Postharvest Quality of Citrus medica L. (cv Liscia-Diamante) Fruit Stored at Different Temperatures: Volatile Profile and Antimicrobial Activity of Essential Oils
Abstract
1. Introduction
2. Methods
2.1. Samples and Storage Conditions
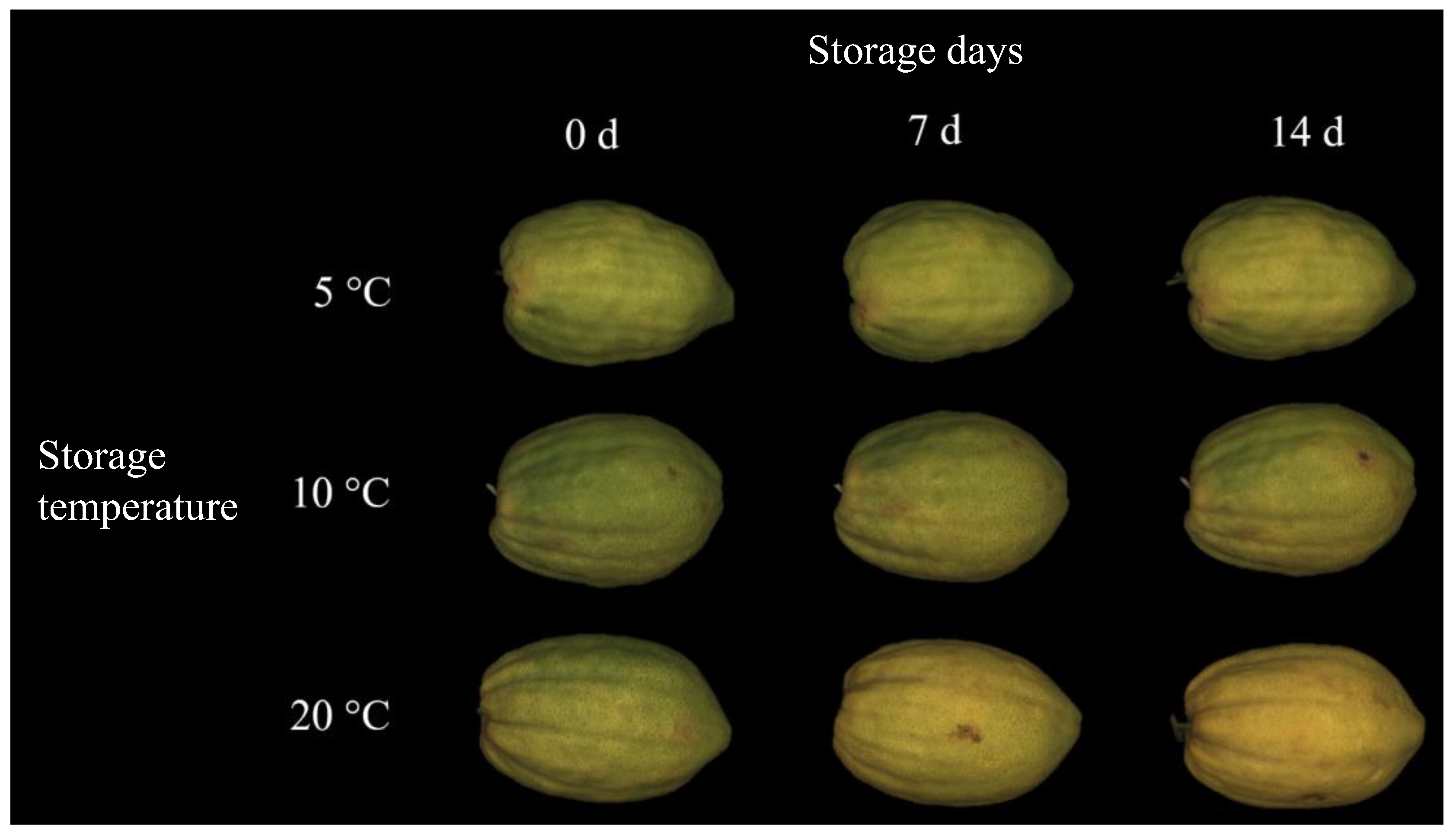
2.2. Visual Quality and Color Attributes
2.3. Respiration Rate and Weight Loss
2.4. Extraction and GC-FID Characterization of the Essential Oils from the Citron Fruit Peel (cv. “Liscia-Diamante”)
2.5. Antimicrobial Activity of Essential Oils Extracted from Fresh and Stored Citron Fruit (cv. “Liscia-Diamante”)
