Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications
Abstract
1. Introduction
2. Structural Composition of Soy, Pulse, Cereal, and Pseudo-Cereal Proteins
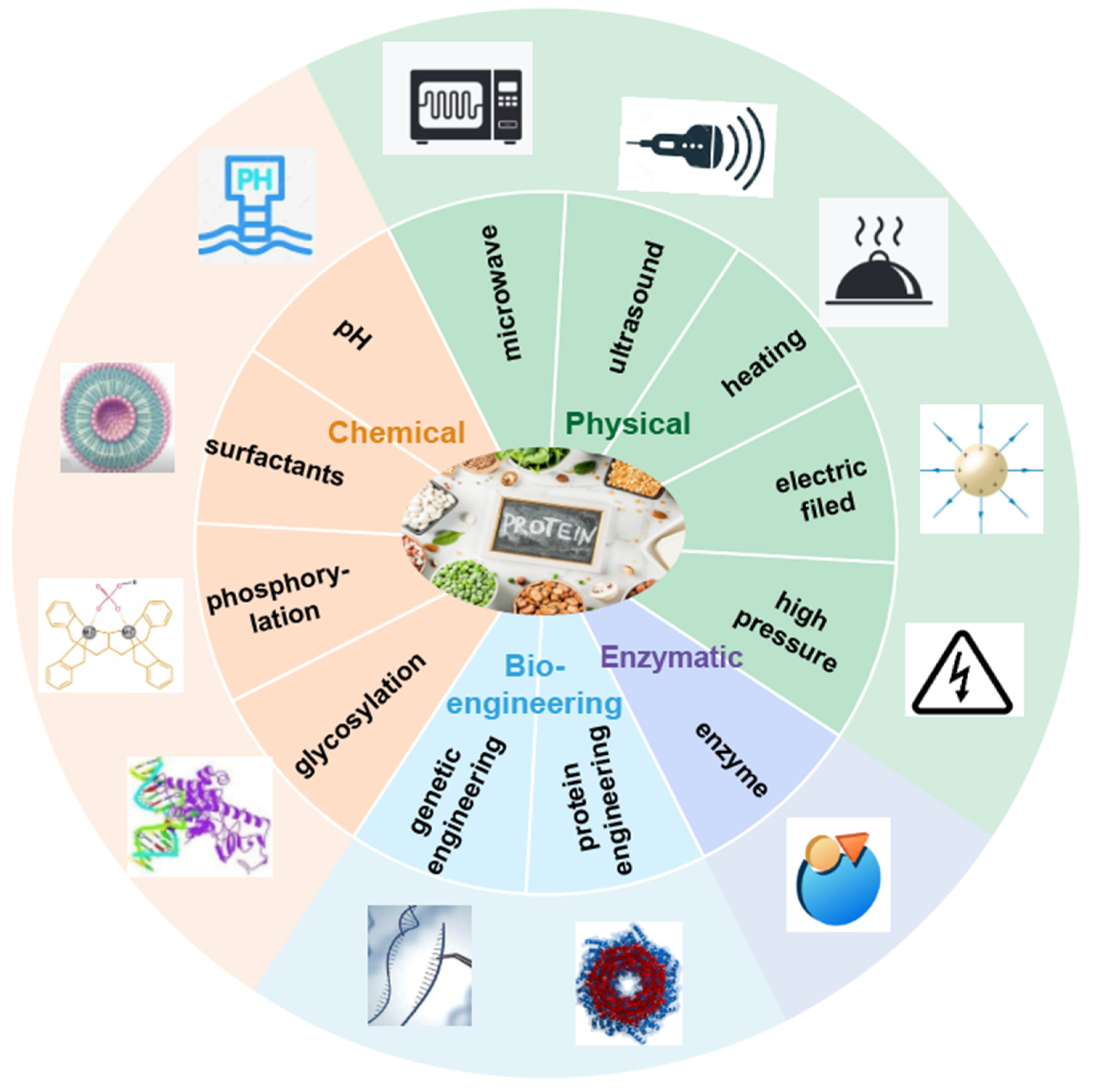
3. Modification Techniques of Soy, Pulse, Cereal, and Pseudo-Cereal Proteins
3.1. Physical Modification
3.2. Chemical Modification
3.3. Enzymatic Modification
3.4. Combined Modification
3.5. Safety Issues of the Modification Technologies
4. Bioactive Properties of Soy, Pulse, Cereal, and Pseudo-Cereal Proteins
4.1. Antioxidant Activity
4.2. Antimicrobial Activity
4.3. Antitumoral Activity
4.4. Other Activities
5. Food Applications of Soy, Pulse, Cereal, and Pseudo-Cereal Proteins
5.1. Dairy Alternatives
5.2. Meat Analogs
5.3. Baked Goods
5.4. 3D Printed Foods
5.5. Other Food Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| GHG | Greenhouse gas |
| LDL | Low-density lipoprotein |
| 11S | Glycinin |
| 7S | β-conglycinin |
| AAs | Amino acids |
| 2S | Albumins |
| MM | Molecular masses |
| S-S | Disulfide bonds |
| PEF | Pulsed electric field |
| MIC | Minimum inhibitory concentration |
| QPHs | Quinoa protein hydrolysates |
| OPCs | Oat protein concentrates |
| CAGR | Compound annual growth rate |
| RBM | Rice bran milk |
| TSS | Total soluble solids |
| MBPI | Mung bean protein isolate |
| WHC | Water-holding capacity |
| FAC | Fat absorption capacity |
| TVP | Textured vegetable protein |
References
- Wang, Y.; Liu, J.; Zhang, Z.; Meng, X.; Yang, T.; Shi, W.; He, R.; Ma, H. Insights into Ultrasonication Treatment on the Characteristics of Cereal Proteins: Functionality, Conformational and Physicochemical Characteristics. Foods 2023, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Boateng, I.D.; Xu, J. How Does Ultrasound-Assisted Ionic Liquid Treatment Affect Protein? A Comprehensive Review of Their Potential Mechanisms, Safety Evaluation, and Physicochemical and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13261. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global Food Supply: Land Use Efficiency of Livestock Systems. Int. J. Life Cycle Assess. 2016, 21, 747–758. [Google Scholar] [CrossRef]
- Zhang, W.; Duah, I.; Xu, J. Trends in Food Science & Technology How Does Ultrasound-Assisted Ionic Liquids ( UA-ILs ) Pretreatment Affect the Functional Properties of Soy Protein Hydrolysates? A Critical Review. Trends Food Sci. Technol. 2024, 143, 104313. [Google Scholar]
- Dent, T.; Maleky, F. Pulse Protein Processing: The Effect of Processing Choices and Enzymatic Hydrolysis on Ingredient Functionality. Crit. Rev. Food Sci. Nutr. 2023, 63, 9914–9925. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health Benefits of Cereal Grain-and Pulse-Derived Proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and Food Security: Dietary Protein, Digestibility, Bioactive and Functional Properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food Proteins from Plants and Fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa Protein: Composition, Structure and Functional Properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Espinoza-Herrera, J.; Martínez, L.M.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems. Foods 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, Properties and Applications of Soy-Protein-Based Materials: A Review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef] [PubMed]
