Analysing the Impact of Resistant Starch Formation in Basmati Rice Products: Exploring Associations with Blood Glucose and Lipid Profiles across Various Cooking and Storage Conditions In Vivo
Abstract
1. Introduction
- To determine the effect of cooking and refrigeration on the resistant starch and dietary fibre content of cereal products;
- To quantify the resistant starch and soluble fibre components in cereal products;
- To assess the impact of resistant starch and soluble fibre components on postprandial glucose responses and appetite ratings;
- To determine the effectiveness of resistant starch on lipid profiles and blood glucose levels in rat models.
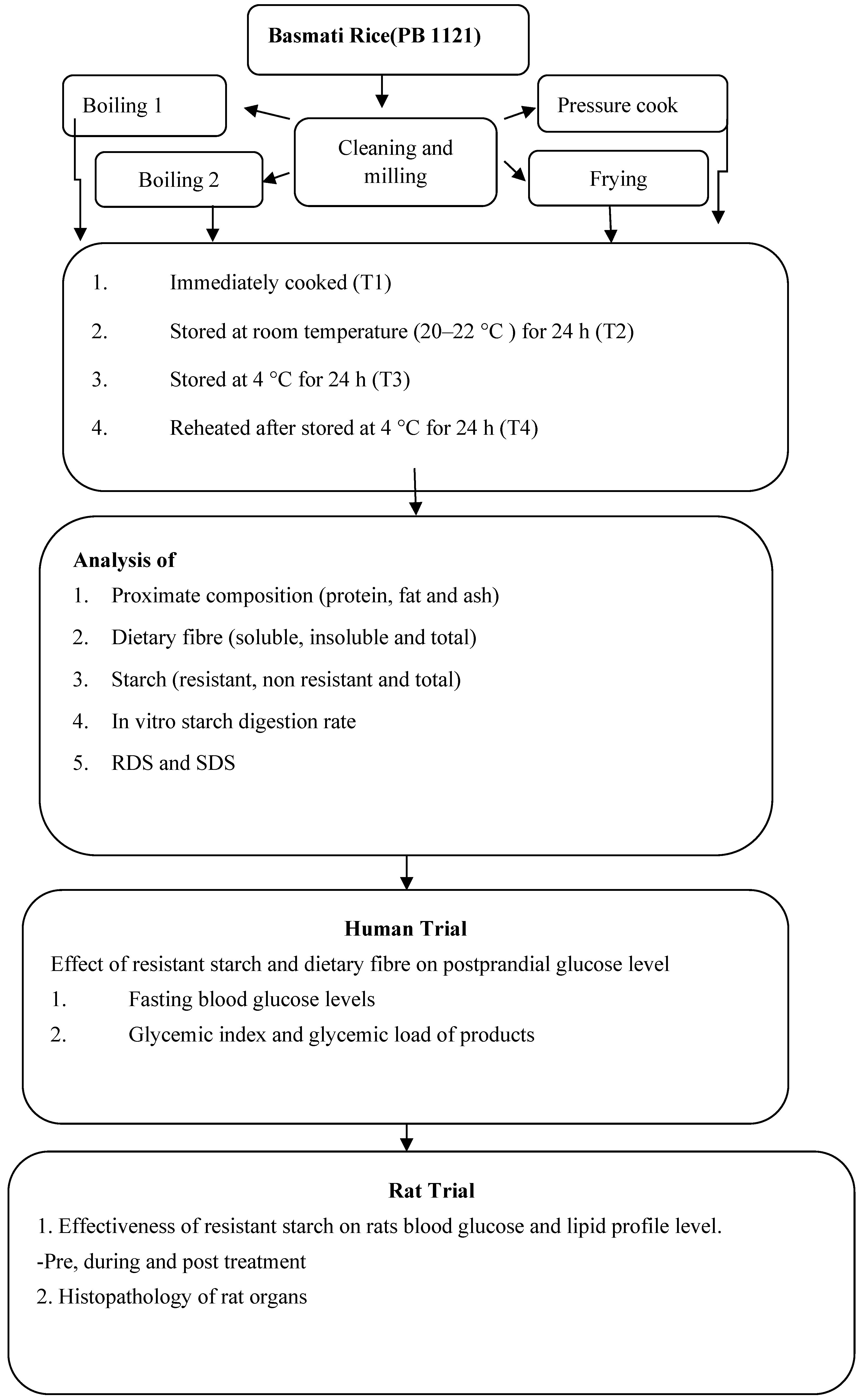
2. Material and Methods
2.1. Procurement and Cooking
2.2. Nutritional Analysis
2.2.1. Proximate Composition
2.2.2. Dietary Fiber
2.2.3. Total Starch and Resistant Starch
2.2.4. In Vitro Starch Digestion Rate
2.2.5. Rapidly Digestible Starch and Slowly Digestible Starch
2.3. Impact of Resistant Starch and Soluble Fibre Components on Postprandial Glucose Response via Measuring Glycaemic Index
2.4. Effectiveness of Resistant Starch on Blood Glucose Levels and Lipid Profiles in Rats
2.4.1. Animal Collection
2.4.2. Induction of Diabetes
2.4.3. Treatment Protocol
- Group-I: (Normal control) Normal rats were fed a normal diet for a period of 28 days.
- Group-II: (Diabetic control) Diabetic rats were fed a regular diet for 28 days following the onset of diabetes.
- Group-III Diabetic rats were fed an FBR (freshly prepared boiled basmati rice) diet for 28 days.
- Group-IV (Treatment group) Diabetic rats were fed 4BR (stored at 4 °C for 24 h boiled basmati rice) for 28 days.
- Group-V (Treatment group) Diabetic rats were fed RehBR (reheated basmati rice after being stored at 4 °C for 24 h) for 28 days.
