LC-QTOF/MS-Based Profiling of the Phytochemicals in Ice Plant (Mesembryanthemum crystallinum) and Their Bioactivities
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Sample Preparation
2.3. UPLC-QTOF-MS Analysis
2.4. Phytochemical Profiling
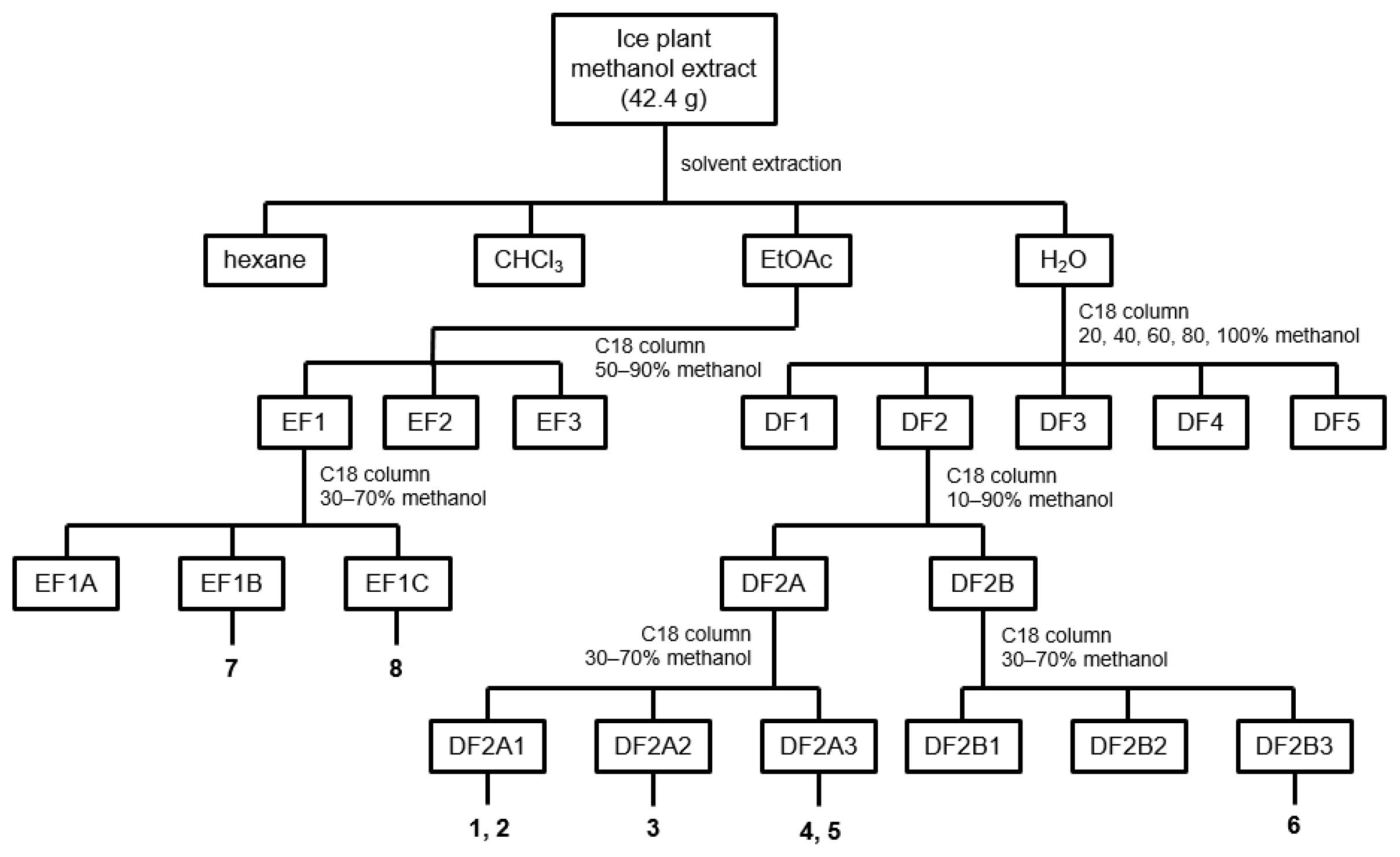
2.5. Extraction and Isolation of Ice Plant Compounds
2.6. Assessment of Antioxidant Activity
2.6.1. Total Antioxidant Capacity (TAC)
2.6.2. DPPH Radical Scavenging Activity
2.6.3. FRAP Assay
2.7. Assessment of Anti-Inflammatory Activity
2.7.1. Cell Culture
2.7.2. Cell Viability Assay
2.7.3. Nitric Oxide (NO) Production
2.8. Statistical Analysis
3. Results and Discussion
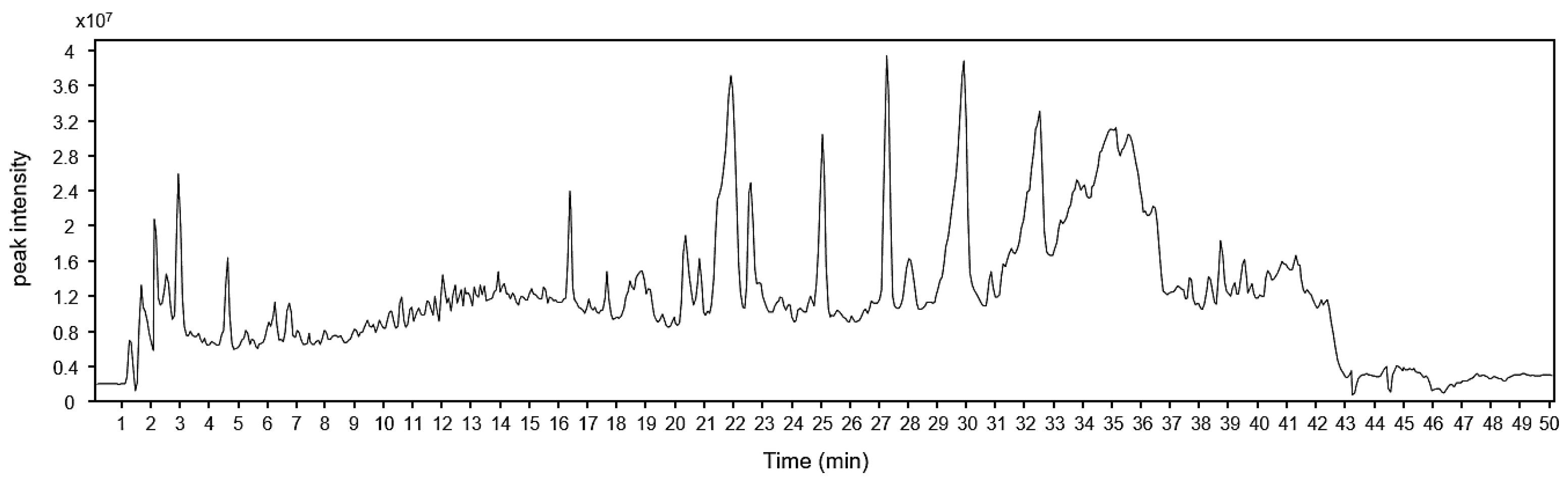
3.1. Chemical Profiling of Ice Plant
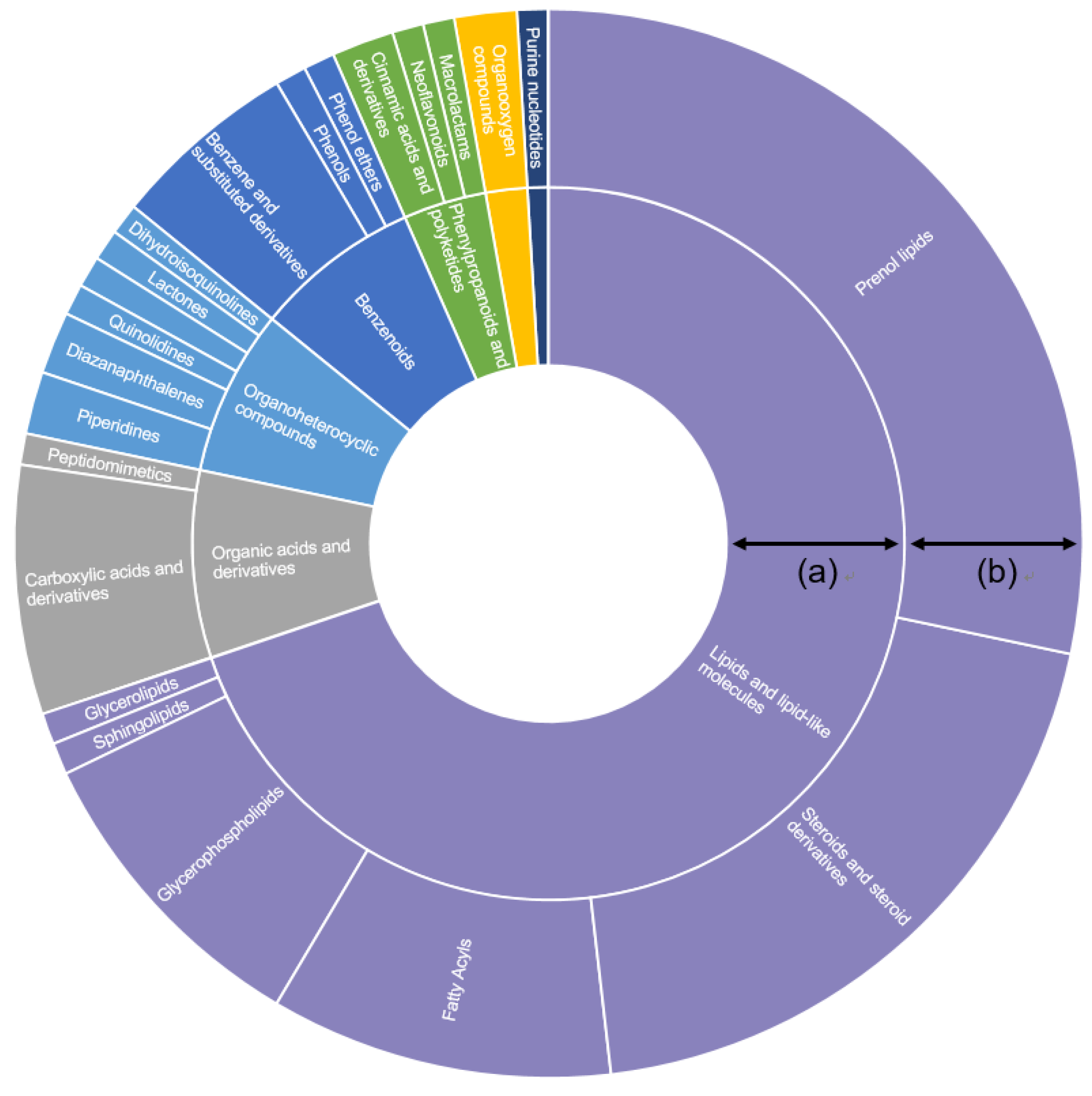
3.2. Identification of Phytochemicals in Ice Plant Extract
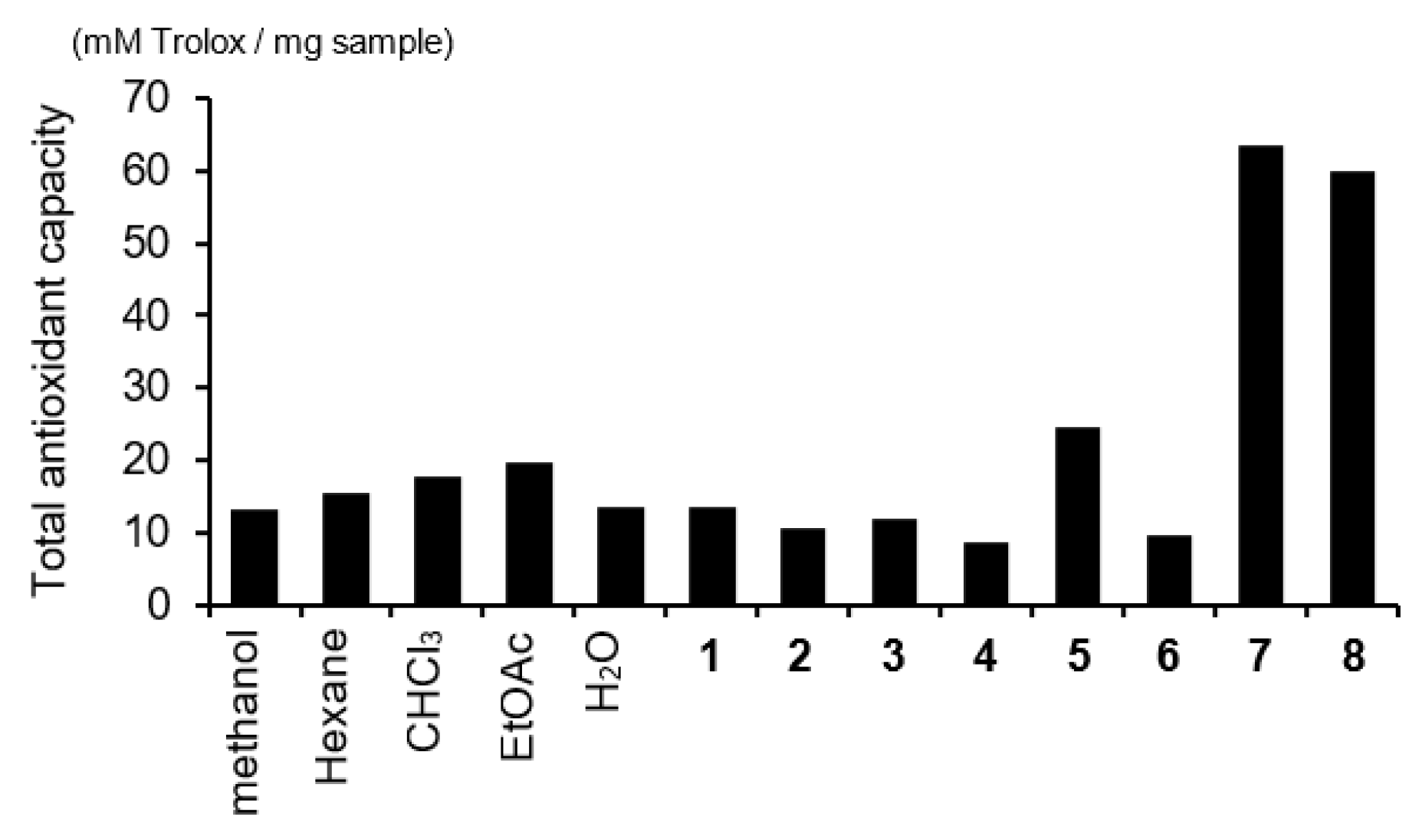
3.3. Antioxidant Activity
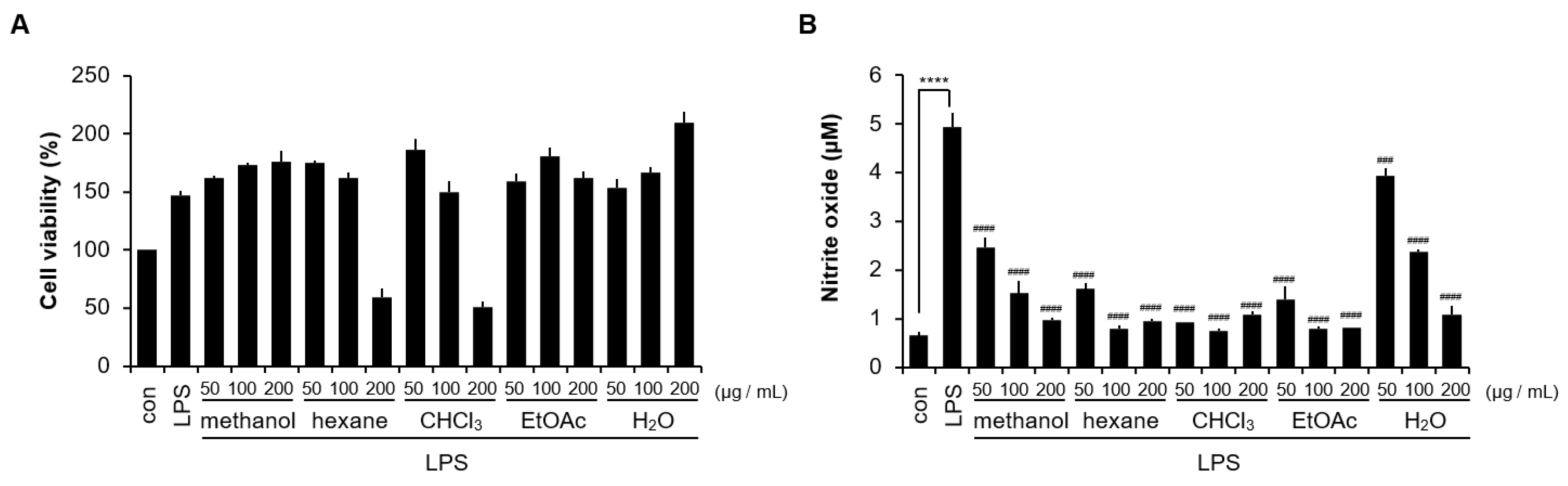
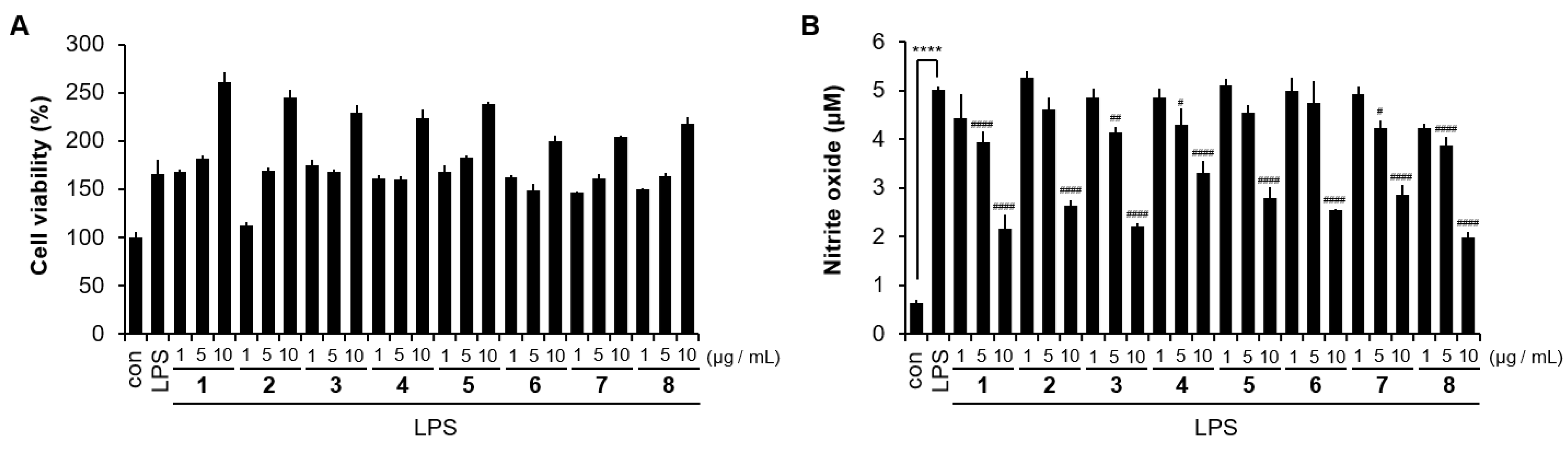
3.4. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Gupta, E.; Mishra, P. Functional food with some health benefits, so called superfood: A review. Curr. Nutr. Food Sci. 2021, 17, 144–166. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Najda, A.; Sharma, R.; Nurzyńska-Wierdak, R.; Dhanjal, D.S.; Sharma, R.; Manickam, S.; Kabra, A.; Kuča, K.; Bhardwaj, P. Fruit and vegetable peel-enriched functional foods: Potential avenues and health perspectives. Evid. Based Complement. Altern. Med. 2022, 2022, 8543881. [Google Scholar] [CrossRef]