- L’hocine, L.; Boye, J.I.; Arcand, Y. Composition and Functional Properties of Soy Protein Isolates Prepared Using Alternative Defatting and Extraction Procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Zungur Bastıoğlu, A.; Tomruk, D.; Koç, M.; Ertekin, F.K. Spray Dried Melon Seed Milk Powder: Physical, Rheological and Sensory Properties. J. Food Sci. Technol. 2016, 53, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant Based Alternatives to Conventional Milk, Production, Potential and Health Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Zannini, E.; Arendt, E.K. Production of Pulse Protein Ingredients and Their Application in Plant-Based Milk Alternatives. Trends Food Sci. Technol. 2021, 110, 364–374. [Google Scholar] [CrossRef]
- Moongngarm, A. Chemical Compositions and Resistant Starch Content in Starchy Foods. Am. J. Agric. Biol. Sci. 2013, 8, 107–113. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Jeske, S.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Wriessnegger, C.L.; O’Mahony, J.A.; Zannini, E.; Arendt, E.K. Membrane Filtration and Isoelectric Precipitation Technological Approaches for the Preparation of Novel, Functional and Sustainable Protein Isolate from Lentils. Eur. Food Res. Technol. 2019, 245, 1855–1869. [Google Scholar] [CrossRef]
- Geerts, M.E.; Strijbos, M.; van der Padt, A.; van der Goot, A.J. Understanding Functional Properties of Mildly Refined Starch Fractions of Yellow Pea. J. Cereal Sci. 2017, 75, 116–123. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Naguleswaran, S. Utilizing side streams of pulse protein processing: A review. Legum. Sci. 2021, 4, e120. [Google Scholar] [CrossRef]
- Mokni Ghribi, A.; Ben Amira, A.; Maklouf Gafsi, I.; Lahiani, M.; Bejar, M.; Triki, M.; Zouari, A.; Attia, H.; Besbes, S. Toward the Enhancement of Sensory Profile of Sausage “Merguez” with Chickpea Protein Concentrate. Meat Sci. 2018, 143, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Pourmohammadi, K. Physical Modifications of Wheat Gluten Protein: An Extensive Review. J. Food Process. Eng. 2021, 44, 128398. [Google Scholar] [CrossRef]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit, R.; Weisz, M.; Trakhtenberg, S. Characterisation of Pseudocereal and Cereal Proteins by Protein and Amino Acid Analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Phongthai, S.; Rawdkuen, S. Fractionation and Characterization of Antioxidant Peptides from Rice Bran Protein Hydrolysates Stimulated by in Vitro Gastrointestinal Digestion. Cereal Chem. 2020, 97, 316–325. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Triticale: Nutritional Composition and Food Uses. Food Chem. 2018, 241, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Bean, S.R.; Wilson, J.D.; Moreau, R.A.; Galant, A.; Awika, J.M.; Kaufman, R.C.; Ioerger, B.P. Structure and Composition of the Sorghum Grain. Sorghum A State Art Future Perspetives 2019, 58, 173–214. [Google Scholar]
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Charrondière Assessment of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ballogou, V.Y.; Soumanou, M.M.; Toukourou, F.; Hounhouigan, J.D. Structure and Nutritional Composition of Fonio (Digitaria exilis) Grains: A Review. Int. Res. J. Biol. Sci. 2013, 2, 73–79. [Google Scholar]
