Pulsed Electric Field Pretreatments Affect the Metabolite Profile and Antioxidant Activities of Freeze− and Air−Dried New Zealand Apricots
Abstract
1. Introduction
2. Materials and Methods
2.1. Apricot Samples
2.2. PEF Treatment and Drying
2.3. Physical Analysis
2.3.1. Texture Evaluation
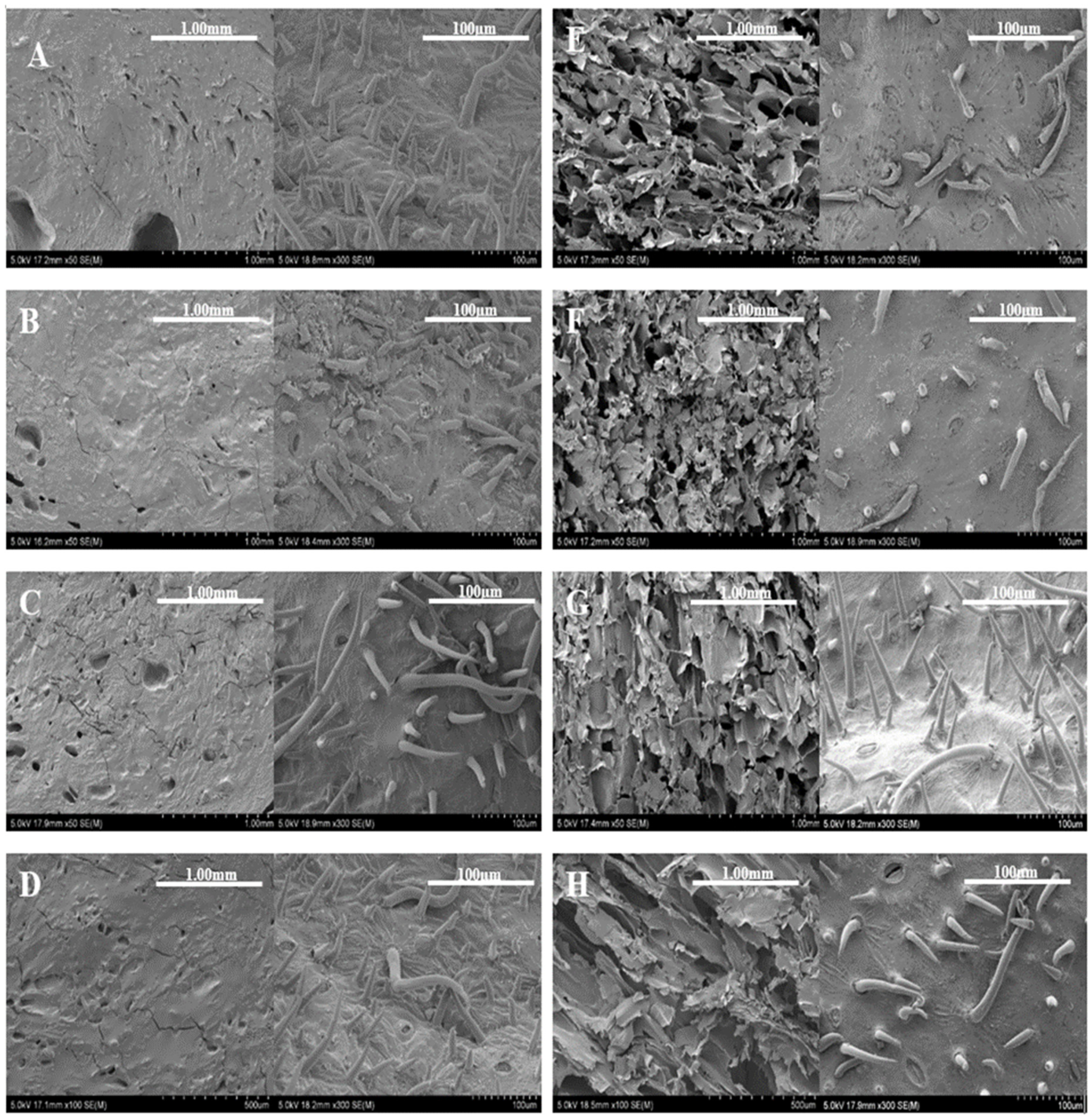
2.3.2. Scanning Electron Microscopy Imaging Analysis
2.3.3. Moisture Content and Water Activity
2.4. Chemical Analysis
2.4.1. Sample Extraction Prior to Antioxidant Analysis
2.4.2. Cupric−Reducing Antioxidant Capacity (CUPRAC)
2.4.3. Ferric−Reducing Antioxidant Power (FRAP)
2.4.4. Total Phenolic Content (TPC)
2.4.5. Metabolite Analysis
TMS Derivatisation
MCF Derivatisation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Analysis
Moisture Content and Water Activity
3.2. Texture
3.3. Scanning Electron Microscopy (SEM) Imaging and Analysis
3.4. Chemical Analysis
3.4.1. Antioxidant Activities
3.4.2. Total Phenol Content (TPC)
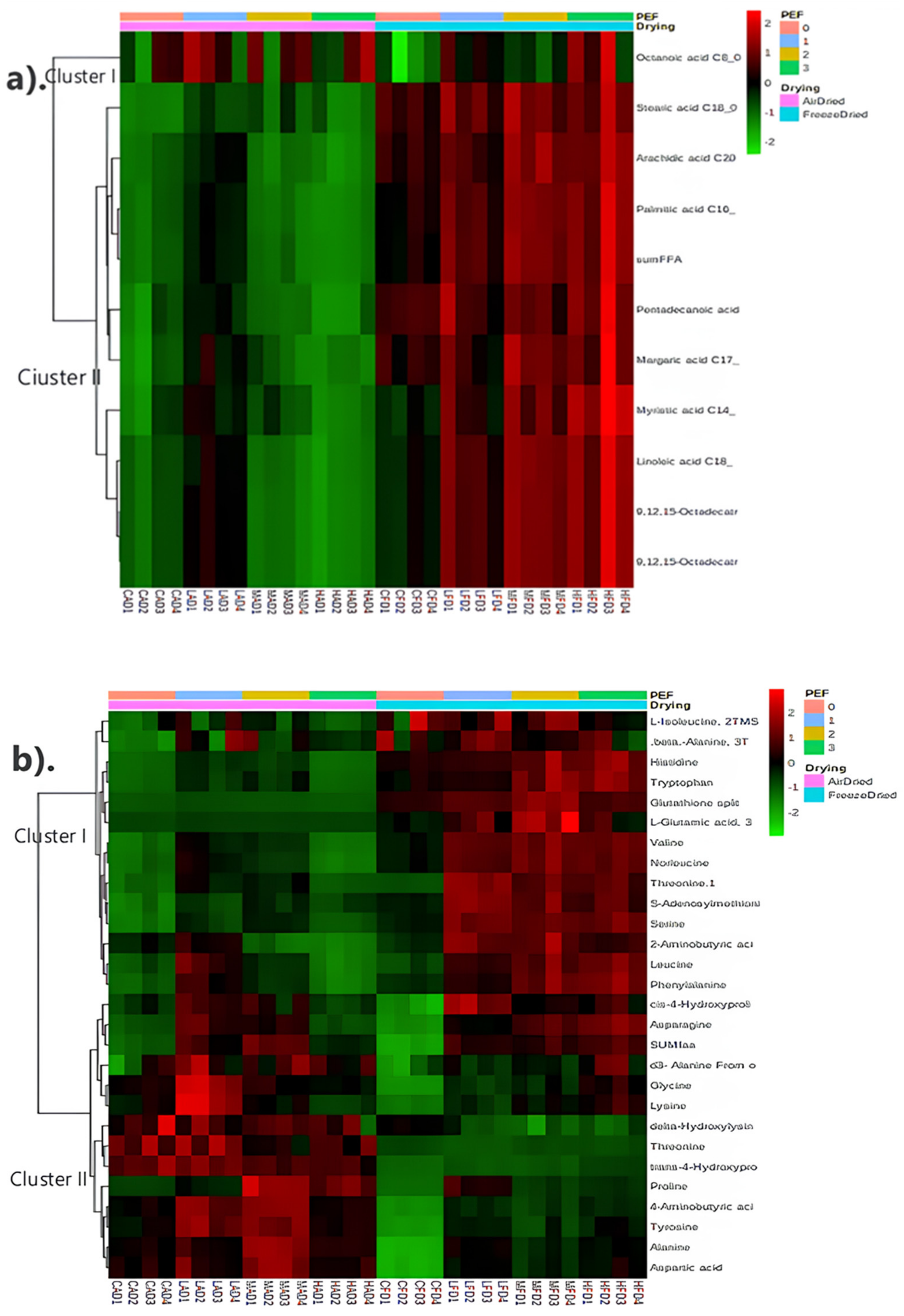
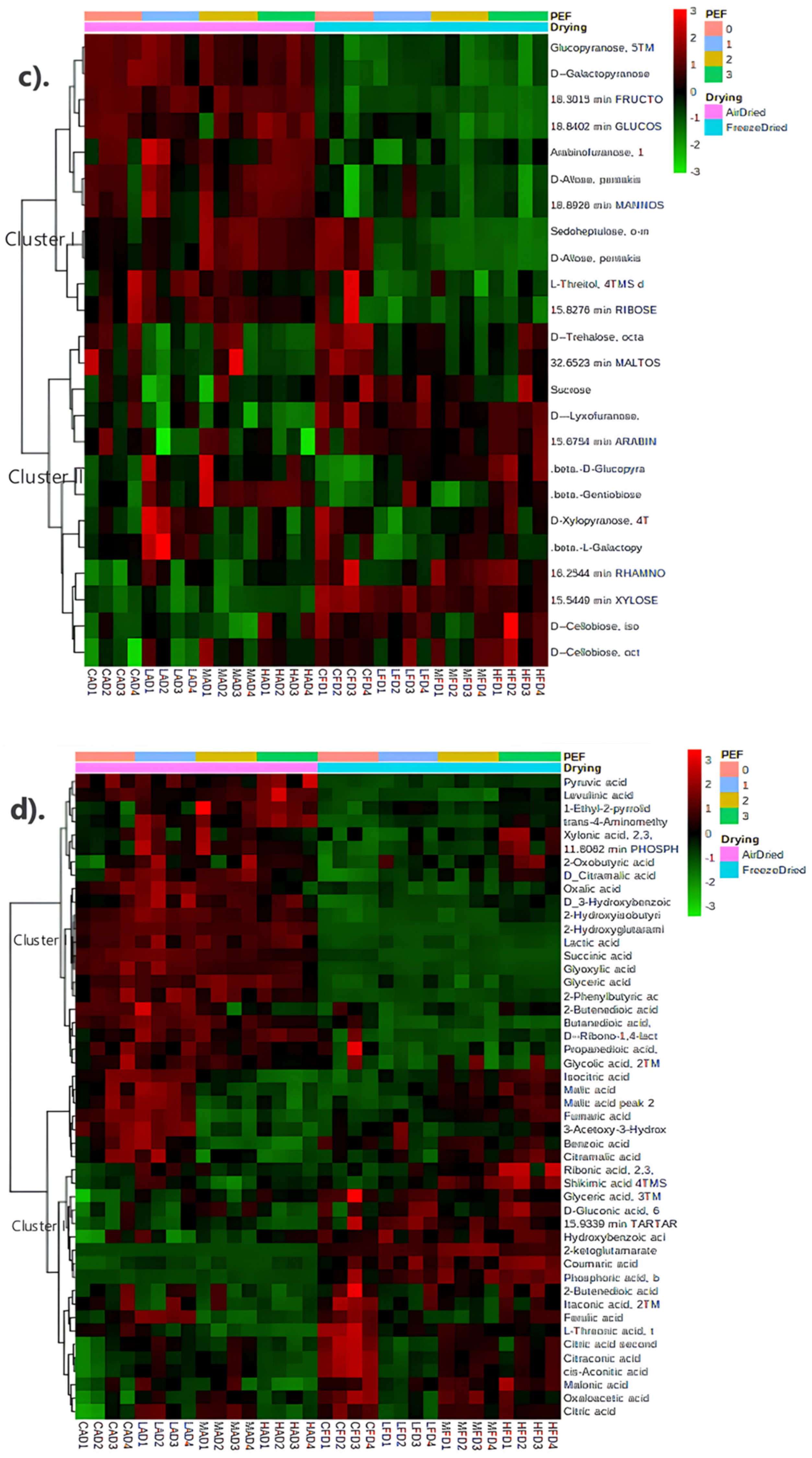
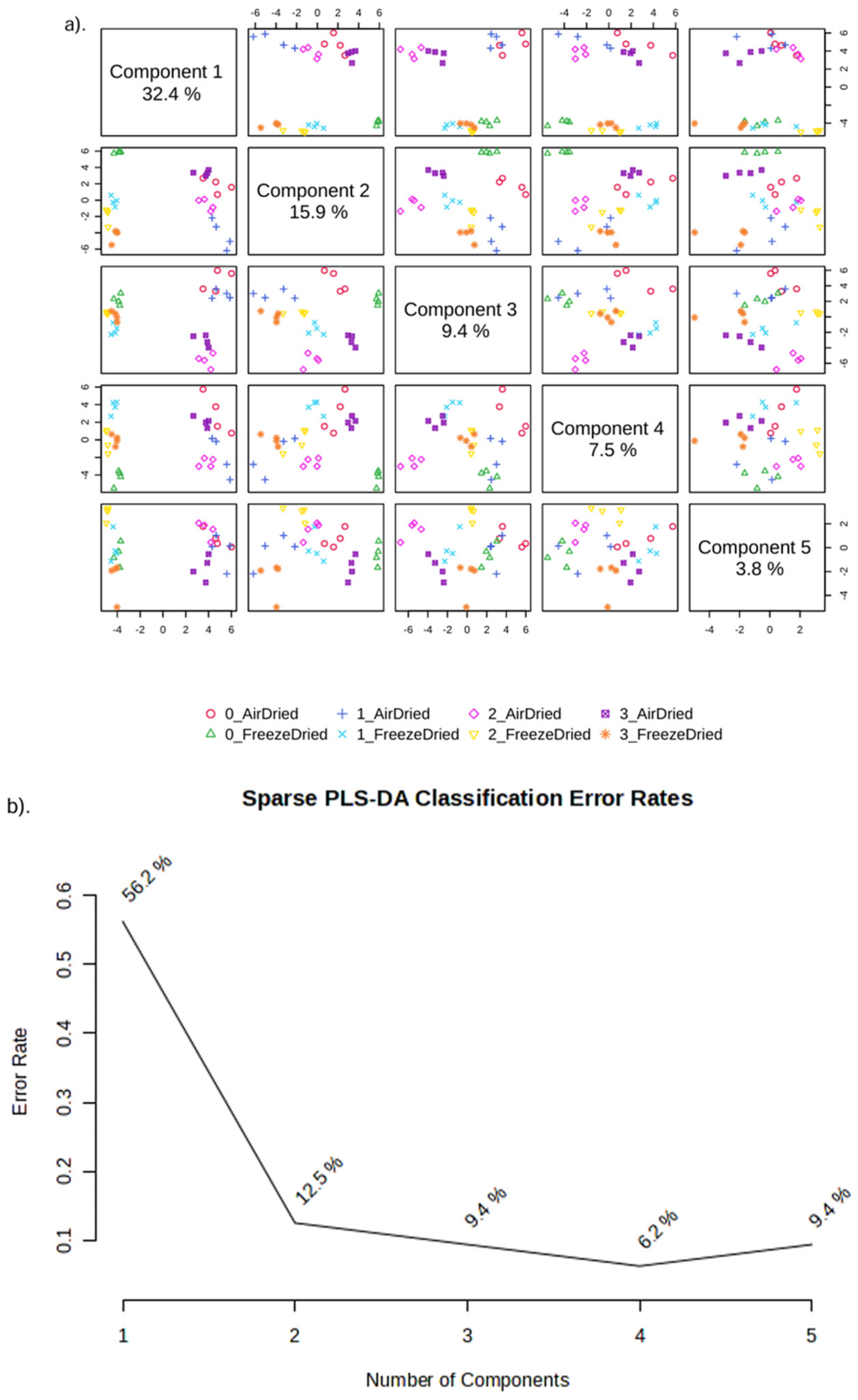
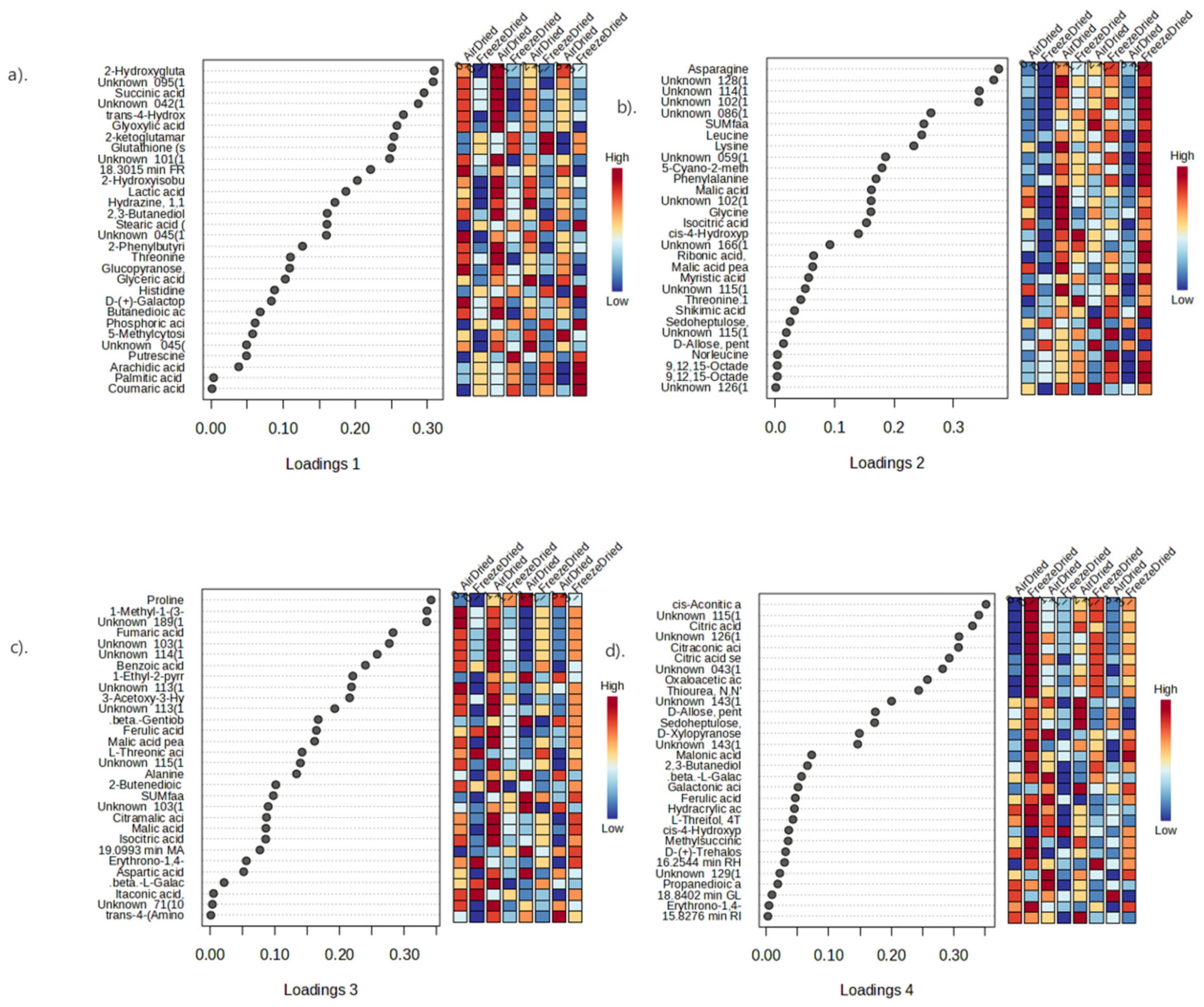
3.4.3. Metabolite Profiling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Category | Threonine | trans-4-Hydroxyproline | Glutathione (Split Peak 3) | Histidine | Palmitic Acid (C16_0) | Stearic Acid (C18_0) | Arachidic Acid (C20_0) | Succinic Acid | Lactic Acid | Glyoxylic Acid |
| air dried | 0.001 a | 0.000 a | 0.000 b | 0.000 b | 0.013 b | 0.017 b | 0.000 b | 0.042 a | 0.015 a | 0.001 a |
| freeze dried | 0.000 b | 0.000 b | 0.001 a | 0.001 a | 0.022 a | 0.028 a | 0.000 a | 0.011 b | 0.004 b | 0.000 b |
| Pr > F(Drying method) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Category | 2-Hydroxyisobutyric Acid | Glyceric Acid | 2-Hydroxyglutaramic Acid MCF1 | 2-Ketoglutamarate | Coumaric Acid | Phosphoric Acid, Bis(trimethylsilyl)monomethyl Ester | Butanedioic Acid, 2TMS Derivative | 2-Phenylbutyric Acid, TBDMS Derivative | Glucopyranose, 5TMS Derivative | D-(+)-Galactopyranose, 5TMS Derivative (Isomer 1) |
| air dried | 0.000 a | 0.002 a | 0.002 a | 0.001 b | 0.001 b | 0.000 b | 0.036 a | 0.002 a | 0.164 a | 0.108 a |
| freeze dried | 0.000 b | 0.001 b | 0.000 b | 0.015 a | 0.002 a | 0.000 a | 0.016 b | 0.000 b | 0.058 b | 0.035 b |