- Agarie, S.; Kawaguchi, A.; Kodera, A.; Sunagawa, H.; Kojima, H.; Nose, A.; Nakahara, T. Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Prod. Sci. 2009, 12, 37–46. [Google Scholar] [CrossRef]
- Atzori, G. The potential of edible halophytes as new crops in saline agriculture: The ice plant (Mesembryanthemum crystallinum L.) case study. In Future of Sustainable Agriculture in Saline Environments; CRC Press: Boca Raton, FL, USA, 2021; Volume 3, pp. 443–460. [Google Scholar]
- Rodríguez-Hernández, M.d.C.; Garmendia, I. Optimum growth and quality of the edible ice plant under saline conditions. J. Sci. Food Agric. 2022, 102, 2686–2692. [Google Scholar] [CrossRef]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, antihypertensive, hypoglycaemic and nootropic activity of a polyphenolic extract from the halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Rowida, Y.E.; Elsebaie, E. Ice plant as antioxidant/anticancer and functional food. WJDFS 2018, 13, 57–62. [Google Scholar]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: An emerging approach to determine the composition of herbal products. Comput. Struct. Biotechnol. J. 2013, 4, e201301007. [Google Scholar] [CrossRef]
- Allwood, J.W.; Williams, A.; Uthe, H.; van Dam, N.M.; Mur, L.A.; Grant, M.R.; Pétriacq, P. Unravelling plant responses to stress—The importance of targeted and untargeted metabolomics. Metabolites 2021, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Park, S.; Kim, H.; Choi, G.J.; Kim, S.H. Application of UPLC-QTOF-MS based untargeted metabolomics in identification of metabolites induced in pathogen-infected rice. Plants 2021, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Perez da Graça, J.; Porto, C.; Martin do Prado, R.; Nunes, E.; Corrêa Marcelino-Guimarães, F.; Conrado Meyer, M.; Jorge Pilau, E. Untargeted metabolomics analysis by UHPLC-MS/MS of soybean plant in a compatible response to Phakopsora pachyrhizi infection. Metabolites 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef]
- da Silva, R.R.; Wang, M.; Nothias, L.-F.; van der Hooft, J.J.; Caraballo-Rodríguez, A.M.; Fox, E.; Balunas, M.J.; Klassen, J.L.; Lopes, N.P.; Dorrestein, P.C. Propagating annotations of molecular networks using in silico fragmentation. PLoS Comput. Biol. 2018, 14, e1006089. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminf. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264. 7 cells: In vitro assessment and a theoretical model. BioMed Res. Int. 2019, 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Król, W.; Czuba, Z.P.; Threadgill, M.D.; Cunningham, B.D.; Pietsz, G. Inhibition of nitric oxide (NO•) production in murine macrophages by flavones. Biochem. Pharmacol. 