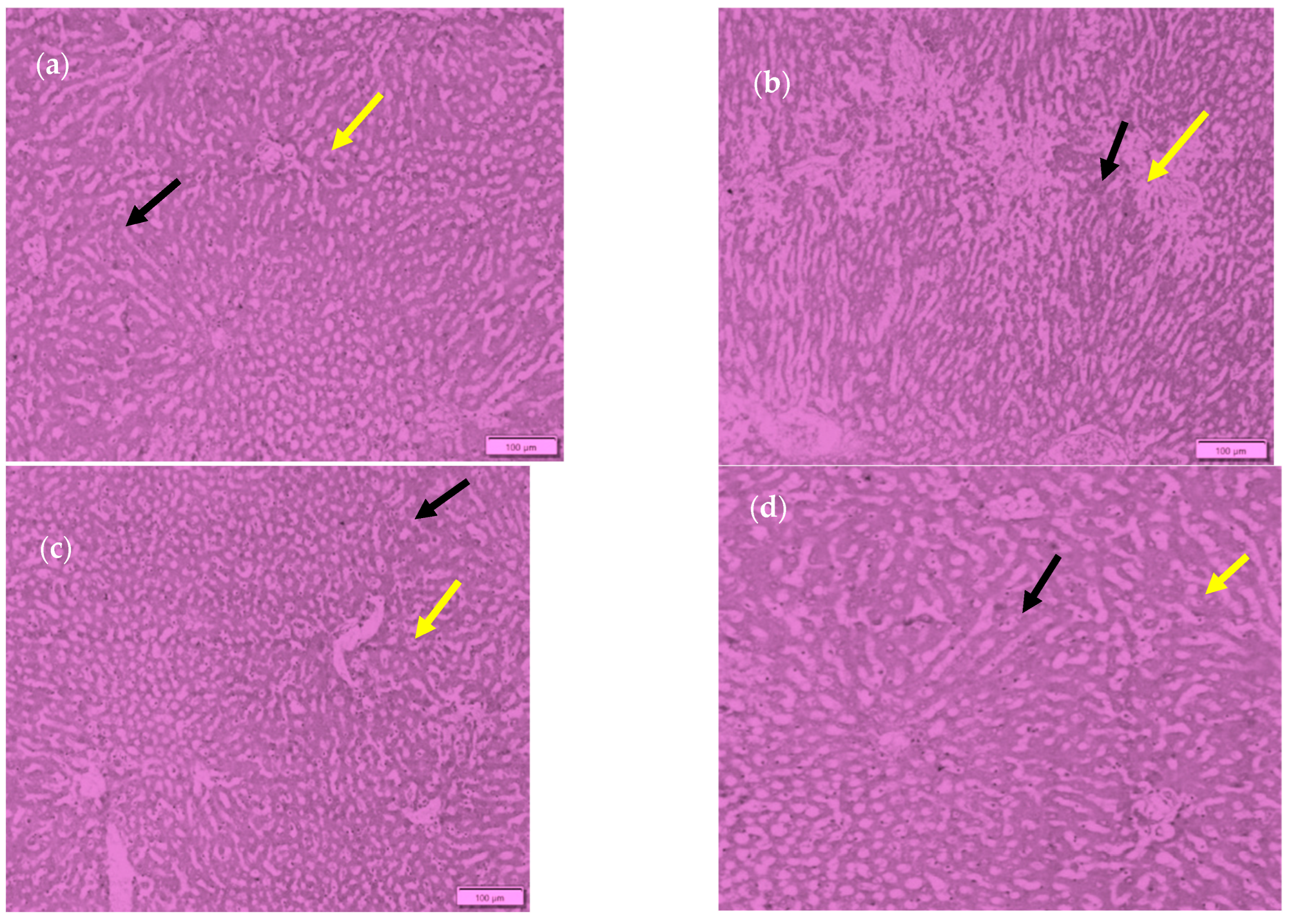
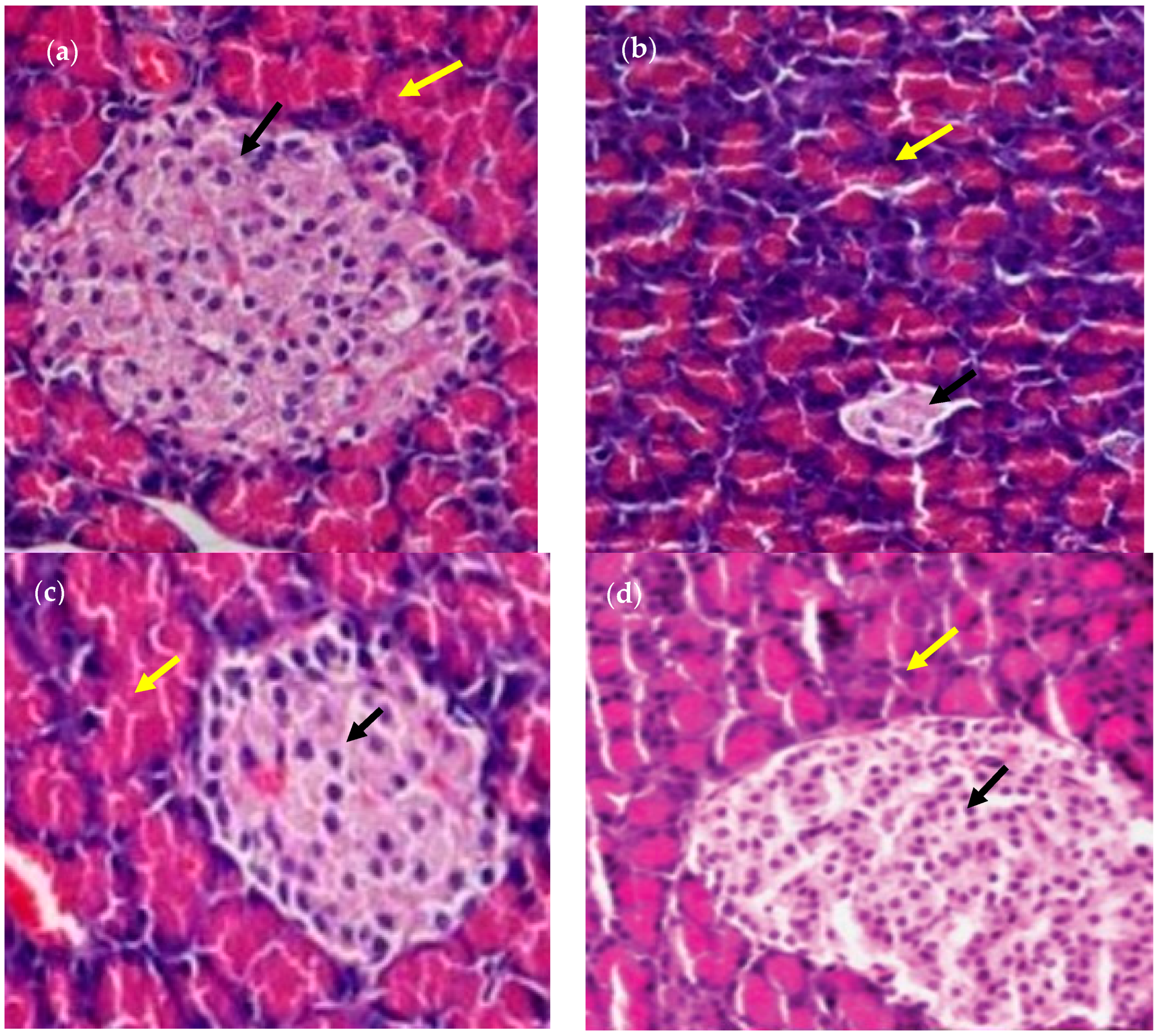
2.5. Histopathological Study of Rat Organs
2.5.1. Preparation of Samples
2.5.2. Haematoxylin–Eosin (HE) Staining
2.6. Statistical Analysis
3. Results
3.1. Proximate Composition
3.2. Dietary Fibre
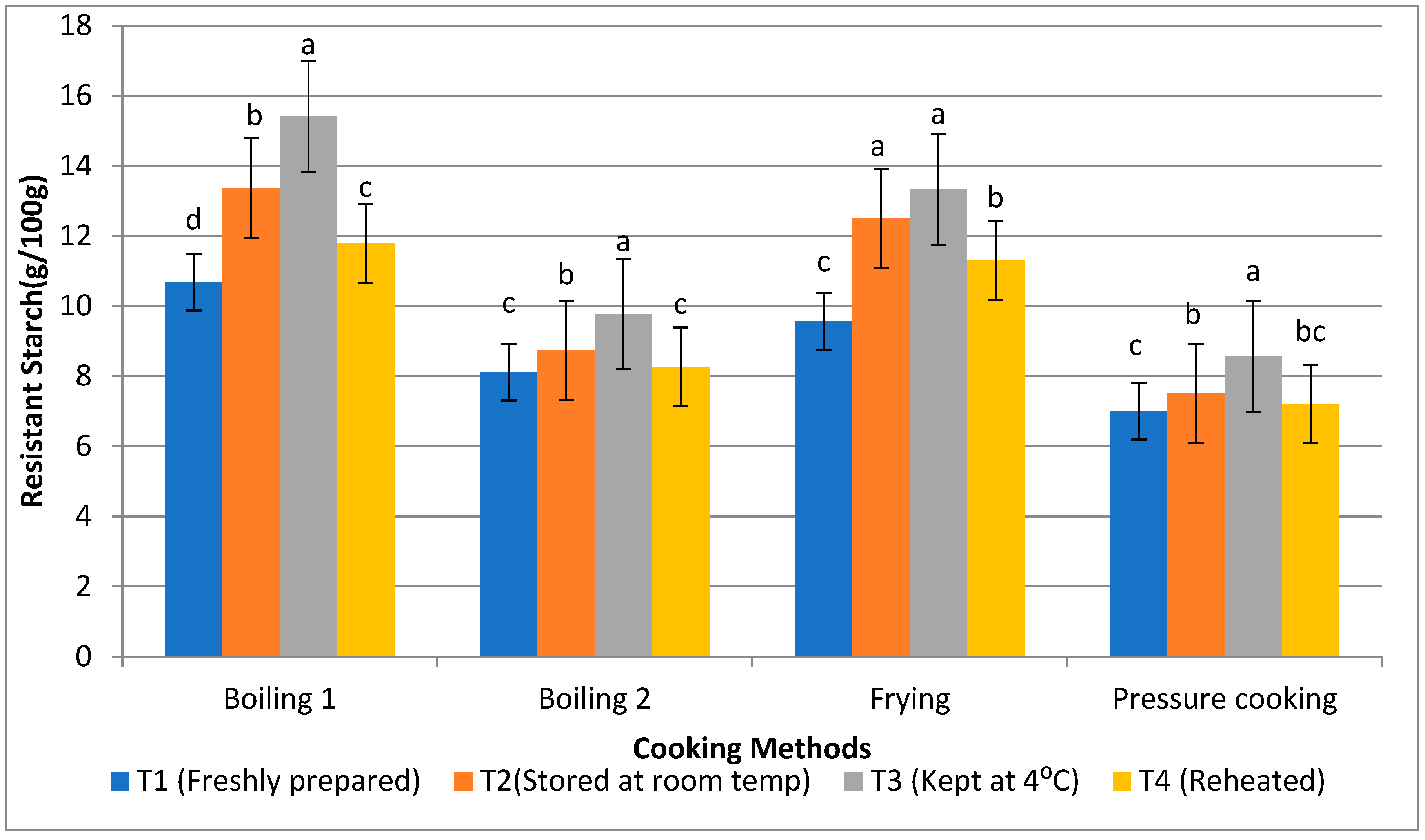
3.3. Resistant Starch, Non-Resistant Starch, and Total Starch
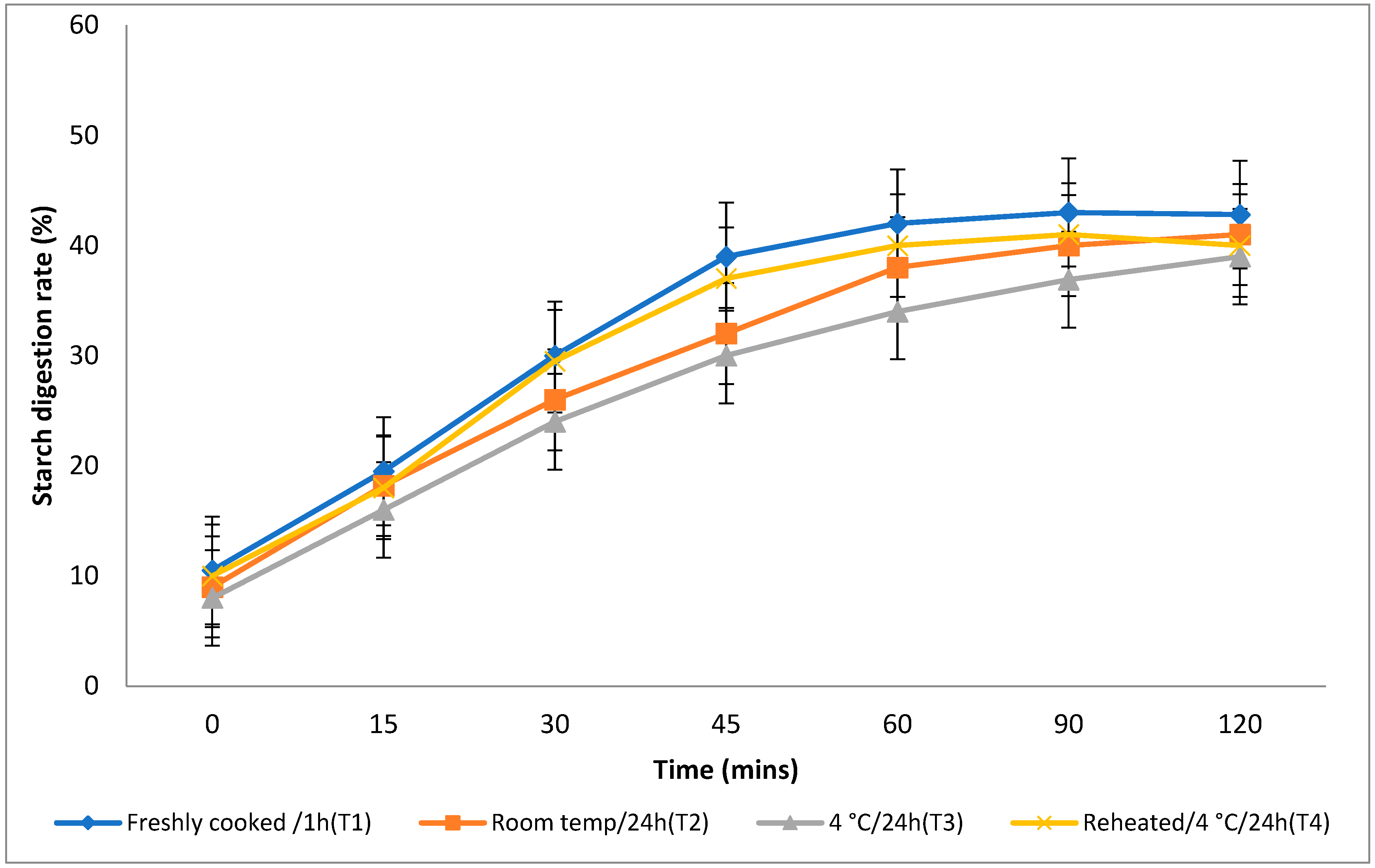
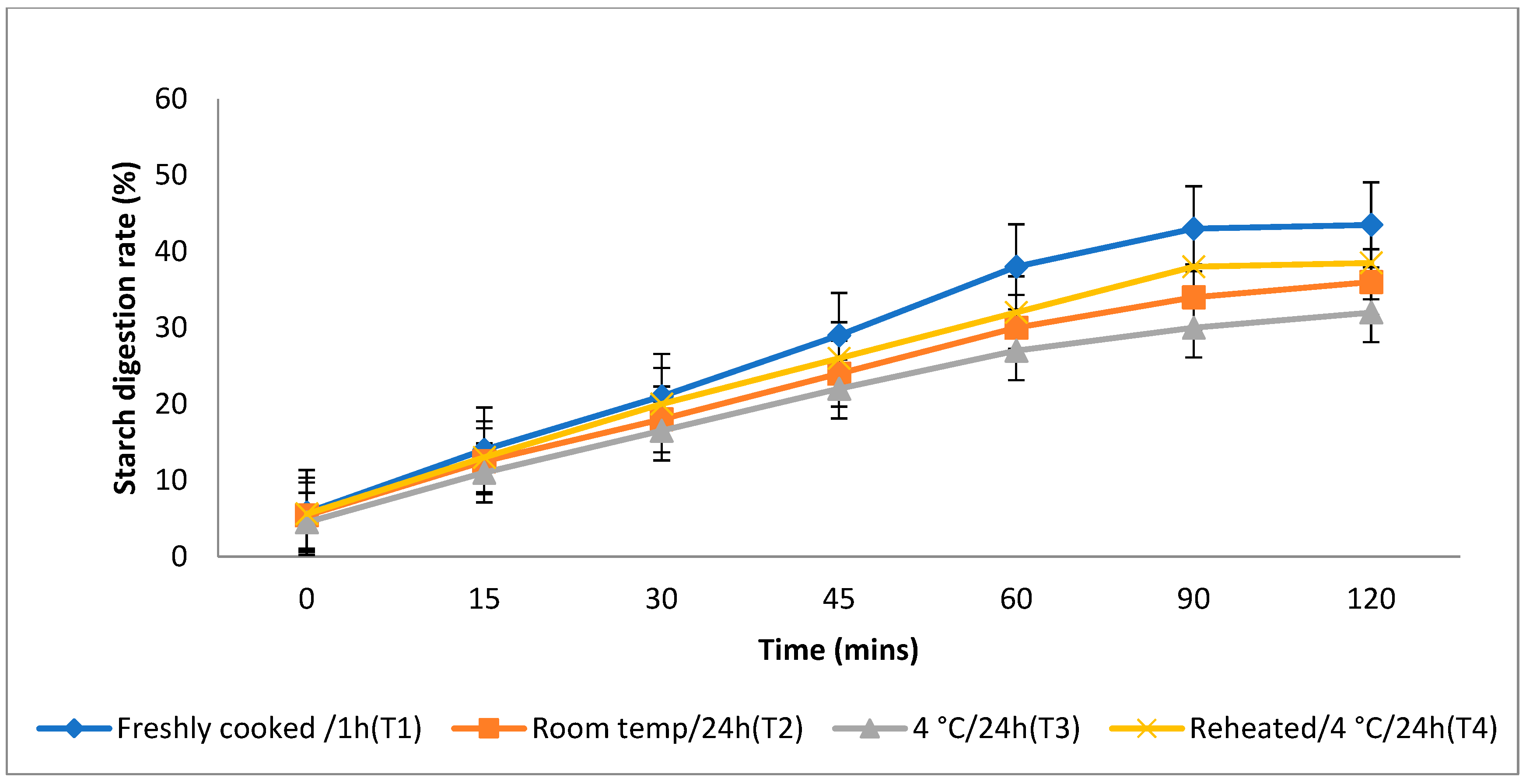
3.4. In Vitro Starch Digestion Rate
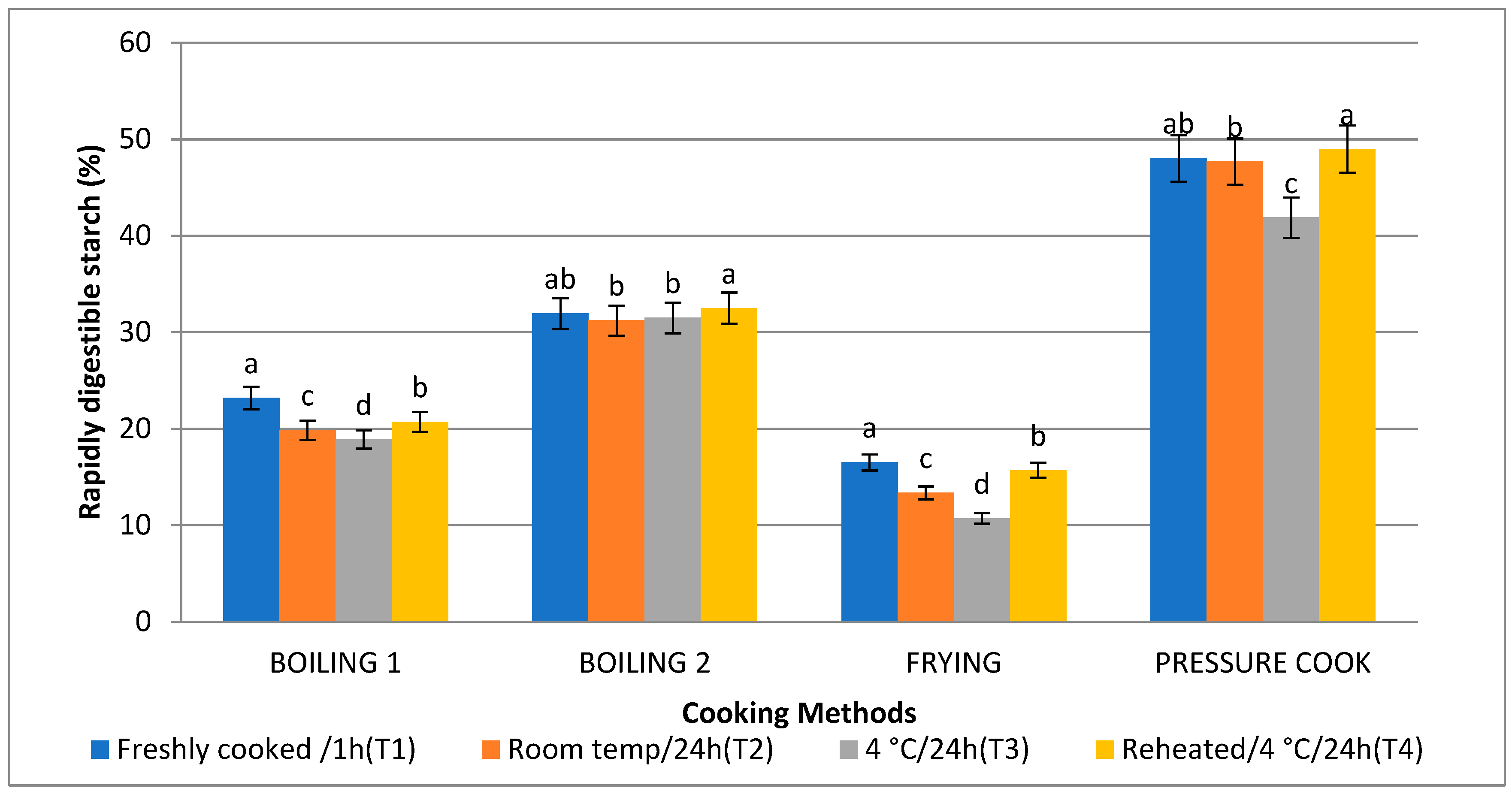
3.5. Rapidly Digestible Starch (RDS) and Slowly Digestible Starch (SDS)
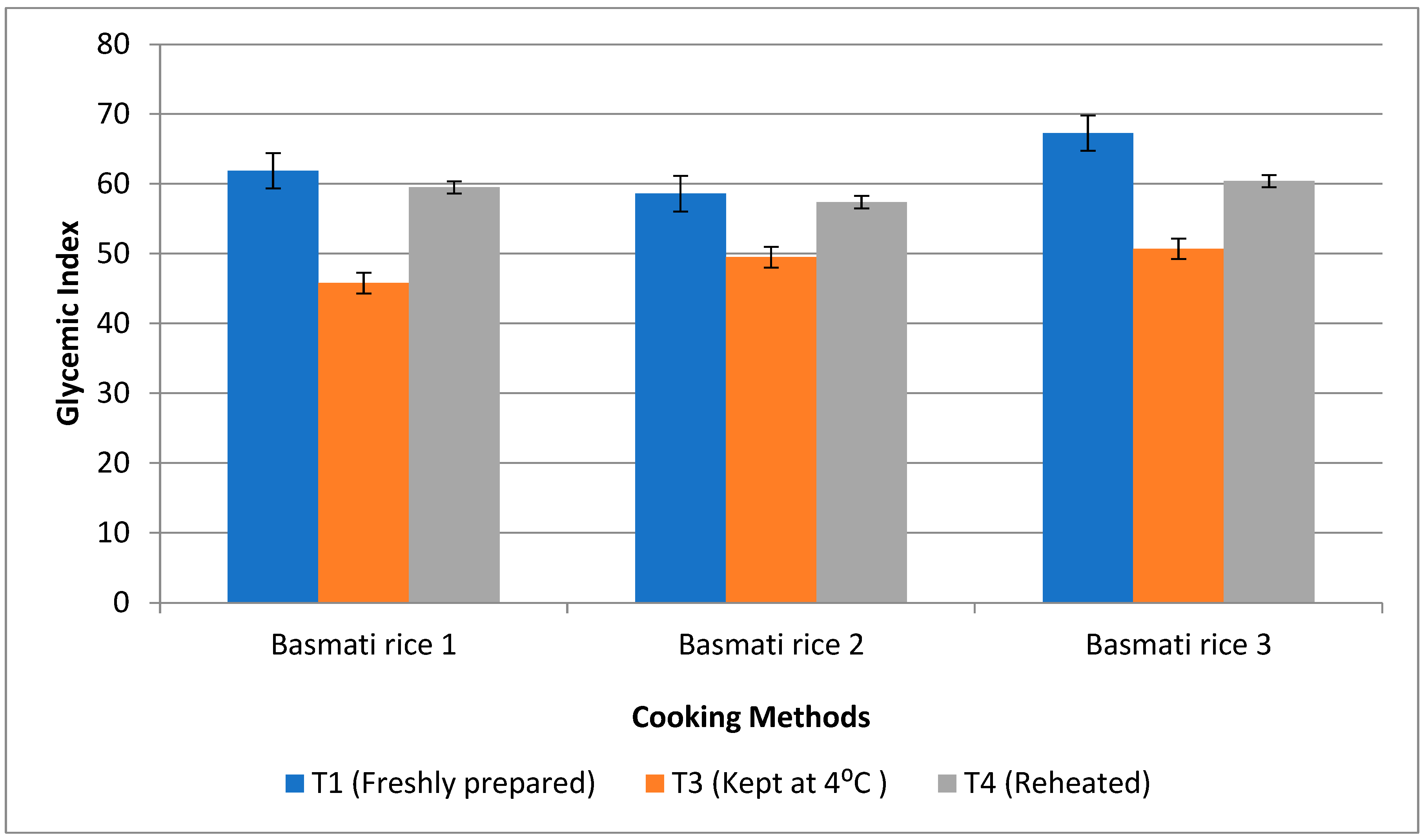
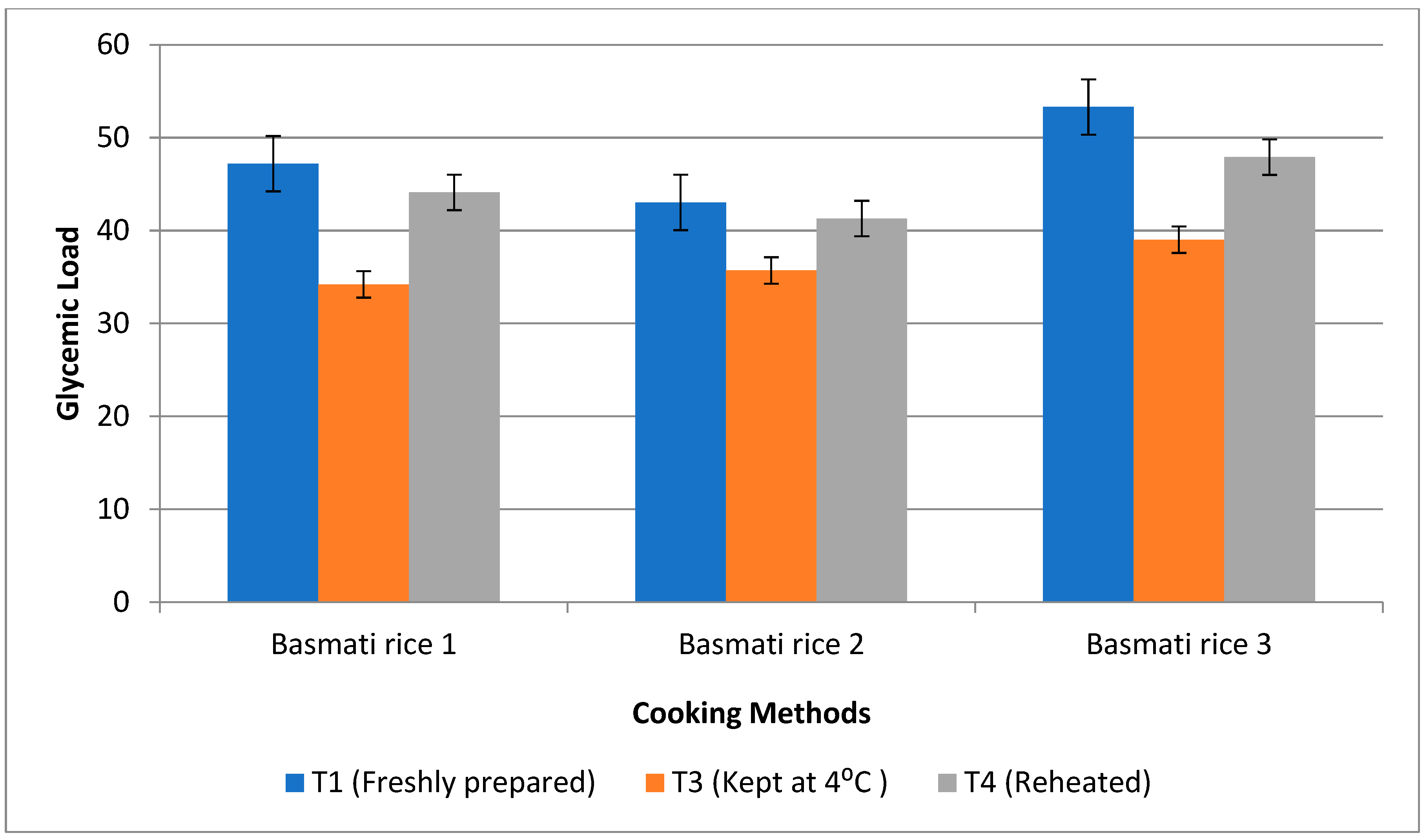
3.6. Impact of Resistant Starch and Soluble Fibre Components on Postprandial Glucose Response
3.7. Effectiveness of Resistant Starch on Blood Glucose Level in Rats
3.8. Effect of Resistant Starch on Lipid Profile in Rats
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sanchez-Zapata, E.; Perez-Alvarez, J.A. Resistant Starch as functional ingredient—A review. Food Res. Int. 2010, 43, 921–942. [Google Scholar] [CrossRef]
- Higgins, J.A.; Higbee, D.R.; Donahoo, W.T.; Brown, I.L.; Bell, M.L.; Bessesen, D.H. Resistant starch consumption promotes lipid oxidation. Nutr. Metab. 2004, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.S.; Kumar, V.; Mishra, A.; Fandade, V.; Kumar, V.; Kondepudi, K.K.; Bishnoi, M.; Roy, J. High resistant starch mutant wheat ‘TAC 35’ reduced glycemia and ameliorated high fat diet induced metabolic dysregulation in mice. J. Cereal Sci. 2022, 105, 103459. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 133–150. [Google Scholar]
- Asp, N.G. Resistant starch. Proceedings of the 2nd plenary meeting of EURESTA: European Flair Concerted Action No. 11 on physiological implications of the consumption of resistant starch in man. Eur. J. Clin. Nutr. 1992, 46, 1–148. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Hernández, O.; Emaldi, U.; Tovar, J. In vitro digestibility of edible films from various starch sources. Carbohydr. Polym. 2008, 71, 648–655. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Sanz, J.; Haros, M. Phytate reduction in bran-enriched bread by phytase producing bifidobacteria. J. Agric. Food Chem. 2009, 57, 10239–10244. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, A.; Shevkani, K. Maize: Grain structure, composition, milling, and starch characteristics. In Nutrition Dynamics and Novel Uses; Springer: New Delhi, India, 2013; pp. 65–76. [Google Scholar] [CrossRef]
- Reader, D.; Johnson, M.L.; Hollander, P.; Franz, M. Response of resistant starch in a food bar vs. two commercially available bars in persons with type II diabetes mellitus. Diabetes 1997, 46, 254. [Google Scholar]