| Pr > F(Drying method) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Category | 18.3015 min FRUCTOSE Oxime1 | 2,3-Butanediol | Hydrazine, 1,1-Dimethyl-2-propyl- (NIST) | Putrescine | 5-Methylcytosine, 2TMS Derivative | Unknown 045(100) 43(14.2) 57(11.6) | Unknown 095(100) 126(31.0) 39(11.2) | Unknown 042(100) 41(57.3) 86(37.5) | Unknown 045(100) 59(89.0) 74(67.8) | Unknown 101(100) 55(35.6) 117(28.9) |
| air dried | 1.873 a | 0.001 a | 0.001 a | 0.001 b | 0.005 a | 0.001 a | 0.001 a | 0.000 a | 0.016 a | 0.000 a |
| freeze dried | 0.937 b | 0.000 b | 0.000 b | 0.001 a | 0.000 b | 0.000 b | 0.000 b | 0.000 b | 0.010 b | 0.000 b |
| Pr > F(Drying method) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Category | Glycine | Leucine | Norleucine | Threonine.1 | Asparagine | cis-4-Hydroxyproline | Phenylalanine | Lysine | SUMfaa | Myristic Acid (C14_0) |
| 3_FreezeDried | 0.009 a | 0.027 a | 0.024 ab | 0.003 b | 0.265 a | 0.001 b | 0.011 a | 0.002 a | 0.559 a | 0.000 a |
| 2_FreezeDried | 0.007 b | 0.027 a | 0.025 a | 0.003 b | 0.243 b | 0.000 b | 0.011 a | 0.002 b | 0.532 a | 0.000 b |
| 1_FreezeDried | 0.006 b | 0.025 b | 0.023 b | 0.003 a | 0.205 c | 0.001 a | 0.009 b | 0.001 c | 0.531 a | 0.000 b |
| 0_FreezeDried | 0.003 c | 0.018 c | 0.018 c | 0.002 c | 0.133 d | 0.000 c | 0.007 c | 0.001 d | 0.317 b | 0.000 c |
| Pr > F(PEF-Drying) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.000 |
| Category | 9,12,15-Octadecatrienoic Acid, Methyl Ester, (Z,Z,Z)- | D_Citramalic Acid | Malic Acid Peak 2 | Isocitric Acid | Ribonic Acid, 2,3,4,5-Tetrakis-O-(trimethylsilyl)-, Trimethylsilyl Ester | Shikimic Acid (4TMS) | Sedoheptulose, o-Methyloxime, Hexakis-O-(trimethylsilyl)- | D-Allose, Pentakis(trimethylsilyl) Ether, Ethyloxime (Isomer 1) | 5-Cyano-2-methyl-4-methylthio-6-phenylpyrimidine | Unknown 126(100) 141(46.7) 54(33.2) |
| 3_FreezeDried | 0.012 a | 0.003 a | 0.237 a | 0.004 a | 0.020 a | 0.004 a | 0.002 b | 0.004 b | 0.001 a | 0.000 a |
| 2_FreezeDried | 0.011 ab | 0.003 b | 0.227 a | 0.004 b | 0.011 b | 0.004 a | 0.002 b | 0.005 b | 0.000 b | 0.000 b |
| 1_FreezeDried | 0.010 b | 0.003 b | 0.210 b | 0.003 c | 0.008 b | 0.003 b | 0.003 b | 0.006 b | 0.000 b | 0.000 b |
| 0_FreezeDried | 0.007 c | 0.002 c | 0.204 b | 0.003 d | 0.006 b | 0.002 b | 0.012 a | 0.020 a | 0.000 c | 0.000 c |
| Pr > F(PEF-Drying) | 0.000 | 0.000 | <0.0001 | <0.0001 | 0.012 | 0.000 | <0.0001 | <0.0001 | <0.0001 | 0.000 |