1995, 50, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Stander, M.A.; Stafford, G.I.; Makunga, N.P. Mass spectrometry metabolomics and feature-based molecular networking reveals population-specific chemistry in some species of the Sceletium genus. Front. Nutr. 2022, 9, 819753. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Quirós-Guerrero, L.; Muribeca, A.; Reis, J.; Pamplona, S.; Lima, A.H.; Trindade, M.; Silva, C.; Souza, J.N.; Boutin, J. Constituents of Chamaecrista diphylla (L.) greene leaves with potent antioxidant capacity: A feature-based molecular network dereplication approach. Pharmaceutics 2021, 13, 681. [Google Scholar] [CrossRef]
- Surprenant, H.L.; Sarneski, J.E.; Key, R.R.; Byrd, J.T.; Reilley, C.N. Carbon-13 NMR studies of amino acids: Chemical shifts, protonation shifts, microscopic protonation behavior. J. Magn. Reson. 1980, 40, 231–243. [Google Scholar] [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G. Biological magnetic resonance data bank. Nucleic Acids Res. 2023, 51, D368–D376. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, P.; Casati, S.; Manzocchi, A. Complete 1H and 13C NMR spectral assignment of α-and β-adenosine, 2′-deoxyadenosine and their acetate derivatives. Magn. Reson. Chem. 2007, 45, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J.; Jimeno, M.L.; Yranzo, G.I. 1H and 13C NMR study of the structure of pyrazoles, imidazoles and their benzo derivatives in sulphuric acid (azolium cations). Magn. Reson. Chem. 1990, 28, 807–811. [Google Scholar] [CrossRef]