- Cummings, J.H.; Englyst, H.N. Measurement of starch fermentation in the human large intestine. Can. J. Physiol. Pharmacol. 1991, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bullock, N.R.; Norton, G. Techniques to assess the fermentation of resistant starch in the mammalian gastrointestinal tract. Carbohydr. Polym. 1999, 38, 225–230. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.J.; Nawrocki, A.R. Butyrate and propionate protect against diet induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- Baghurst, K.; Baghurst, P.A.; Record, S.J. CRC Handbook of Dietary Fiber in Human Health; CRC Press: Boca Raton, FL, USA, 2001; pp. 583–591. [Google Scholar]
- Alsaffar, A.A. Effect of food processing on the resistant starch content of cereals and cereal products: A review. Int. J. Food Sci. Technol. 2011, 46, 455–462. [Google Scholar] [CrossRef]
- Kale, C.K.; Kotecha, P.M.; Chavan, J.K.; Kadam, S. Effect of processing conditions of bakery products on formation of resistant starch. J. Food Sci. Technol. 2002, 39, 520–524. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Official Methods of Analysis, 17th ed.; AOAC International: Rockville, MD, USA, 2000.
- Sopade, P.A.; Gidley, M.J. A rapid in-vitro digestibility assay based on glucometry for investigating the kinetics of starch digestion. Starch-Stärke 2009, 61, 245–255. [Google Scholar] [CrossRef]
- Goni, I.; Garia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Ndaong, N.A. Efek Pemaparan Deltamethrin Pada Broiler Terhadap Aktivitas Enzim Alanine Amino Transferase, Asparat Aminotransferase, Gambaran Histopatologi Hepar. Ph.D. Thesis, Fakultas Kedokteran Hewan, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2013. [Google Scholar]
- Selan, Y.N. Morfologi Dan Momfometri Saluran Pencernaan Kalong Kapauk (Pteropus vampyrus) Beserta Distribusi Sarafnya. Ph.D. Thesis, Fakultas Kedokteran Hewan, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2013. [Google Scholar]
- Ebuehi, O.; Oyewole, C.A. Effect of cooking and soaking on physical, nutrient composition and sensory evaluation of indigenous and foreign rice varieties in Nigeria. Nutr. Food Sci. 2008, 38, 15–21. [Google Scholar] [CrossRef]
- Tarade, K.M.; Singhal, R.S.; Jayram, R.V.; Andit, A.B. Kinetics of degradation of ODAP in Lathyrus sativus L. flour during food processing. Food Chem. 2007, 104, 643–649. [Google Scholar] [CrossRef]
- Siregar, K.; Sofyan, H.; Nasution, I.S.; Ichwana, R.; Syafriandi, S.; Sholihati Sofiah, I.; Miharza, T. Study of life cycle assessment in biodiesel production from crude palm oil and its benefits for the sustainability of oil palm industry in Aceh province Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 644, 012017. [Google Scholar] [CrossRef]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Polonor. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef]