2.6. Statistical Data Analysis
3. Results and Discussion
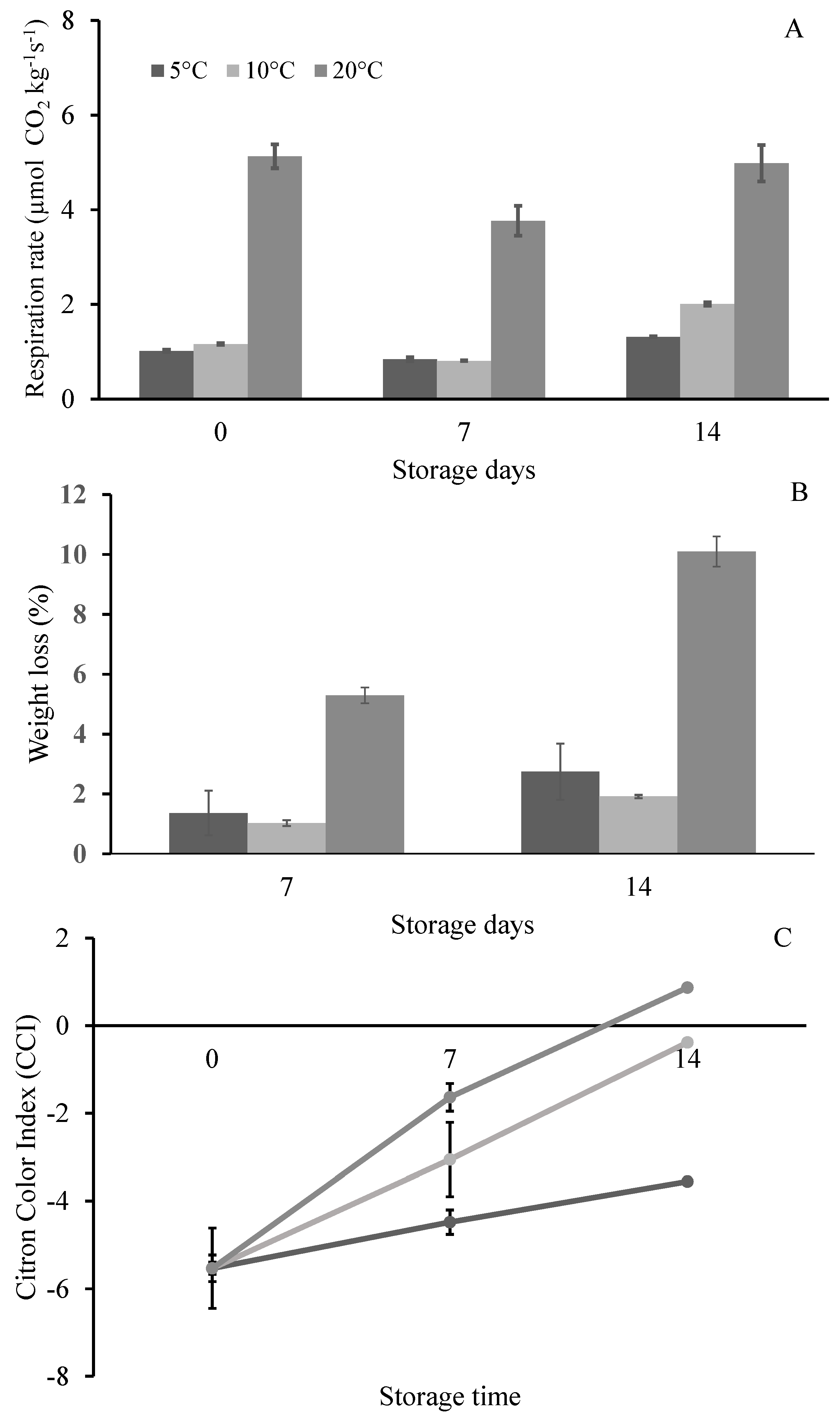
3.1. Postharvest Quality Parameters of Citrons Fruit (cv. “Liscia-Diamante”) Stored at Three Different Temperatures
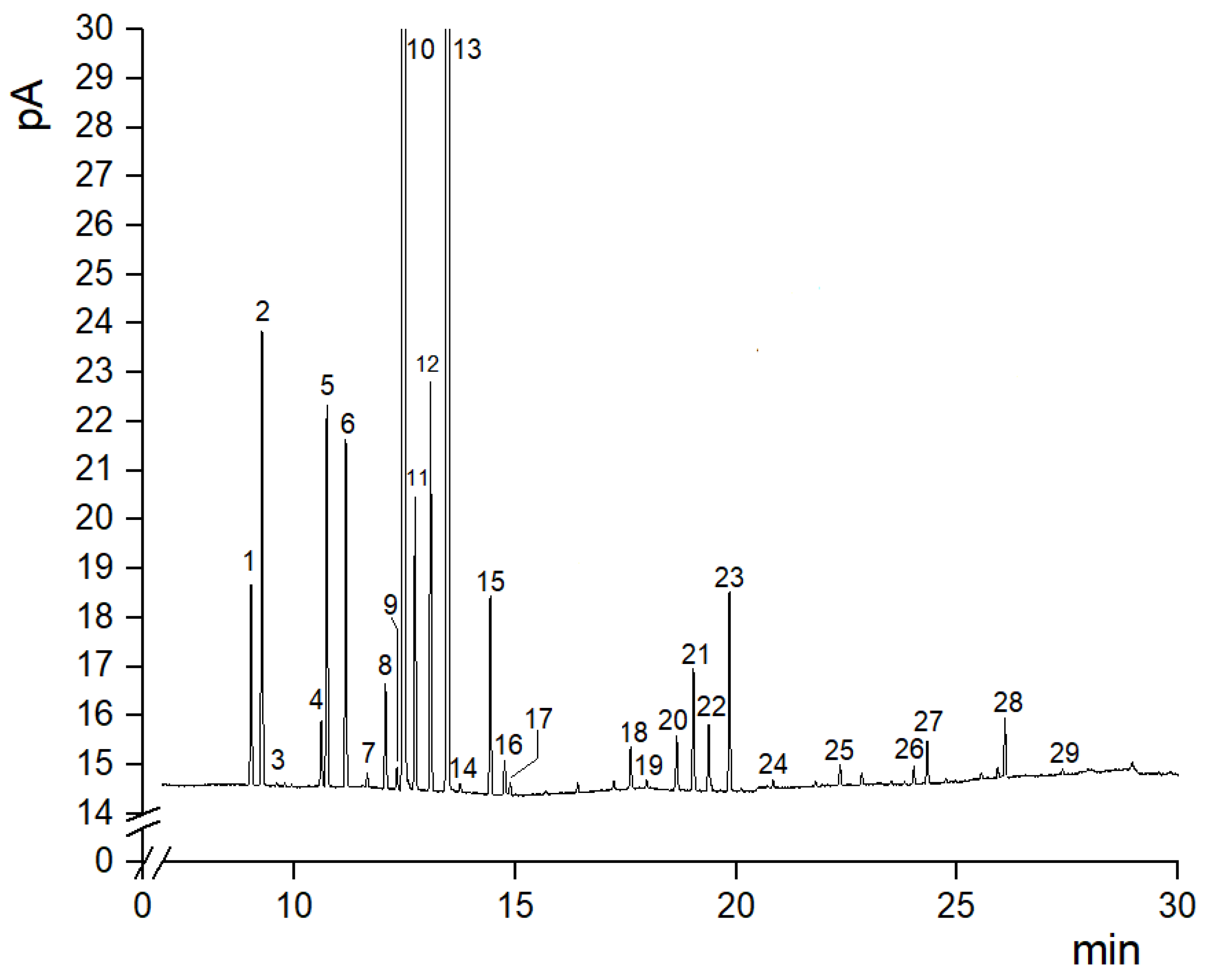
3.2. Changes in the Profile of Essential Oils in Fresh and Stored Citron Fruit (cv. “Liscia-Diamante”)
3.3. Antimicrobial Activity of Essential Oils against Pathogens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDO | Protected Designation of Origin |
| EOs | Essential oils |
| MHs | Monoterpene hydrocarbons |
| CCI | Citron Color Index |
| GC-FID | Gas Chromatography–Flame Ionization Detector |
| ANOVA | Analysis of variance |
| MDR | Multidrug resistant |
References
- Reg. Ue 2023/971, 10/05/2023—COMMISSION IMPLEMENTING REGULATION (EU) 2023/971 of 10 May 2023 Entering a Name in the Register of Protected Designations of Origin and Protected Geographical Indications (‘Cedro di Santa Maria del Cedro’ (PDO)). Official Journal of the European Union on the 10/05/2023. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2023:132:TOC (accessed on 12 May 2024).