- Yang, F.-L.; Zhu, X.; Rao, D.-K.; Cao, X.-N.; Li, K.; Xu, Y.; Hao, X.-Q.; Song, M.-P. Highly efficient synthesis of primary amides via aldoximes rearrangement in water under air atmosphere catalyzed by an ionic ruthenium pincer complex. RSC Adv. 2016, 6, 37093–37098. [Google Scholar] [CrossRef]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), Thermogravimetric and Antimicrobial Studies of Ca (II), Mn (II), Cu (II), Zn (II) and Cd (II) Complexes of Ferulic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Karker, M.; Falleh, H.; Msaada, K.; Smaoui, A.; Abdelly, C.; Legault, J.; Ksouri, R. Antioxidant, anti-inflammatory and anticancer activities of the medicinal halophyte Reaumuria vermiculata. EXCLI J. 2016, 15, 297. [Google Scholar]
- Mazouz, W.; Haouli, N.E.H.; Gali, L.; Vezza, T.; Bensouici, C.; Mebrek, S.; Hamel, T.; Galvez, J.; Djeddi, S. Antioxidant, anti-alzheimer, anti-diabetic, and anti-inflammatory activities of the endemic halophyte Limonium spathulatum (Desf.) kuntze on LPS-stimulated RAW264 macrophages. S. Afr. J. Bot. 2020, 135, 101–108. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Kang, Y.-W.; Joo, N.-M. Comparative analysis on phytochemical properties, anti-oxidative, and anti-inflammatory activities of the different organs of the common ice plant Mesembryanthemum crystallinum L. Appl. Sci. 2023, 13, 2527. [Google Scholar] [CrossRef]