- Kate, F. Everything You Need to Know about Soluble Fiber. 2020. Available online: http://www.katefarms.com (accessed on 1 July 2020).
- Cheng, L.; Zhang, X.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. Characterisation of physicochemical and functional properties of soluble dietary fibre from potato pulp obtained by enzyme-assisted extraction. Int. J. Biol. Macromol. 2017, 101, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Akinola, O.O.; Raji, T.J.; Olawoye, B. Lignocellulose, dietary fibre, inulin and their potential application in food. Heliyon 2022, 29, 104–159. [Google Scholar]
- Chang, M.C.; Morris, W.C. Effect of heat treatments on chemical analysis of dietary fiber. J. Food Sci. 1990, 55, 1647–1650. [Google Scholar] [CrossRef]
- Herranz, J.; Vidal-Valverde, C.; Rojas-Hidalgo, E. Cellulose, Hemicellulose and Lignin Content of Raw and Cooked Spanish Vegetables. J. Food Sci. 1981, 46, 1927–1933. [Google Scholar] [CrossRef]
- Thed, S.T.; Philips, R.D. Changes of dietary fibre and starch composition of processed potato products during domestic cooking. Food Chem. 1995, 52, 301–304. [Google Scholar] [CrossRef]
- Devi, K.; Geervani, P. Rice processing effect on dietary fiber components and in vitro starch digestibility. J. Food Sci. Technol. 2000, 37, 315–318. [Google Scholar]
- Ramulu, P.; Udayasekhararao, P. Effect of processing on dietary fibre content of cereals and pulses. Plant Foods Hum. Nutr. 1997, 50, 249–257. [Google Scholar] [CrossRef]
- Chung, H.; Hoover, R.; Liu, Q. The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch. Int. J. Biol. Macromol. 2009, 44, 203–210. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, T.; Gawalko, E.J. Effect of cooking on the consumption of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Ek, K.L.; Brand-Miller, J.; Copelan, L. Glycemic effect of potatoes. Food Chem. 2012, 133, 1230–1240. [Google Scholar] [CrossRef]
- Kolaric, L.; Minarovicova, L.; Lauková, M.; Karovicovaand, J.; Kohajdova, Z. Pasta enriched with sweet potato starch: Impact on quality parameters and resistant starch content. J. Text. Stud. 2020, 51, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Katawal, S.B.; Shrestha, A.K. Formation of resistant starch during processing and storage of instant noodles. Int. J. Food Prop. 2010, 13, 454–463. [Google Scholar] [CrossRef]