- Urbizo-Reyes, U.; San Martin-González, M.F.; Garcia-Bravo, J.; López Malo Vigil, A.; Liceaga, A.M. Physicochemical Characteristics of Chia Seed (Salvia Hispanica) Protein Hydrolysates Produced Using Ultrasonication Followed by Microwave-Assisted Hydrolysis. Food Hydrocoll. 2019, 97, 105187. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, T.; Jiang, L. Soy Protein: Molecular Structure Revisited and Recent Advances in Processing Technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, L.; Zhang, X.; Geng, H.; Xue, W.; Chen, Z. Assembly Behavior, Structural Characterization and Rheological Properties of Legume Proteins Based Amyloid Fibrils. Food Hydrocoll. 2021, 111, 106396. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying Plants with the Essential Amino Acids Lysine and Methionine to Improve Nutritional Quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Boateng, I.D.; Xu, J. Novel Marine Proteins as a Global Protein Supply and Human Nutrition: Extraction, Bioactivities, Potential Applications, Safety Assessment, and Deodorization Technologies. Trends Food Sci. Technol. 2024, 143, 104283. [Google Scholar] [CrossRef]
- Al-Doury, M.K.W.; Hettiarachchy, N.S.; Horax, R. Rice-Endosperm and Rice-Bran Proteins: A Review. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 943–956. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and Zein -Based Nano-Materials for Food and Nutrition Applications: A Review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B. Pseudocereals as Super Foods of 21st Century: Recent Technological Interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional Constituents of Pseudo Cereals and Their Potential Use in Food Systems: A Review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Taylor, J.; Campanella, O.H.; Hamaker, B.R. Functionality of the Storage Proteins in Gluten-Free Cereals and Pseudocereals in Dough Systems. J. Cereal Sci. 2016, 67, 22–34. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the Chemical and Technological Characteristics of Wholemeal Flours Obtained from Amaranth (Amaranthus sp.), Quinoa (Chenopodium quinoa) and Buckwheat (Fagopyrum sp.) Seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Naowarojna, N.; Cheng, R.; Lopez, J.; Wong, C.; Qiao, L.; Liu, P. Chemical Modifications of Proteins and Their Applications in Metalloenzyme Studies. Synth. Syst. Biotechnol. 2021, 6, 32–49. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, W.; Chen, B.; Zhu, J.; Wang, L. Enhancing the Solubility and Foam Ability of Rice Glutelin by Heat Treatment at PH12: Insight into Protein Structure. Food Hydrocoll. 2020, 103, 105626. [Google Scholar] [CrossRef]
- Chao, D.; Jung, S.; Aluko, R.E. Physicochemical and Functional Properties of High Pressure-Treated Isolated Pea Protein. Innov. Food Sci. Emerg. Technol. 2018, 45, 179–185. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Ding, X.; Wu, Z.; Li, Y. Effects of Dual-Frequency Ultrasound with Different Energy Irradiation Modes on the Structural and Emulsifying Properties of Soy Protein Isolate. Food Bioprod. Process. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Mo, H. Effects of Pulsed Electric Fields on Physicochemical Properties of Soybean Protein Isolates. LWT 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. PH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different PH, Salt Concentration, and Temperature Conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef]
- Kathuria, D.; Gautam, S.; Thakur, A. Maillard Reaction in Different Food Products: Effect on Product Quality, Human Health and Mitigation Strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Wang, T.; Yi, K.; Li, Y.; Wang, H.; Fan, Z.; Jin, H.; Xu, J. Esterified Soy Proteins with Enhanced Antibacterial Properties for the Stabilization of Nano-Emulsions under Acidic Conditions. Molecules 2023, 28, 3078. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.W.; Tang, C.H.; Cao, J.S.; Hu, E.K.; Wen, Q.B.; Yang, X.Q. Effects of Limited Enzymatic Hydrolysis with Trypsin on the Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. Food Chem. 2008, 106, 1004–1013. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and Gel Textural Properties of Soy Protein Isolate When Subjected to Extreme Acid PH-Shifting and Mild Heating Processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of High Intensity Ultrasound on Structure and Foaming Properties of Pea Protein Isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An Efficient Ultrasound-Assisted Extraction Method of Pea Protein and Its Effect on Protein Functional Properties and Biological Activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, C.; Feng, Y.; Zhang, J.; He, Y.; Duan, Y.; Zhang, H.; Ma, H. Effects of Ultrasound-Assisted Extraction on the Structural, Functional and Antioxidant Properties of Dolichos Lablab L. Protein. Process. Biochem. 2021, 101, 274–284. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochem 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Melchior, S.; Calligaris, S.; Bisson, G.; Manzocco, L. Understanding the Impact of Moderate-Intensity Pulsed Electric Fields (MIPEF) on Structural and Functional Characteristics of Pea, Rice and Gluten Concentrates. Food Bioproc Tech. 2020, 13, 2145–2155. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.J.; Jiang, W.; Qian, J.Y. Effect of Pulsed Electric Field on Functional and Structural Properties of Canola Protein by Pretreating Seeds to Elevate Oil Yield. LWT 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Fernando, S. Pulse Protein Ingredient Modification. J. Sci. Food Agric. 2022, 102, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y.L. Interfacial Structural Role of PH-Shifting Processed Pea Protein in the Oxidative Stability of Oil/Water Emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jin, Y.; Xiong, Y.L. Heating-Aided PH Shifting Modifies Hemp Seed Protein Structure, Cross-Linking, and Emulsifying Properties. J. Agric. Food Chem. 2018, 66, 10827–10834. [Google Scholar] [CrossRef] [PubMed]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of Alkaline Deamidation on the Structure, Surface Hydrophobicity, and Emulsifying Properties of the Z19 α-Zein. J. Agric. Food Chem. 2007, 55, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.E.; Martínez, N.E.; Añón, M.C. Influence of PH on Structure and Function of Amaranth (Amaranthus Hypochondriacus) Protein Isolates. Cereal Chem. 2010, 87, 448–453. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Yang, W. Protein Glycosylation: A Promising Way to Modify the Functional Properties and Extend the Application in Food System. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of Citrus Pectin and Apple Pectin in Conjugation with Soy Protein Isolate (SPI) under Controlled Dry-Heating Conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gan, J.; Li, Y.; Nirasawa, S.; Cheng, Y. Conformation and Emulsifying Properties of Deamidated Wheat Gluten-Maltodextrin/Citrus Pectin Conjugates and Their Abilities to Stabilize β-Carotene Emulsions. Food Hydrocoll. 2019, 87, 129–141. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea Protein Isolate-Gum Arabic Maillard Conjugates Improves Physical and Oxidative Stability of Oil-in-Water Emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Zhang, W.; Azizi-Lalabadi, M.; Roy, S.; Salim, S.A.; Castro-Muñoz, R.; Jafari, S.M. Maillard-Reaction (Glycation) of Biopolymeric Packaging Films; Principles, Mechanisms, Food Applications. Trends Food Sci. Technol. 2023, 138, 523–538. [Google Scholar] [CrossRef]