| Category | Unknown 114(100) 59(15.3) 82(14.4) | Unknown 086(100) 59(56.6) 128(39.3) | Unknown 166(100) 139(94.5) 111(42.6) | Unknown 115(100) 69(73.8) 59(62.9) | Unknown 115(100) 55(17.7) 59(9.5) | Unknown 059(100) 73(69.3) 77(45.8) | Unknown 102(100) 58(0.86) 59(0.75) | Unknown 102(100) 103(26.6) 44(19.0) | Unknown 128(100) 129(0.69) 42(0.52) | |
| 3_FreezeDried | 0.001 a | 0.001 a | 0.000 a | 0.001 a | 0.006 a | 0.002 a | 0.005 a | 0.004 a | 0.002 a | |
| 2_FreezeDried | 0.001 b | 0.001 b | 0.000 b | 0.001 a | 0.006 b | 0.001 a | 0.004 a | 0.004 a | 0.002 a | |
| 1_FreezeDried | 0.000 c | 0.001 b | 0.000 a | 0.001 a | 0.005 c | 0.001 b | 0.004 a | 0.003 b | 0.001 b | |
| 0_FreezeDried | 0.000 d | 0.000 c | 0.000 b | 0.000 b | 0.005 d | 0.001 b | 0.002 b | 0.001 c | 0.001 c | |
| Pr > F(PEF-Drying) | <0.0001 | <0.0001 | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Category | Alanine | Aspartic Acid | Proline | SUMfaa | Fumaric Acid | Benzoic Acid | 3-Acetoxy-3-Hydroxy-2-methylpropionic Acid (NIST Match 88.6) | Malic Acid | Citramalic Acid | Isocitric Acid |
| 1_AirDried | 0.093 b | 0.001 b | 0.050 c | 0.529 b | 0.106 a | 0.014 a | 0.002 a | 0.252 a | 0.000 a | 0.004 a |
| 0_AirDried | 0.089 b | 0.001 b | 0.038 c | 0.391 d | 0.103 a | 0.013 a | 0.002 a | 0.230 b | 0.000 ab | 0.004 a |
| 2_AirDried | 0.121 a | 0.002 a | 0.117 a | 0.584 a | 0.069 b | 0.006 b | 0.001 b | 0.204 c | 0.000 bc | 0.003 b |
| 3_AirDried | 0.095 b | 0.001 b | 0.092 b | 0.451 c | 0.075 b | 0.005 b | 0.001 b | 0.201 c | 0.000 c | 0.003 c |
| Pr > F(PEF-Drying) | <0.0001 | 0.003 | <0.0001 | <0.0001 | <0.0001 | 0.001 | <0.0001 | 0.000 | 0.015 | <0.0001 |
| Category | Ferulic Acid | 2-Butenedioic Acid, (E)-, 2TMS Derivative | Itaconic Acid, 2TMS Derivative | 1-Ethyl-2-pyrrolidinecarboxylic Acid, Methyl Ester | L-Threonic Acid, Tris(trimethylsilyl) Ether, Trimethylsilyl Ester | trans-4-(Aminomethyl)cyclohexanecarboxylic Acid, N,N-Tris(trimethylsilyl), Trimethylsilyl Ester | .beta.-L-Galactopyranoside, Methyl 6-Deoxy-, (S,S,R,R,S)-, 3TMS Derivative | .beta.-Gentiobiose, Octakis(trimethylsilyl) Ether, Methyloxime (Isomer 1) | 19.0993 min MANNITOL | 1-Methyl-1-(3-octenoyloxy)-1-silacyclohexane (NIST) |
| 1_AirDried | 0.002 a | 0.015 a | 0.012 a | 0.001 bc | 0.009 b | 0.003 ab | 0.004 a | 0.004 ab | 0.004 b | 0.019 a |
| 0_AirDried | 0.002 b | 0.013 a | 0.017 a | 0.000 c | 0.011 a | 0.001 c | 0.001 b | 0.003 b | 0.003 b | 0.019 a |
| 2_AirDried | 0.001 c | 0.010 b | 0.010 a | 0.001 a | 0.007 c | 0.003 b | 0.001 b | 0.005 a | 0.004 a | 0.008 b |