- Mangala, S.L.; Vidyasankar, K.; Tharanathan, R.N. Resistant starch from processed cereals. The influence of amylopectin and non-carbohydrate constituents on its formation. Food Chem. 1999, 64, 391–396. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. High pressure phase transition kinetics of maize starch. J. Food Eng. 2007, 81, 469–475. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Stewart, M.L. Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac. J. Clin. Nutr. 2013, 22, 372–379. [Google Scholar]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Englyst, K.N.; Liu, S.; Englyst, H.N. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2006, 61, 19–39. [Google Scholar] [CrossRef]
- Lehmann, U.; Robin, F. Slowly digestible starch—Its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Williams, M.R.; Bowler, P. Starch gelatinization—A morphological study of Triticeae and other starches. Starch-Stärke 1982, 34, 221–223. [Google Scholar] [CrossRef]
- Goddard, M.S.; Young, G.; Marcus, R. The effect of amylase content on insulin and glucose responses to ingested rice. Am. J. Clin. Nutr. 1984, 39, 388–392. [Google Scholar] [PubMed]
- Sagum, R.; Arcot, J. Effect of domestic processing methods on the starch, non starch polysaccharides and in vitro starch and protein digestibility of three varieties of rice with varying levels of amylose. Food Chem. 2000, 70, 107–111. [Google Scholar] [CrossRef]
- Ahmed, N.; Tetlow, I.J.; Nawaz, S.; Iqbal, A.; Mubin, M.; Nawaz, U.; Rehman, M.S.; Butt, A.; Lightfoot, D.A.; Maekawa, M. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice: Effect of high temperature in basmati rice. J. Sci. Food Agric. 2015, 95, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2011, 75, 436–447. [Google Scholar] [CrossRef]
- Sonia, S.; Witjaksono, F.; Ridwan, R. Effect of cooling of cooked white rice on resistant starch content and glycemic response. Asia Pac. J. Clin. Nutr. 2015, 24, 620–625. [Google Scholar] [PubMed]
- Kim, W.K.; Chung, M.K.; Kang, N.E.; Kim, M.H.; Park, O.J. Effect of resistant starch from corn or rice on glucose control, colonic events, and blood lipid concentrations in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2003, 14, 166–172. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Lopez, H.W.; Levrat-Verny, M.A.; Coudray, C.; Besson, C.; Krespine, V.; Messager, A. Class 2 resistant starches lower plasma and liver lipids and improve mineral retention in rats. J. Nutr. 2001, 131, 1283–1289. [Google Scholar] [CrossRef]
- Wood, L.; Wilbourne, J.; Kyne-Grzebalski, D. Administration of insulin by injection. Pract. Diabetes Int. 2002, 19, S1–S4. [Google Scholar] [CrossRef]
- Graham, K.L.; Krishnamurthy, B.; Fynch, S.; Mollah, Z.U.; Slattery, R.; Santamaria, P.; Kay, T.W.; Thomas, H.E. Autoreactive cytotoxic T lymphocytes acquire higher expression of cytotoxic effector markers in the islets of nod mice after priming in pancreatic lymph nodes. Am. J. Pathol. 2011, 178, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.A. Studies on the surgical induction of endometriosis in the rat. Fertil. Steril. 1985, 4, 684–694. [Google Scholar]