- Zha, F.; Rao, J.; Chen, B. Modification of Pulse Proteins for Improved Functionality and Flavor Profile: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Osman, A. Antimicrobial Activity of Native and Esterified Legume Proteins against Gram-Negative and Gram-Positive Bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional Properties and Structural Characteristics of Phosphorylated Pea Protein Isolate. Int. J. Food Sci. Technol. 2020, 55, 2002–2010. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Ren, L.; Li, M.; Lv, Y.; Guo, S.; Waqar, K. Physical-Chemical Properties and in Vitro Digestibility of Phosphorylated and Glycosylated Soy Protein Isolate. LWT 2021, 152, 112380. [Google Scholar] [CrossRef]
- Mo, J.; Wang, F.; Xu, Z.; Feng, C.; Fang, Y.; Tang, X.; Shen, X. Characterization and Performance of Soybean Protein Modified by Tyrosinase. Int. J. Adhes. Adhes. 2019, 92, 111–118. [Google Scholar] [CrossRef]
- Liu, K.; Xu, X.; Liu, H.; Liu, Z.; Zhao, K.; Ma, Y.; Zhang, K. Mechanical Properties and Water Sensitivity of Soybean Protein Isolate Film Improved by Incorporation of Sodium Caseinate and Transglutaminase. Prog. Org. Coat. 2021, 153, 106154. [Google Scholar] [CrossRef]
- Awosika, T.; Aluko, R.E. Enzymatic Pea Protein Hydrolysates Are Active Trypsin and Chymotrypsin Inhibitors. Foods 2019, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Daliri, H.; Ahmadi, R.; Pezeshki, A.; Hamishehkar, H.; Mohammadi, M.; Beyrami, H.; Khakbaz Heshmati, M.; Ghorbani, M. Quinoa Bioactive Protein Hydrolysate Produced by Pancreatin Enzyme- Functional and Antioxidant Properties. LWT 2021, 150, 111853. [Google Scholar] [CrossRef]
- Kamal, H.; Mudgil, P.; Bhaskar, B.; Fisayo, A.F.; Gan, C.Y.; Maqsood, S. Amaranth Proteins as Potential Source of Bioactive Peptides with Enhanced Inhibition of Enzymatic Markers Linked with Hypertension and Diabetes. J. Cereal Sci. 2021, 101, 103308. [Google Scholar] [CrossRef]
- Liang, G.; Chen, W.; Qie, X.; Zeng, M.; Qin, F.; He, Z.; Chen, J. Modification of Soy Protein Isolates Using Combined Pre-Heat Treatment and Controlled Enzymatic Hydrolysis for Improving Foaming Properties. Food Hydrocoll. 2020, 105, 105764. [Google Scholar] [CrossRef]
- Figueroa-González, J.J.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Aguirre-Mandujano, E.; Alvarez-Ramirez, J.; Martínez-Velasco, A. Modifying the Structure, Physicochemical Properties, and Foaming Ability of Amaranth Protein by Dual PH-Shifting and Ultrasound Treatments. LWT 2022, 153, 112561. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Shi, L.; Liu, W. Comprehensive Analyses of Advanced Glycation End Products and Heterocyclic Amines in Peanuts during the Roasting Process. Molecules 2023, 28, 7012. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Clark, K. Trends in Extracting Agro-Byproducts’ Phenolics Using Non-Thermal Technologies and Their Combinative Effect: Mechanisms, Potentials, Drawbacks, and Safety Evaluation. Food Chem. 2024, 437, 137841. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Zhang, W.; Greco, I.; De Gobba, C.; Olsen, K.; Lund, M.N. Lund Liquid Chromatography Quadrupole-Orbitrap Mass Spectrometry for the Simultaneous Analysis of Advanced Glycation End Products and Protein-Derived Cross-Links in Food and Biological Matrices. J. Chromatogr. A 2020, 1615, 460767. [Google Scholar] [CrossRef] [PubMed]