| 3_AirDried | 0.001 bc | 0.009 b | 0.007 a | 0.001 ab | 0.007 c | 0.004 a | 0.001 b | 0.005 a | 0.004 ab | 0.010 b |
| Pr > F(PEF-Drying) | <0.0001 | 0.002 | 0.283 | 0.013 | 0.001 | 0.006 | 0.014 | 0.019 | 0.045 | <0.0001 |
| Category | Erythrono-1,4-lactone, (E)-, 2TMS Derivative | Unknown 113(100) 85(48.1) 59(21.2) | Unknown 103(100) 88(73.4) 71(66.9) | Unknown 71(100) 103(73.4) 131(66.9) | Unknown 115(100) 55(17.7) 59(9.5) | Unknown 113(100) 59(23.1) 85(16.0) | Unknown 114(100) 147(31.9) 115(27.2) | Unknown 103(100) 114(81.7) 71(55.6) | Unknown 189(100) 75(99.0) 113(90.9) | |
| 1_AirDried | 0.002 a | 0.041 a | 0.002 ab | 0.003 a | 0.007 a | 0.006 a | 0.009 a | 0.028 a | 0.004 a | |
| 0_AirDried | 0.002 a | 0.039 a | 0.002 b | 0.003 a | 0.007 a | 0.006 a | 0.009 a | 0.027 a | 0.004 a | |
| 2_AirDried | 0.001 a | 0.026 b | 0.003 a | 0.002 b | 0.006 b | 0.003 b | 0.004 b | 0.012 b | 0.002 b | |
| 3_AirDried | 0.001 a | 0.026 b | 0.002 a | 0.002 b | 0.004 c | 0.003 b | 0.005 b | 0.015 b | 0.002 b | |
| Pr > F(PEF-Drying) | 0.202 | <0.0001 | 0.013 | 0.006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Category | cis-4-Hydroxyproline | Malonic Acid | Oxaloacetic Acid | Citraconic Acid | cis-Aconitic Acid | Citric Acid Secondary Peak | Citric Acid | Ferulic Acid | Propanedioic Acid, 2TMS Derivative | Hydracrylic Acid, 2TMS Derivative |
| 0_FreezeDried | 0.000 c | 0.003 a | 0.000 a | 0.026 a | 0.092 a | 0.046 a | 0.353 a | 0.002 a | 0.001 a | 0.001 a |
| 2_FreezeDried | 0.000 b | 0.003 ab | 0.000 ab | 0.021 b | 0.077 b | 0.040 b | 0.341 a | 0.001 b | 0.001 b | 0.001 ab |
| 3_FreezeDried | 0.001 b | 0.003 a | 0.000 bc | 0.020 b | 0.076 b | 0.039 b | 0.343 a | 0.002 a | 0.001 ab | 0.001 ab |
| 1_FreezeDried | 0.001 a | 0.003 b | 0.000 c | 0.017 c | 0.066 c | 0.034 c | 0.309 b | 0.002 b | 0.001 b | 0.000 b |
| Pr > F(PEF-Drying) | <0.0001 | 0.069 | 0.001 | <0.0001 | <0.0001 | 0.000 | 0.001 | <0.0001 | 0.097 | 0.132 |
| Category | Galactonic Acid, .Gamma.-Lactone, 4TMS Derivavative | Methylsuccinic Acid, 2TMS Derivative | D-Xylopyranose, 4TMS Derivative | .beta.-L-Galactopyranoside, Methyl 6-Deoxy-, (S,S,R,R,S)-, 3TMS Derivative | Sedoheptulose, o-Methyloxime, Hexakis-O-(trimethylsilyl)- | L-Threitol, 4TMS Derivative | D-(+)-Trehalose, Octakis(trimethylsilyl) Ether | D-Allose, Pentakis(trimethylsilyl) Ether, Ethyloxime (Isomer 1) | 18.8402 min GLUCOSE Oxime1 | 16.2544 min RHAMNOSE Oxime1 |