- Continella, A. The Citron in Italy and Its Cultivation in Calabria. In The Citron Compendium: The Citron (Etrog) Citrus medica L.: Science and Tradition; Goldschmidt, E.E., Bar-Joseph, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 265–286. ISBN 978-3031257742. [Google Scholar]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential oil composition of Citrus medica L. cv. Diamante (Diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G. Ricerca: Cedro. In Gli agrumi: Botanica, Storia e arte, Alimentazione, Paesaggio, Coltivazione, Ricerca, Utilizzazione, Mondo e Mercato; Tribulato, E., Inglese, P., Eds.; Collana Coltura & Cultura—Angelini R. Bayer CropScience E. Script: Bologna, Italy, 2012; pp. 386–395. [Google Scholar]
- Brigand, J.P.; Nahon, P. Gastronomy and the citron tree (Citrus medica L.). Int. J. Gastron. Food Sci. 2016, 3, 12–16. [Google Scholar] [CrossRef]
- Klein, J.D.; Hebbe, Y.; Shapovalov, A.; Shklyar, G.; Korol, L.; Cohen, S. Changes in peel color of citron fruits from different genetic origins in response to postharvest copper and gibberellic acid treatments. In VII International Postharvest Symposium, Kuala Lumpur (Malaysia); ISHS Publisher: Leuven, Belgium, 2012; Volume 1012, pp. 385–390. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Silva, A.S.; Carreiró, F.; Loizzo, M.R. Health-Promoting Properties and Potential Application in the Food Industry of Citrus medica L. and Citrus × clementina Hort. Ex Tan. Essential Oils and Their Main Constituents. Plants 2023, 12, 991. [Google Scholar] [CrossRef]
- Klein, J.D. Citron Cultivation, Production and Uses in the Mediterranean Region. In Medicinal and Aromatic Plants of the Middle-East. Medicinal and Aromatic Plants of the World; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 199–214. [Google Scholar] [CrossRef]
- Zacarias, L.; Cronje, P.J.; Palou, L. Postharvest technology of citrus fruits. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 421–446. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A.; Agabbio, M. Response of Three Citrus Species to Different Hygrometric Conditions. In Proceedings of the International Conference on Quality in Chains. An Integrated View on Fruit and Vegetable Quality, Wageningen, The Netherlands, 6–9 July 2003; Tijskens, L.M.M., Vollebregt, H.M., Eds.; ISHS Publisher: Leuven, Belgium, 2003; Volume 604, pp. 631–637. [Google Scholar]
- Ladaniya, M. Preharvest Factors Affecting Fruit Quality and Postharvest Life. In Citrus Fruit: Biology, Technology, and Evaluation; Ladaniya, M., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 79–101. ISBN 978-0-12-374130-1. [Google Scholar]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon, and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 437e–516e. [Google Scholar] [CrossRef]
- Patel, B.; Tandel, Y.N.; Patel, A.H.; Patel, B.L. Chilling injury in tropical and subtropical fruits: A cold storage problem and its remedies: A review. Int. J. Sci. Environ. 2016, 5, 1882–1887. [Google Scholar]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Bonesi, M.; de Cindio, B.; Loizzo, M.R.; Conforti, F.; Statti, G.A.; Menabeni, R.; Bettini, R.; Menichini, F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. Antimicrobial and antibiofilm activities of citrus water-extracts obtained by microwave-assisted and conventional methods. Biomedicines 2018, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Nikolaou, A.; Santarmaki, V.; Sgouros, G.; Kourkoutas, Y. Citrus medica and Cinnamomum zeylanicum essential oils as potential biopreservatives against spoilage in low alcohol wine products. Foods 2020, 9, 577. [Google Scholar] [CrossRef]