- Shahali, Y.; Dadar, M. Plant Food Allergy: Influence of Chemicals on Plant Allergens. Food Chem. Toxicol. 2018, 115, 365–374. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of Fourteen Polyphenols in Pulses by High Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and Correlation Study with Antioxidant Activity and Colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Legume Proteins and Peptides as Compounds in Nutraceuticals: A Structural Basis for Dietary Health Effects. Nutrients 2022, 14, 1188. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Ismail Antioxidative Peptides from Food Proteins: A Review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Antioxidant Peptides from Enzymatically Hydrolyzed Rice Bran Protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, Identification and Characterization of Two Novel Antioxidant Peptides from Finger Millet (Eleusine Coracana) Protein Hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Patil, P.J.; Mehmood, A.; Rehman, A.; Shah, H.; Haider, J.; Xu, K.; Zhang, C.; Li, X. Comparative Evaluation of Pseudocereal Peptides: A Review of Their Nutritional Contribution. Trends Food Sci. Technol. 2022, 122, 287–313. [Google Scholar] [CrossRef]
- Osman, A.; Elaraby, G.M.; Taha, H. Potential Use as a Bio-Preservative from Lupin Protein Hydrolysate Generated by Alcalase in Food System. J. Appl. Biol. Biotechnol. 2016, 4, 76–81. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial Potential of Legume Extracts against Foodborne Pathogens: A Review. Trends Food Sci. Technol. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Ben Mlouka, M.A.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, Antityrosinase and Antibiofilm Activities of Synthesized Peptides Derived from Vicia Faba Protein Hydrolysate: A Powerful Agents in Cosmetic Application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Osman, A.; Enan, G.; Al-Mohammadi, A.R.; Abdel-Shafi, S.; Abdel-Hameid, S.; Sitohy, M.Z.; El-Gazzar, N. Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins. Antibiotics 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Omar, L.S.; Kamal, H.; Kilari, B.P.; Maqsood, S. Multi-Functional Bioactive Properties of Intact and Enzymatically Hydrolysed Quinoa and Amaranth Proteins. LWT 2019, 110, 207–213. [Google Scholar] [CrossRef]
- Fu, H.; Shan, D.; Li, J.; Swallah, M.S.; Yang, X.; Ji, L.; Yu, H. Potential Functionality of β-Conglycinin with Subunit Deficiencies: Soy Protein May Regulate Glucose and Lipid Metabolism. Food Funct. 2022, 13, 12291–12303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive Effects of Some Popular Phytochemicals on Human Colon Cancer: A Review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Gonzalez de Mejia, E.; García-Lara, S.; Aguilar, O.; Lopez-Castillo, L.M.; Otero-Pappatheodorou, J.T. Antiproliferative Effect of Peptide Fractions Isolated from a Quality Protein Maize, a White Hybrid Maize, and Their Derived Peptides on Hepatocarcinoma Human HepG2 Cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In Vitro Chemopreventive Properties of Peptides Released from Quinoa (Chenopodium Quinoa Willd.) Protein under Simulated Gastrointestinal Digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.T.; Ju, Z.; Wang, L.; Qiu, J.; Liu, L.; Zhou, X.; Liang, T.; Geng, D.; Zhou, S. Peptides Derived from Rice α-Globulin Reduce Atherosclerosis in Apolipoprotein E-Deficient Mice by Inhibiting TNF-α-Induced Vascular Endothelial Cells Injury. J. Funct. Foods 2019, 63, 3582. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Shan, K.; Sun, T.; Lin, M.; Jia, L.; Chen, Y.Q. Effect of Different Cereal Peptides on the Development of Type 1 Diabetes Is Associated with Their Anti-Inflammatory Ability: In Vitro and In Vivo Studies. Mol. Nutr. Food Res. 2019, 63, 1800987. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qu, Y.; Hua, X.; Wang, F.; Jia, X.; Yin, L. Recent Advances in Soybean Protein Processing Technologies: A Review of Preparation, Alterations in the Conformational and Functional Properties. Int. J. Biol. Macromol. 2023, 2023, 125862. [Google Scholar] [CrossRef] [PubMed]