| 0_FreezeDried | 0.007 ab | 0.004 a | 0.022 a | 0.002 a | 0.012 a | 0.003 a | 0.014 a | 0.020 a | 1.503 ab | 0.007 ab |
| 2_FreezeDried | 0.007 ab | 0.003 ab | 0.016 ab | 0.002 ab | 0.002 b | 0.001 ab | 0.005 b | 0.005 b | 1.393 bc | 0.007 a |
| 3_FreezeDried | 0.009 a | 0.004 ab | 0.017 ab | 0.001 ab | 0.002 b | 0.001 b | 0.008 b | 0.004 b | 1.358 c | 0.007 ab |
| 1_FreezeDried | 0.006 b | 0.003 b | 0.011 b | 0.001 b | 0.003 b | 0.001 b | 0.006 b | 0.006 b | 1.599 a | 0.006 b |
| Pr > F(PEF-Drying) | 0.089 | 0.082 | 0.026 | 0.044 | <0.0001 | 0.104 | 0.001 | <0.0001 | 0.003 | 0.139 |
| Category | 15.8276 min RIBOSE Oxime1 | Thiourea, N,N′-Di-2-propenyl- (NIST) | Erythrono-1,4-lactone, (E)-, 2TMS Derivative | 2,3-Butanediol, O-(trimethylsilyl)-, Monoacetate | Unknown 043(100) 59(16.5) 74(15.0) | Unknown 126(100) 127(46.7) 59(33.2) | Unknown 143(100) 157(92.0) 101(60.9) | Unknown 129(100) 157(62.8) 97(29.6) | Unknown 115(100) 59(65.5) 189(50.1) | Unknown 143(100) 101(42.3) 201(15.1) |
| 0_FreezeDried | 0.006 a | 0.003 a | 0.003 a | 0.002 a | 0.016 a | 0.001 a | 0.000 a | 0.000 b | 0.050 a | 0.007 a |
| 2_FreezeDried | 0.005 ab | 0.002 b | 0.002 ab | 0.002 ab | 0.012 b | 0.001 b | 0.000 b | 0.000 b | 0.042 b | 0.005 b |
| 3_FreezeDried | 0.005 ab | 0.002 b | 0.002 ab | 0.002 ab | 0.012 bc | 0.001 b | 0.000 b | 0.000 a | 0.040 b | 0.005 b |
| 1_FreezeDried | 0.005 b | 0.001 b | 0.002 b | 0.001 b | 0.010 c | 0.001 c | 0.000 b | 0.000 b | 0.036 c | 0.005 b |
| Pr > F(PEF-Drying) | 0.133 | 0.007 | 0.061 | 0.099 | <0.0001 | <0.0001 | 0.019 | 0.018 | <0.0001 | 0.001 |
References
- İncedayi, B.; Tamer, C.E.; Sinir, G.Ö.; Suna, S.; Çopur, Ö.U. Impact of different drying parameters on color, β−carotene, antioxidant activity and minerals of apricot (Prunus armeniaca L.). Food Sci. Technol. 2016, 36, 171–178. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, A.; Abrol, G.S.; Joshi, V.K. Value addition of wild apricot fruits grown in North–West Himalayan regions−a review. J. Food Sci. Technol. 2014, 51, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Butt, M.S.; Greiby, I. Apricots production, processing, and nutrition. In Handbook of Fruits and Fruit Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 385–398. [Google Scholar]
- Food and Agriculture Organization. Fruit Production in the World. Available online: https://www.fao.org/faostat (accessed on 21 June 2022).