- Rimm, E.B. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease Among Men. JAMA 1996, 275, 447. [Google Scholar] [CrossRef] [PubMed]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Levrat, M.A.; Moundras, C.; Younes, H. Effectiveness of resistant starch, compared to guar gum, in depressing plasma cholesterol and enhancing fecal steroid excretion. Lipids 1996, 31, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Dinesen, B.; Jorgensen, P.N.; Poulsen, F.; Roder, M.F. Enzyme immunoassay for intact human insulin in serum or plasma. Clin. Chem. 1993, 39, 578–582. [Google Scholar] [CrossRef]
- Sasaik, Y.; Weekes, T.E.C.; Bruice, J.B. Effects of glucose and butyrate on insulin release from perifused fragments of sheep pancreas. J. Endocr. 1977, 72, 415–416. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary Resistant Starch Upregulates Total GLP-1 and PYY in a Sustained Day-Long Manner through Fermentation in Rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1160–1166. [Google Scholar] [CrossRef]



| Cereal Product | Ratio of Ingredients | Method |
|---|---|---|
| Boiling 1 (rice was boiled in an equal amount of water i.e., boiling by absorption) | Basmati rice to water (2:4 w/v) | Basmati rice was boiled in water for 15 min. |
| Boiling 2 (after boiling, extra water was drained out) | Basmati rice to water (2:7–8 w/v) | Basmati rice was boiled in an extra amount of water separately for 15 min, and then the extra water was drained out after cooking. |
| Frying (fried rice) | Basmati rice to water (2:4 w/v) | Wok was heated on a high flame and 10 mL of oil was used to fry rice. Then the rice was cooked for about 20–25 min. |
| Pressure cooking (pressure cooked rice) | Basmati rice to water (2:4 w/v) | Basmati rice was pressure cooked separately in a pressure cook (15 psi) for 10 min. |
| Cooking Methods | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | Treatment Mean | |
| Crude protein | |||||
| Boiling 1 | 8.68 ± 0.22 b | 9.32 ± 0.57 b | 10.44 ± 0.19 b | 9.74 ± 0.52 a | 9.54 A |
| Boiling 2 | 9.51 ± 0.36 b | 7.62 ± 0.07 c | 9.90 ± 0.10 b | 6.87 ± 0.42 cd | 8.22 B |
| Frying | 7.59 ± 0.10 c | 8.80 ± 0.10 b | 9.34 ± 0.14 c | 7.40 ± 0.46 b | 8.28 B |
| Pressure cooking | 6.34 ± 0.44 de | 7.54 ± 0.43 a | 8.21 ± 0.26 cd | 5.57 ± 0.20 e | 7.49 C |
| Storage Mean | 8.03 B | 8.32 B | 9.47 A | 7.39 C | |
| Crude fat | |||||
| Boiling 1 | 2.01 ± 0.04 b | 2.37 ± 0.15 b | 2.47 ± 0.07 b | 2.38 ± 0.83 b | 2.18 B |
| Boiling 2 | 1.91 ± 0.08 b | 1.84 ± 0.07 bc | 1.93 ± 0.06 b | 1.95 ± 0.05 b | 1.90 B |
| Frying | 10.60 ± 0.40 a | 10.98 ± 0.49 a | 11.31 ± 0.30 a | 10.57 ± 0.64 a | 10.86 A |
| Pressure cooking | 0.62 ± 0.05 d | 0.83 ± 0.14 d | 0.90 ± 0.10 cd | 0.80 ± 0.10 d | 0.78 C |