- Ferreira ED, S.; Silva, M.A.; Demonte, A.; Neves, V.A. Soy β-Conglycinin (7S Globulin) Reduces Plasma and Liver Cholesterol in Rats Fed Hypercholesterolemic Diet. J. Med. Food 2011, 14, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Boachie, R.; Yao, S.; Udenigwe, C.C. Molecular Mechanisms of Cholesterol-Lowering Peptides Derived from Food Proteins. Curr. Opin. Food Sci. 2018, 20, 58–63. [Google Scholar] [CrossRef]
- Rajpurohit, B.; Li, Y. Overview on Pulse Proteins for Future Foods: Ingredient Development and Novel Applications. J. Future Foods 2023, 3, 340–356. [Google Scholar] [CrossRef]
- Spaen, J.; Silva, J.V.C. Oat Proteins: Review of Extraction Methods and Techno-Functionality for Liquid and Semi-Solid Applications. LWT 2021, 147, 111478. [Google Scholar] [CrossRef]
- Beniwal, A.S.; Singh, J.; Kaur, L.; Hardacre, A.; Singh, H. Meat Analogs: Protein Restructuring during Thermomechanical Processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1221–1249. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Barba, F.J.; Rezek-Jambrak, A.; Gálvez, F.; Zamuz, S.; Granato, D.; Lorenzo, J.M. Effects of Pulses and Microalgal Proteins on Quality Traits of Beef Patties. J. Food Sci. Technol. 2018, 55, 4544–4553. [Google Scholar] [CrossRef] [PubMed]
- Kendler, C.; Duchardt, A.; Karbstein, H.P.; Emin, M.A. Effect of Oil Content and Oil Addition Point on the Extrusion Processing of Wheat Gluten-Based Meat Analogues. Foods 2021, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative Analysis of Plant-Based High-Protein Ingredients and Their Impact on Quality of High-Protein Bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Campbell, L.; Euston, S.R.; Ahmed, M.A. Effect of Addition of Thermally Modified Cowpea Protein on Sensory Acceptability and Textural Properties of Wheat Bread and Sponge Cake. Food Chem. 2016, 194, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Vittadini, E.; Vodovotz, Y. Effect of Chickpea Protein Concentrate on the Loaf Quality of Composite Soy-Wheat Bread. LWT 2018, 89, 400–402. [Google Scholar] [CrossRef]
- Duta, D.E.; Culetu, A.; Sozer, N. Effect of Dry Fractionated Hybrid Protein Ingredients on the Structural, Textural, Thermal and Sensory Properties of Gluten-Free Oat and Faba Pasta. Int. J. Food Sci. Technol. 2019, 54, 3205–3215. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Bhandari, B. 3D Printing of Steak-Like Foods Based on Textured Soybean Protein. Foods 2021, 10, 2011. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Stability of 3D Printing Using a Mixture of Pea Protein and Alginate: Precision and Application of Additive Layer Manufacturing Simulation Approach for Stress Distribution. J. Food Eng. 2020, 288, 110127. [Google Scholar] [CrossRef]
- Qiu, Y.; McClements, D.J.; Chen, J.; Li, C.; Liu, C.; Dai, T. Construction of 3D Printed Meat Analogs from Plant-Based Proteins: Improving the Printing Performance of Soy Protein- and Gluten-Based Pastes Facilitated by Rice Protein. Food Res. Int. 2023, 167, 112635. [Google Scholar] [CrossRef]
- Bedoya, M.G.; Montoya, D.R.; Tabilo-Munizaga, G.; Pérez-Won, M.; Lemus-Mondaca, R. Promising Perspectives on Novel Protein Food Sources Combining Artificial Intelligence and 3D Food Printing for Food Industry. Trends Food Sci. Technol. 2022, 128, 38–52. [Google Scholar] [CrossRef]