- Pawar, K.R.; Nema, P.K. Apricot kernel characterization, oil extraction, and its utilization: A review. Food Sci. Biotechnol. 2023, 32, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Masud, T.; Abbasi, K.S. Physico−chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.-D.A. Effect of pulsed electric fields (PEF) on physico−chemical properties, β−carotene and antioxidant activity of air−dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, C.F.; Fourie, P.C. Dehydration of apricots without sulphur dioxide. In Proceedings of the XI International Symposium on Apricot Culture, Veroia, Greece, 25 May 1997; Volume 488. [Google Scholar]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Fauster, T.; Giancaterino, M.; Pittia, P.; Jaeger, H. Effect of pulsed electric field pretreatment on shrinkage, rehydration capacity and texture of freeze−dried plant materials. LWT 2020, 121, 108937. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined effect of pulsed electric field and osmotic dehydration pre−treatments on mass transfer and quality of air dried goji berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Siemer, C.; Toepfl, S.; Mykhailyk, V.; Gondek, E.; Rybak, K.; Witrowa-Rajchert, D.; Parniakov, O. The effects of pulsed electric fields on the quality parameters of freeze−dried apples. J. Food Eng. 2019, 252, 36–43. [Google Scholar] [CrossRef]
- Ciurzynska, A.; Trusinska, M.; Rybak, K.; Wiktor, A.; Nowacka, M. The Influence of Pulsed Electric Field and Air Temperature on the Course of Hot−Air Drying and the Bioactive Compounds of Apple Tissue. Molecules 2023, 28, 2970. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of thermal and non−thermal technologies on kinetics and the main quality parameters of red bell pepper dried with convective and microwave–convective methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Almeida, R.L.; Brito, A.C.; Silva, V.M.; Albuquerque, J.C.; Saraiva, M.M.; Santos, R.M.; De Sousa, F.M.; Ribeiro, V.H.; Carvalho, R.D.; et al. Effect of pulse electric field (PEF) intensity combined with drying temperature on mass transfer, functional properties, and in vitro digestibility of dehydrated mango peels. J. Food Meas. Charact. 2023, 17, 5219–5233. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Praporscic, I.; Vorobiev, E. Effect of moderate thermal and pulsed electric field treatments on textural properties of carrots, potatoes and apples. Innov. Food Sci. Emerg. Technol. 2004, 5, 9–16. [Google Scholar] [CrossRef]
- Tylewicz, U.; Mannozzi, C.; Castagnini, J.M.; Genovese, J.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Application of PEF−and OD−assisted drying for kiwifruit waste valorisation. Innov. Food Sci. Emerg. Technol. 2022, 77, 102952. [Google Scholar] [CrossRef]
- Alam, M.R.; Lyng, J.G.; Frontuto, D.; Marra, F.; Cinquanta, L. Effect of pulsed electric field pretreatment on drying kinetics, color, and texture of parsnip and carrot. J. Food Sci. 2018, 83, 2159–2166. [Google Scholar] [CrossRef]
- Ozkan, G.; Stübler, A.S.; Aganovic, K.; Dräger, G.; Esatbeyoglu, T.; Capanoglu, E. Retention of polyphenols and vitamin C in cranberrybush purée (Viburnum opulus) by means of non−thermal treatments. Food Chem. 2021, 360, 129918. [Google Scholar] [CrossRef] [PubMed]
- Lakka, A.; Bozinou, E.; Stavropoulos, G.; Samanidis, I.; Athanasiadis, V.; Dourtoglou, V.G.; Lalas, S.I. Enhancement of polyphenols recovery from Rosa canina, Calendula officinalis and Castanea sativa using Pulsed Electric Field. Beverages 2021, 7, 63. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Evaluation of pulsed electric field polyphenol extraction from Vitis vinifera, Sideritis scardica and Crocus sativus. ChemEngineering 2021, 5, 25. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non−thermal processing treatments on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI (–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Villas-Bôas, S.G.; Noel, S.; Lane, G.A.; Attwood, G.; Cookson, A. Extracellular metabolomics: A metabolic footprinting approach to assess fiber degradation in complex media. Anal. Biochem. 2006, 349, 297–305. [Google Scholar] [CrossRef]
- Smart, K.F.; Aggio, R.B.M.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air−drying and freeze−drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Fontana, A.J., Jr. D: Minimum Water Activity Limits for Growth of Microorganisms. Water Act. Foods 2007, 406, 571–572. [Google Scholar]
- Acevedo, N.C.; Briones, V.; Buera, P.; Aguilera, J.M. Microstructure affects the rate of chemical, physical and color changes during storage of dried apple discs. J. Food Eng. 2008, 85, 222–231. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, L.; Vorobiev, E.; Kechaou, N. Effect of pulsed electric field and osmotic dehydration pretreatment on the convective drying of carrot tissue. Dry. Technol. 2008, 26, 231–238. [Google Scholar] [CrossRef]
- Amami, E.; Vorobiev, E.; Kechaou, N. Effect of pulsed electric field on the osmotic dehydration and mass transfer kinetics of apple tissue. Dry. Technol. 2005, 23, 581–595. [Google Scholar] [CrossRef]