- Chouhan, H.S.; Sahu, A.N.; Singh, S.K. Fatty acid composition, antioxidant, anti-inflammatory and antibacterial activities of seed oil from Crotalaria juncea Linn. J. Med. Plants Res. 2011, 5, 984–991. [Google Scholar]
- de Morais, S.M.; do Nascimento, J.E.T.; de Sousa Silva, A.A.; Junior, J.E.R.H.; Pinheiro, D.C.S.N.; de Oliveira, R.V. Fatty acid profile and anti-inflammatory activity of fixed plant oils. Acta Sci. Vet. 2017, 45, 8. [Google Scholar] [CrossRef][Green Version]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential antiinflammatory agents: Current status and future perspectives. J. Phytopharmacol. 2015, 4, 121–125. [Google Scholar] [CrossRef]


| Sample | Concentration (µg/mL) | DPPH (% Inhibition) | FRAP (µg/mL) |
|---|---|---|---|
| 5 | 20 | 0.68 ± 0.60 | 1.50 ± 0.05 |
| 40 | 4.61 ± 3.15 | 2.03 ± 0.14 | |
| 60 | 2.81 ± 1.54 | 2.74 ± 0.05 | |
| 80 | 4.07 ± 2.87 | 3.67 ± 0.09 | |
| 100 | 7.97 ± 0.92 | 4.04 ± 0.28 | |
| 7 | 20 | 1.74 ± 1.00 | 7.37 ± 0.14 |
| 40 | 5.59 ± 2.05 | 10.40 ± 0.66 | |
| 60 | 3.64 ± 3.33 | 13.79 ± 0.42 | |
| 80 | 7.06 ± 2.42 | 17.07 ± 0.82 | |
| 100 | 8.52 ± 2.35 | 14.53 ± 0.31 | |
| 8 | 20 | 2.36 ± 1.58 | 9.20 ± 0.44 |
| 40 | 7.63 ± 1.23 | 15.39 ± 0.80 | |
| 60 | 10.75 ± 2.22 | 18.83 ± 0.52 | |