| Storage Mean | 3.78 A | 4.0 A | 4.03 A | 3.92 A | |
| Ash | |||||
| Boiling 1 | 1.79 ± 0.09 ab | 1.82 ± 0.04 a | 1.90 ± 0.03 a | 1.95 ± 0.05 a | 1.86 A |
| Boiling 2 | 1.72 ± 0.14 ab | 1.71 ± 0.05 abc | 1.87 ± 0.03 a | 1.71 ± 0.11 abc | 1.75 B |
| Frying | 1.89 ± 0.01 a | 1.92 ± 0.07 a | 1.85 ± 0.06 a | 1.74 ± 0.21 ab | 1.85 AB |
| Pressure cooking | 1.43 ± 0.10 cd | 1.53 ± 0.13 bc | 1.22 ± 0.12 d | 1.69 ± 0.08 abc | 1.46 C |
| Storage Mean | 1.70 A | 1.74 A | 1.70 A | 1.77 A | |
| Cooking Methods | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | Treatment Mean | |
| Soluble dietary fibre | |||||
| Boiling 1 | 1.10 ± 0.10 ab | 0.89 ± 0.09 bcde | 0.59 ± 0.02 fg | 0.93 ± 0.06 bcd | 0.87 A |
| Boiling 2 | 1.16 ± 0.06 a | 0.80 ± 0.00 cdef | 0.63 ± 0.06 fg | 0.97 ± 0.06 abcd | 0.89 A |
| Frying | 0.89 ± 0.10 bcde | 0.77 ± 0.03 def | 0.55 ± 0.05 g | 0.90 ± 0.00 bcd | 0.78 B |
| Pressure cooking | 1.17 ± 0.15 a | 0.87 ± 0.06 cde | 0.68 ± 0.09 efg | 0.99 ± 0.01 abc | 0.92 A |
| Storage Mean | 1.08 A | 0.83 C | 0.61 D | 0.94 B | |
| Insoluble dietary fibre | |||||
| Boiling 1 | 2.43 ± 0.06 defg | 3.10 ± 0.10 ab | 3.33 ± 0.12 a | 2.33 ± 0.15 defgh | 2.80 A |
| Boiling 2 | 2.23 ± 0.06 fghi | 2.50 ± 0.00 cdef | 2.87 ± 0.06 bc | 2.30 ± 0.10 efgh | 2.47 B |
| Frying | 1.92 ± 0.07 i | 2.50 ± 0.24 def | 2.62 ± 0.26 cde | 1.99 ± 0.01 hi | 2.25 C |
| Pressure cooking | 2.00 ± 0.10 hi | 2.36 ± 0.14 defgh | 2.70 ± 0.10 cd | 2.07 ± 0.06 ghi | 2.28 C |
| Storage Mean | 2.14 C | 2.61 B | 2.88 A | 2.17 C | |
| Total dietary fibre | |||||
| Boiling 1 | 3.53 ± 0.15 bc | 3.99 ± 0.01 a | 3.92 ± 0.14 ab | 3.27 ± 0.15 cde | 3.67 A |
| Boiling 2 | 3.40 ± 0.10 cd | 3.30 ± 0.00 cd | 3.50 ± 0.10 c | 3.27 ± 0.06 cde | 3.36 B |
| Frying | 2.82 ± 0.16 f | 3.27 ± 0.21 cde | 3.17 ± 0.21 cdef | 2.89 ± 0.01 ef | 3.03 D |
| Pressure cooking | 3.17 ± 0.21 cdef | 3.23 ± 0.13 cde | 3.38 ± 0.16 cd | 3.06 ± 0.05 def | 3.20 C |
| Storage Mean | 3.22 B | 3.44 A | 3.49 A | 3.12 B | |
| Blood Glucose Level (mg/dL) | Plasma Insulin (µ/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Diet Group | Pre Treatment | During Treatment | Post Treatment | Treatment Mean | Initial (Pre) | Final (Post) | p Value |
| G1 | 114.3 ± 6.86 g | 115 ± 5.4 g | 112.6 ± 4.32 g | 114.0 E | 24.33 ± 0.82 | 24.833 ± 1.17 a | 0.36 NS |
| G2 | 286.5 ± 16.5 a | 285.6 ± 13.8 a | 283.3 ± 8.35 a | 285.1 A | 12.67 ± 1.63 | 10.00 ± 0.89 d | ≤0.001 * |
| G3 | 286.3 ± 16.4 a | 268 ± 15.9 abcd | 244.2 ± 11.0 bcde | 266.1 B | 12.00 ± 1.26 | 16.833 ± 1.72 c | 0.005 * |
| G4 | 282.1 ± 18.4 ab | 238.17 ± 19.4 cde | 198.3 ± 18.1 f | 239.5 D | 13.00 ± 1.79 | 21.500 ± 0.54 b | ≤0.001 * |
| G5 | 276.6 ± 30.1 abc | 257.7 ± 28.4 abcde | 232.5 ± 25.8 def | 255.6 BC | 12.33 ± 1.03 | 17.167 ± 0.75 c | ≤0.001 * |
| Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | HDL (High Density Lipoprotein) (mg/dL) | LDL (Low Density Lipoprotein)(mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet Group | Initial (Pre) | Final (Post) | p Value | Initial (Pre) | Final (Post) | p Value | Initial (Pre) | Final (Post) | p Value | Initial (Pre) | Final (Post) | p Value |
| G1 | 85.17 ± 3.97 | 85.500 ± 2.58 cd | 0.805 NS | 72.33 ± 5.96 | 73.833 ± 6.145 d | 0.122 NS | 54.16 ± 4.57 | 56.167 ± 1.16 a | 0.356 NS | 23.5 ± 1.048 | 24.333 ± 1.032 f | 0.201 NS |
| G2 | 137.3 ± 11.05 | 165.50 ± 05.57 a | 0.003 * | 126.83 ± 1.47 | 124.50 ± 02.66 a | 0.084 NS | 24.66 ± 1.36 | 23.333 ± 1.21 e | 0.034 * | 74.33 ± 1.632 | 77.500 ± 3.78 a | 0.141 NS |
| G3 | 134.83 ± 7.22 | 91.000 ± 0.89 b | ≤0.001 * | 129.66 ± 3.66 | 103.167 ± 1.47 b | ≤0.001 * | 23.5 ± 2.58 | 46.000 ± 0.89 c | ≤0.001 * | 70.5 ± 2.88 | 62.500 ± 0.54 b | 0.019 * |
| G4 | 138.33 ± 10.74 | 83.000 ± 0.89 d | ≤0.001 * | 128.16 ± 1.72 | 88.000 ± 1.89 c | ≤0.001 * | 24.66 ± 1.86 | 54.500 ± 1.64 a | ≤0.001 * | 68.5 ± 2.25 | 47.667 ± 2.06 e | ≤0.001 * |
| G5 | 134.0 ± 8.31 | 89.000 ± 0.89 bc | ≤0.001 * | 125.83 ± 3.76 | 99.333 ± 1.50 b | ≤0.001 * | 23.66 ± 1.21 | 47.500 ± 0.54 c | ≤0.001 * | 68.66 ± 2.65 | 56.500 ± 1.76 c | ≤0.001 * |
| 180–200 | <150 | >50 | <100 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Kaur, H.; Aggarwal, R.; Bains, K.; Mahal, A.K.; Singla, L.D.; Gupta, K. Analysing the Impact of Resistant Starch Formation in Basmati Rice Products: Exploring Associations with Blood Glucose and Lipid Profiles across Various Cooking and Storage Conditions In Vivo. Foods 2024, 13, 1669. https://doi.org/10.3390/foods13111669
Kaur P, Kaur H, Aggarwal R, Bains K, Mahal AK, Singla LD, Gupta K. Analysing the Impact of Resistant Starch Formation in Basmati Rice Products: Exploring Associations with Blood Glucose and Lipid Profiles across Various Cooking and Storage Conditions In Vivo. Foods. 2024; 13(11):1669. https://doi.org/10.3390/foods13111669
Chicago/Turabian StyleKaur, Prabhjot, Harpreet Kaur, Renuka Aggarwal, Kiran Bains, Amrit Kaur Mahal, Lachhman Das Singla, and Kuldeep Gupta. 2024. "Analysing the Impact of Resistant Starch Formation in Basmati Rice Products: Exploring Associations with Blood Glucose and Lipid Profiles across Various Cooking and Storage Conditions In Vivo" Foods 13, no. 11: 1669. https://doi.org/10.3390/foods13111669
APA StyleKaur, P., Kaur, H., Aggarwal, R., Bains, K., Mahal, A. K., Singla, L. D., & Gupta, K. (2024). Analysing the Impact of Resistant Starch Formation in Basmati Rice Products: Exploring Associations with Blood Glucose and Lipid Profiles across Various Cooking and Storage Conditions In Vivo. Foods, 13(11), 1669. https://doi.org/10.3390/foods13111669