- Wiktor, A.; Śledź, M.; Nowacka, M.; Chudoba, T.; Witrowa-Rajchert, D. Pulsed electric field pretreatment for osmotic dehydration of apple tissue: Experimental and mathematical modeling studies. Dry. Technol. 2014, 32, 408–417. [Google Scholar] [CrossRef]
- Ramos, I.N.; Brandão, T.R.; Silva, C.L. Structural changes during air drying of fruits and vegetables. Food Sci. Technol. Int. 2003, 9, 201–206. [Google Scholar] [CrossRef]
- Mujumdar, A.S. Book Review: Handbook of Industrial Drying: A Review of “Publisher: CRC Press. Boca Raton, FL, 2007”. Dry. Technol. 2007, 25, 1133–1134. [Google Scholar]
- Ratti, C. Hot air and freeze−drying of high−value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Kovacs, E.; Meresz, P.; Kristof, Z.; Nemeth-Szerdahelyi, E. Ripening and microstructure of apricot (Prunus Armeniaca L.). Acta Aliment. 2008, 37, 23–39. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted vacuum freeze−drying of apple tissue. Innov. Food Sci. Emerg. Technol. 2016, 35, 52–57. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food processing and maillard reaction products: Effect on human health and nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum−drying, hot air−drying and freeze−drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of pulsed electric field treatment for the extraction of bioactive compounds from blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Influence of pulsed electric fields processing on the bioaccessible and non−bioaccessible fractions of apple phenolic compounds. J. Funct. Foods 2019, 59, 206–214. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Zammit, N.; Munro, B.R.; Murchie, S.; Bowyer, M.C.; Scarlett, C.J. Effect of Drying Conditions on Physicochemical and Antioxidant Properties of V itex agnus−castus Leaves. J. Food Process. Preserv. 2015, 39, 2562–2571. [Google Scholar] [CrossRef]
- Siddeeg, A.; Zeng, X.; Rahaman, A.; Manzoor, M.F.; Ahmed, Z.; Ammar, A. Effect of pulsed electric field pretreatment of date palm fruits on free amino acids, bioactive components, and physicochemical characteristics of the alcoholic beverage. J. Food Sci. 2019, 84, 3156–3162. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Akdemir Evrendilek, G. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.-A.; Rahaman, A.; Aadil, R.M.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Mtaoua, H.; Sánchez-Vega, R.; Ferchichi, A.; Martín-Belloso, O. Impact of high−intensity pulsed electric fields or thermal treatment on the quality attributes of date juice through storage. J. Food Process. Preserv. 2017, 41, e13052. [Google Scholar] [CrossRef]
- Wang, L.; Wen, H.; Yang, N.; Li, H. Effect of vacuum freeze drying and hot air drying on dried mulberry fruit quality. PLoS ONE 2023, 18, e0283303. [Google Scholar] [CrossRef]
- Hu, J.; Sun, X.; Yang, F.; Vidyarthi, S.K.; Xiao, H.; Liu, C.; Duan, X.; Wang, H. Changes in, and correlation analysis of, volatile compounds, key enzymes, and fatty acids in lemon juice vesicles during freeze drying and hot−air drying. J. Sci. Food Agric. 2023, 103, 6330–6339. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Wang, M.; Lu, R. Effects of pulsed electric fields on bioactive components, colour and flavour of green tea infusions. Int. J. Food Sci. Technol. 2009, 44, 312–321. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, R.; Liu, C.; Wu, Y.; Duan, X.; Hu, J.; Wang, H. Dynamic changes in volatile flavor compounds, amino acids, organic acids, and soluble sugars in lemon juice vesicles during freeze−drying and hot−air drying. Foods 2022, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Sikora, F.; Lachowicz, S.; Kapusta, I.; Dylawerski, M.; Zyska, W.; Ochmian, I. Changes in the Quality of Old Apple Cultivars After Freeze−Drying. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 175–185. [Google Scholar] [CrossRef]
- Dönmez, A.; Kadakal, Ç. Hot−air drying and degradation kinetics of bioactive compounds of gilaburu (Viburnum opulus L.) fruit. Chem. Ind. Chem. Eng. Q. 2023, 30, 59–72. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmed, Z.; Ahmad, N.; Aadil, R.M.; Rahaman, A.; Roobab, U.; Siddeeg, A. Novel processing techniques and spinach juice: Quality and safety improvements. J. Food Sci. 2020, 85, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, X.; Dong, X.; Yang, H.-F. Effects of aging−acceleration by electric field on free amino acid of claret. Liquor Mak. Sci. Technol. 2004, 4, 80–81. [Google Scholar]
- Zeng, X.; Gao, D.; Li, G.; Yu, S. The effect of high electric field on enhancing pork’s delicate flavor. Food Sci. 1997, 18, 37–40. [Google Scholar]

| No | ID | PEF | Drying | Replicate |
|---|---|---|---|---|
| 1 | C−AD1 | No PEF | Air drying | 1 |
| 2 | C−AD2 | No PEF | Air drying | 2 |
| 3 | C−AD3 | No PEF | Air drying | 3 |
| 4 | L−AD1 | Low PEF | Air drying | 1 |
| 5 | L−AD2 | Low PEF | Air drying | 2 |
| 6 | L−AD3 | Low PEF | Air drying | 3 |
| 7 | M−AD1 | Medium PEF | Air drying | 1 |
| 8 | M−AD2 | Medium PEF | Air drying | 2 |
| 9 | M−AD3 | Medium PEF | Air drying | 3 |
| 10 | H−AD1 | High PEF | Air drying | 1 |
| 11 | H−AD2 | High PEF | Air drying | 2 |
| 12 | H−AD3 | High PEF | Air drying | 3 |
| 13 | FDC1 | No PEF | Freeze drying | 1 |