| 80 | 15.81 ± 2.96 | 24.36 ± 0.16 | |
| 100 | 21.88 ± 2.29 | 71.00 ± 3.05 | |
| Trolox | 80 | 50.73 ± 0.26 | - |
| Ascorbic acid | 80 | - | 85.56 ± 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.; Han, A.-R.; Lee, J.; Choi, S.Y.; Choi, J.W.; Song, N.-E.; Hong, H.-D.; Rhee, Y.K.; Cho, C.-W. LC-QTOF/MS-Based Profiling of the Phytochemicals in Ice Plant (Mesembryanthemum crystallinum) and Their Bioactivities. Foods 2024, 13, 1820. https://doi.org/10.3390/foods13121820
Oh M, Han A-R, Lee J, Choi SY, Choi JW, Song N-E, Hong H-D, Rhee YK, Cho C-W. LC-QTOF/MS-Based Profiling of the Phytochemicals in Ice Plant (Mesembryanthemum crystallinum) and Their Bioactivities. Foods. 2024; 13(12):1820. https://doi.org/10.3390/foods13121820
Chicago/Turabian StyleOh, Mira, Ah-Ram Han, Jaeyoun Lee, Sang Yoon Choi, Jae Woong Choi, Nho-Eul Song, Hee-Do Hong, Young Kyoung Rhee, and Chang-Won Cho. 2024. "LC-QTOF/MS-Based Profiling of the Phytochemicals in Ice Plant (Mesembryanthemum crystallinum) and Their Bioactivities" Foods 13, no. 12: 1820. https://doi.org/10.3390/foods13121820
APA StyleOh, M., Han, A.-R., Lee, J., Choi, S. Y., Choi, J. W., Song, N.-E., Hong, H.-D., Rhee, Y. K., & Cho, C.-W. (2024). LC-QTOF/MS-Based Profiling of the Phytochemicals in Ice Plant (Mesembryanthemum crystallinum) and Their Bioactivities. Foods, 13(12), 1820. https://doi.org/10.3390/foods13121820






