 0.54 ± 0.01 | 0.73 ± 0.03 | 0.57 ± 0.07 |
| C24:0 (lignoceric acid) | 0.28 ± 0.01 | ND | ND |
| Total number of fatty acids extracted | 16 | 15 | 13 |
| SFAs | 13.5 | 13.7 | 12.8 |
| MUFAs | 39.71 | 41.17 | 41.02 |
| PUFAs | 46.76 | 45.10 | 46.15 |
| Tocopherols mg/kg of dw | |||
|---|---|---|---|
| Sample | Delta (δ) | Gamma (γ) | Alpha (α) |
| TPE | 11.3 ± 0.20 | 22.2 ± 1.43 | 11.5 ± 0.05 |
| THE | 13.9 ± 0.19 | 161.6 ± 2.90 | 15.5 ± 0.11 |
| TET | 13.5 ± 0.10 | 205.1 ± 0.53 | 16.6 ± 0.43 |
| CPE | 13.6 ± 0.13 | 13.8 ± 2.73 | 15.6 ± 0.47 |
| CHE | 22.5 ± 0.58 | 26.1 ± 0.95 | 20.1 ± 0.46 |
| CET | 23.7 ± 0.50 | 28.3 ± 1.99 | 21.0 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Sempértegui, J.; Ibieta, G.; Tullberg, C.; Peñarrieta, J.M.; Linares-Pastén, J.A. Chemical Characterisation of New Oils Extracted from Cañihua and Tarwi Seeds with Different Organic Solvents. Foods 2024, 13, 1982. https://doi.org/10.3390/foods13131982
Ortiz-Sempértegui J, Ibieta G, Tullberg C, Peñarrieta JM, Linares-Pastén JA. Chemical Characterisation of New Oils Extracted from Cañihua and Tarwi Seeds with Different Organic Solvents. Foods. 2024; 13(13):1982. https://doi.org/10.3390/foods13131982
Chicago/Turabian StyleOrtiz-Sempértegui, Jimena, Gabriela Ibieta, Cecilia Tullberg, J. Mauricio Peñarrieta, and Javier A. Linares-Pastén. 2024. "Chemical Characterisation of New Oils Extracted from Cañihua and Tarwi Seeds with Different Organic Solvents" Foods 13, no. 13: 1982. https://doi.org/10.3390/foods13131982
APA StyleOrtiz-Sempértegui, J., Ibieta, G., Tullberg, C., Peñarrieta, J. M., & Linares-Pastén, J. A. (2024). Chemical Characterisation of New Oils Extracted from Cañihua and Tarwi Seeds with Different Organic Solvents. Foods, 13(13), 1982. https://doi.org/10.3390/foods13131982