| 14 | FDC2 | No PEF | Freeze drying | 2 |
| 15 | FDC3 | No PEF | Freeze drying | 3 |
| 16 | FDL1 | Low PEF | Freeze drying | 1 |
| 17 | FDL2 | Low PEF | Freeze drying | 2 |
| 18 | FDL3 | Low PEF | Freeze drying | 3 |
| 19 | FDM1 | Medium PEF | Freeze drying | 1 |
| 20 | FDM2 | Medium PEF | Freeze drying | 2 |
| 21 | FDM3 | Medium PEF | Freeze drying | 3 |
| 22 | FDH1 | High PEF | Freeze drying | 1 |
| 23 | FDH2 | High PEF | Freeze drying | 2 |
| 24 | FDH3 | High PEF | Freeze drying | 3 |
| PEF Intensity | Moisture Content | Aw | |
|---|---|---|---|
| Air drying | |||
| 1 | Control | 27.256 ± 1.868 Aa | 0.544 ± 0.009 Aa |
| 2 | Low PEF | 27.231 ± 0.869 Aa | 0.559 ± 0.014 Aa |
| 3 | Medium PEF | 27.47 ± 0.507 Aa | 0.57 ± 0.014 Aa |
| 4 | High PEF | 27.354 ± 0.943 Aa | 0.571 ± 0.016 Aa |
| p value (PEF condition) | Pr > F | p > 0.05 | p > 0.05 |
| p value (drying method) | Pr > F | ** | *** |
| Freeze drying | |||
| 1 | Control | 19.716 ± 0.460 Ab | 0.384 ± 0.010 Ab |
| 2 | Low PEF | 19.853 ± 0.302 Ab | 0.399 ± 0.024 Ab |
| 3 | Medium PEF | 19.546 ± 0.165 Ab | 0.392 ± 0.010 Ab |
| 4 | High PEF | 19.42 ± 0.865 Ab | 0.399 ± 0.017 Ab |
| p value (PEF condition) | Pr > F | p > 0.05 | p > 0.05 |
| PEF Intensity | Texture | |||||||
|---|---|---|---|---|---|---|---|---|
| Hardness | Adhesiveness | Resilience | Cohesion | Springiness | Gumminess | Chewiness | ||
| Air drying | ||||||||
| 1 | Control | 137.153 ± 23.637 Ca | 0.032 ± 0.187 Aa | 35.844 ± 4.027 Aa | 0.931 ± 0.140 Aa | 190.442 ± 177.722 Aa | 126.961 ± 22.028 Ca | 254.287 ± 253.539 Ba |
| 2 | Low PEF | 716.382 ± 111.191 Ba | −1.108 ± 0.824 Aa | 29.005 ± 0.377 Ba | 0.778 ± 0.010 Aa | 90.716 ± 2.023 Aa | 556.648 ± 78.866 Ba | 505.374 ± 76.017 ABa |
| 3 | Medium PEF | 827.646 ± 67.560 Ba | −9.289 ± 3.96 Bb | 24.788 ± 1.093 Ba | 0.753 ± 0.020 Aa | 92.709 ± 2.226 Aa | 624.145 ± 67.131 Ba | 579.48 ± 74.965 ABa |
| 4 | High PEF | 1052.633 ± 11.842 Aa | 3.673 ± 1.681 Aa | 25.715 ± 2.754 Ba | 0.743 ± 0.056 Aa | 93.798 ± 11.797 Aa | 781.163 ± 50.504 Aa | 731.158 ± 83.063 Aa |
| p value (PEF condition) | Pr > F | *** | * | * | p > 0.05 | p > 0.05 | *** | * |
| p value (drying method) | Pr > F | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Freeze drying | ||||||||
| 1 | Control | 237.689 ± 62.479 Ca | −0.958 ± 2.972 Aa | 25.467 ± 5.551 Aa | 0.709 ± 0.041 Aa | 82.639 ± 14.783 Aa | 167.448 ± 37.869 Ba | 139.356 ± 43.717 Ba |
| 2 | Low PEF | 342.77 ± 12.242 Bb | −0.016 ± 0.675 Aa | 20.134 ± 2.539 Ab | 0.598 ± 0.068 Ab | 64.274 ± 2.671 Ab | 205.134 ± 28.058 Bb | 132.34 ± 23.158 Bb |
| 3 | Medium PEF | 209.267 ± 2.109 Cb | −0.939 ± 0.695 Aa | 19.219 ± 1.541 Ab | 0.634 ± 0.166 Aa | 75.807 ± 20.476 Aa | 132.583 ± 34.2 Bb | 104.974 ± 51.574 Bb |
| 4 | High PEF | 472.137 ± 27.969 Ab | −0.329 ± 0.923 Ab | 25.048 ± 2.55 Aa | 0.695 ± 0.012 Aa | 73.038 ± 2.854 Ab | 328.234 ± 20.657 Ab | 240.129 ± 24.603 Ab |
| p value (PEF condition) | Pr > F | p > 0.05 | *** | p > 0.05 | p > 0.05 | p > 0.05 | ** | * |
| PEF Intensity | Antioxidant Ability | Total Polyphenol Content | ||
|---|---|---|---|---|
| mg of Trolox Equivalent/g Dried Powder (FRAP) | mg of Trolox Equivalent/g Dried Powder (CUPRAC) | mg of Gallic Acid Equivalent/g Dried Powder | ||
| Air drying | ||||
| 1 | Control | 0.447 ± 0.016 Bb | 4.742 ± 0.27 Ca | 0.445 ± 0.015 Ba |
| 2 | Low PEF | 0.565 ± 0.021 Ab | 6.626 ± 0.174 Ba | 0.466 ± 0.016 Ba |
| 3 | Medium PEF | 0.567 ± 0.016 Ab | 7.031 ± 0.115 ABa | 0.493 ± 0.030 ABa |
| 4 | High PEF | 0.607 ± 0.02 Ab | 7.417 ± 0.129 Aa | 0.527 ± 0.020 Aa |
| p value (PEF condition) | Pr > F | * | * | * |
| p value (drying method) | Pr > F | p > 0.05 | p > 0.05 | p > 0.05 |
| Freeze drying | ||||
| 1 | Control | 0.936 ± 0.111 Ba | 4.363 ± 0.498 Aa | 0.576 ± 0.139 Aa |
| 2 | Low PEF | 1.458 ± 0.161 Aa | 4.617 ± 0.154 Ab | 0.503 ± 0.024 Aa |
| 3 | Medium PEF | 1.315 ± 0.074 Aa | 4.062 ± 0.353 Ab | 0.466 ± 0.014 Aa |
| 4 | High PEF | 1.479 ± 0.048 Aa | 5.963 ± 0.061 Ab | 0.522 ± 0.006 Aa |
| p value (PEF condition) | Pr > F | * | p > 0.05 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Oey, I.; Leong, S.Y.; Kam, R.; Kantono, K.; Hamid, N. Pulsed Electric Field Pretreatments Affect the Metabolite Profile and Antioxidant Activities of Freeze− and Air−Dried New Zealand Apricots. Foods 2024, 13, 1764. https://doi.org/10.3390/foods13111764
Liu Y, Oey I, Leong SY, Kam R, Kantono K, Hamid N. Pulsed Electric Field Pretreatments Affect the Metabolite Profile and Antioxidant Activities of Freeze− and Air−Dried New Zealand Apricots. Foods. 2024; 13(11):1764. https://doi.org/10.3390/foods13111764
Chicago/Turabian StyleLiu, Ye, Indrawati Oey, Sze Ying Leong, Rothman Kam, Kevin Kantono, and Nazimah Hamid. 2024. "Pulsed Electric Field Pretreatments Affect the Metabolite Profile and Antioxidant Activities of Freeze− and Air−Dried New Zealand Apricots" Foods 13, no. 11: 1764. https://doi.org/10.3390/foods13111764
APA StyleLiu, Y., Oey, I., Leong, S. Y., Kam, R., Kantono, K., & Hamid, N. (2024). Pulsed Electric Field Pretreatments Affect the Metabolite Profile and Antioxidant Activities of Freeze− and Air−Dried New Zealand Apricots. Foods, 13(11), 1764. https://doi.org/10.3390/foods13111764