- Fan, J.M.; Ma, W.; Liu, G.Q.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Preparation and Characterization of Kidney Bean Protein Isolate (KPI)-Chitosan (CH) Composite Films Prepared by Ultrasonic Pretreatment. Food Hydrocoll. 2014, 36, 60–69. [Google Scholar] [CrossRef]
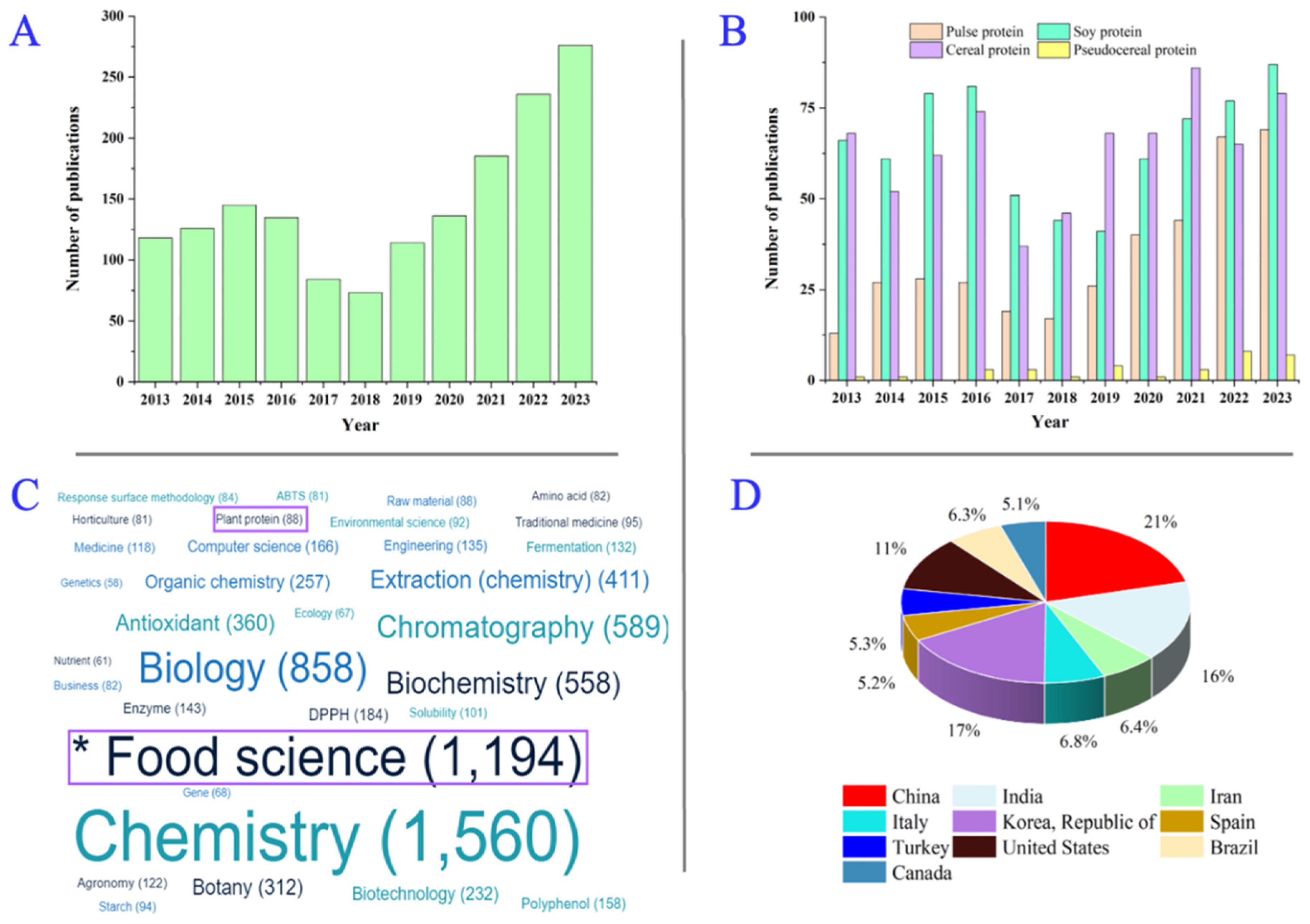


| Classification | Type | Protein | Carbohydrate/Starch | Fiber (Crude) | Fat | Ash | Reference |
|---|---|---|---|---|---|---|---|
| Soybeans | Full fat soy flour | 35–40 | 30–35 | 6–7 | 18–22 | 4–6 | [13] |
| Defatted soy flour | 50–60 | 30–40 | 5–6 | 1–3 | 5–7 | [14] | |
| Soy protein concentrate | 65–70 | 20–30 | 3–5 | 1–2 | 6–8 | [15] | |
| Soy protein isolate | 90–95 | 1–3 | <1 | <1 | <1 | [16] | |
| Pulses | Pea | 14–31 | 55–72 | 3–20 | 1–4 | 2.3–3.7 | [17] |
| Lupin | 32–55.3 | 4.5–47 | 14–55 | 5–15 | 2.6–5.09 | [18] | |
| Chickpea | 19–27 | 52–71 | 6–15 | 1–3 | 1.8–3.5 | [18] | |
| Cowpea/black- eyed pea | 23.6–33 | 37–52 | 2–5 | 1–2.1 | 2–5 | [19] | |
| Lentil | 23–31 | 42–72 | 7.0–23.0 | 1–3 | 2.1–3.2 | [20] | |
| Yellow pea/field pea | 21.4–25.9 | 49.3–56.6 | 11.6–18.4 | 0.8–2.1 | 1.1–2.8 | [21] | |
| Black bean | 22.9–23.2 | 60–65 | 3.4 | 1.6–3.4 | 4.6–5.0 | [22] | |
| Faba bean | 26.4–39.7 | 36.9–61.6 | 6.4–23.7 | 1.5–2.1 | 2.9–4.3 | [23] | |
| Great Northern bean | 20.8–23.6 | 45.5–47.2 | 15–20 | 1.3–1.7 | 3.8–4.4 | [17] | |
| Lima bean | 14.5–24.0 | 47.1–50.5 | 32.6–33.6 | 0.6–0.8 | 2.4–3.9 | [22] | |
| Mung bean | 25.8–27.5 | 52.2–52.8 | 2.2 | 1.6 | 2.9 | [23] | |
| Navy bean | 19–27 | 67–75 | 14–25 | 1.7–2.0 | 4.0–4.9 | [18] | |
| Pinto bean | 17.5–21.6 | 60–65 | 15–20 | 1.2–2.8 | 3.5–4.7 | [22] | |
| Kidney bean | 17–27 | 63–74 | 18–30 | 1–5 | 3.2–5.2 | [18] | |
| Cereals | Wheat | 8.0–17.5 | 70–75 | 2–5 | 1–2 | 1.5–2 | [24] |
| Maize | 8.8–11.9 | 70–80 | 2–4 | 2–5 | 1–2 | [25] | |
| Rice | 6.6–8.4 | 70–80 | 1.6–2.8 | <2 | 0.4–0.7 | [26] | |
| Oats | 8.7–16.0 | 60–70 | 10–15 | 5–10 | 1.5–2.0 | [27] | |
| Rye | 8.0–17.7 | 60–70 | 10–15 | <2 | 1.5–2.0 | [27] | |
| Triticale | 11.7–16.3 | 60–70 | 2–5 | <2 | 1.5–2.0 | [28] | |
| Teff | 8.7–11.1 | 70–75 | 7–10 | 1–3 | 2–3 | [27] | |
| Sorghum | 7.0–15.0 | 70–75 | 6–12 | 2–4 | 1.5–2.0 | [29] | |
| Millet | 8.3–13.3 | 70–75 | 6–10 | 1–5 | 2–3 | [27] | |
| Pseudo-cereals | Buckwheat | 21.6–25.3 | 70–75 | 8–12 | 2–4 | 2–3 | [30] |
| Amaranth | 11.7–18.4 | 65–75 | 6–8 | 5–7 | 2–3 | [27] | |
| Quinoa | 13.0–14.0 | 60–70 | 2.5–4.0 | 4–7 | 2–3 | [31] | |
| Fonio | 9.0–10.0 | 70–75 | 2–4 | 1–2 | 2–3 | [32] | |
| Chia | 19.0–23.0 | 35–40 | 30–40 | 30–35 | <5 | [33] |
| Modification Category | Modification Method | Description | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Physical | Heat treatment |
|
|
| [46] |
| Microwave |
|
|
| [47] | |
| Ultrasound |
|
|
| [48] | |
| Pulsed electric field (PEF) |
|
|
| [49] | |
| Chemical | Acid–base treatment |
|
|
| [50] |
| Glycosylation |
|
|
| [51] | |
| Enzymatic | Enzymatic methods |
|
|
| [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Boateng, I.D.; Xu, J.; Zhang, Y. Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods 2024, 13, 1974. https://doi.org/10.3390/foods13131974
Zhang W, Boateng ID, Xu J, Zhang Y. Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods. 2024; 13(13):1974. https://doi.org/10.3390/foods13131974
Chicago/Turabian StyleZhang, Wenxue, Isaac Duah Boateng, Jinsheng Xu, and Yi Zhang. 2024. "Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications" Foods 13, no. 13: 1974. https://doi.org/10.3390/foods13131974
APA StyleZhang, W., Boateng, I. D., Xu, J., & Zhang, Y. (2024). Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods, 13(13), 1974. https://doi.org/10.3390/foods13131974