- Wu, K.; Jin, R.; Bao, X.; Yu, G.; Yi, F. Potential roles of essential oils from the flower, fruit and leaf of Citrus medica L. var. sarcodactylis in preventing spoilage of Chinese steamed bread. Food Biosci. 2021, 43, 101271–101278. [Google Scholar] [CrossRef]
- Jangi, F.; Ebadi, M.T.; Ayyari, M. Qualitative changes in hyssop (Hyssopus officinalis L.) as affected by cold plasma, packaging method and storage duration. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100289–100296. [Google Scholar] [CrossRef]
- Obenland, D.M.; Collin, S.H.; Sievert, J.; Fjeld, K.; Doctor, J.; Arpaia, M.L. Commercial packing and storage of navel oranges alters aroma volatiles and reduces flavour quality. Postharvest Biol. Technol. 2008, 47, 159–167. [Google Scholar] [CrossRef]
- Obenland, D.; Collin, S.; Sievert, J.; Arpaia, M.L. Mandarin flavor and aroma volatile composition are strongly influenced by holding temperature. Postharvest Biol. Technol. 2013, 82, 6–14. [Google Scholar] [CrossRef]
- Lado, J.; Gurrea, A.; Zacarías, L.; Rodrigo, M.J. Influence of the storage temperature on volatile emission, carotenoid content and chilling injury development in Star Ruby red grapefruit. Food Chem. 2019, 295, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rosado, L.D.S.; Pinto, J.E.B.P.; Bertolucci, S.K.V.; Jesus, H.C.R.D.; Alves, P.B. Changes in the content and composition of the essential oil of Ocimum basilicum L. during storage. J. Essent. Oil Res. 2013, 25, 227–232. [Google Scholar] [CrossRef]
- Ebadi, M.T.; Sefidkon, F.; Azizi, M.; Ahmadi, N. Packaging methods and storage duration affect essential oil content and composition of lemon verbena (Lippia citriodora Kunth.). Food Sci. Nutr. 2017, 5, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Comp. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Digital Image Processing Using MATLAB. 2004. Available online: https://www.cin.ufpe.br/~sbm/DEN/Digital%20Image%20Processing%20Using%20Matlab%20(Gonzalez).pdf (accessed on 12 May 2024).
- Jiménez-Cuesta, M.; Cuquerella, J.; Martinez-Javaga, J.M. Determination of a color index for citrus fruit degreening. In Proceedings of the International Society of Citriculture. International Citrus Congress, Tokyo, Japan, 9–12 November 1981; Matsumoto, K., Ed.; International Society of Citriculture: Shimizu, Japan, 1982; pp. 1982–1983. [Google Scholar]
- DOGV, Diari Oficial de la Comunitat Valenciana. 2006, 5346, 30321–30328. Available online: http://hdl.handle.net/10251/38426 (accessed on 12 May 2024).
- Kader, A.A. Methods of gas mixing, sampling and analysis. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 145–148. [Google Scholar]
- Cavalluzzi, M.M.; Budriesi, R.; De Salvia, M.A.; Quintieri, L.; Piarulli, M.; Milani, G.; Gualdani, R.; Micucci, C.I.; Rosato, A.; Viale, M.; et al. Lubeluzole: From anti-ischemic drug to preclinical antidiarrheal studies. Pharmacol. Rep. 2021, 73, 172–184. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Arfin, M.S.; Rahman, M.A.; Molla, M.M.; Sabuz, A.A.; Matin, M.A. Influence of novel coconut oil and beeswax edible coating and MAP on postharvest shelf life and quality attributes of lemon at low temperature. Meas. Food 2023, 10, 100084–100093. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Otsuki, T.; Okada, R.; Obitsu, S.; Masuda, K.; Hojo, Y.; Matsuura, T.; Mori, I.C.; Abe, D.; Asiche, W.O.; et al. Low temperature modulates natural peel degreening in lemon fruit independently of endogenous ethylene. J. Exp. Bot. 2020, 71, 4778–4796. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Venturini, N.; Barboni, T.; Curk, F.; Costa, J.; Paolini, J. Volatile and flavonoid composition of the peel of Citrus medica L. var. Corsican fruit for quality assessment of its liqueur. Food Technol. Biotechnol. 2014, 52, 403–410. [Google Scholar] [CrossRef]
- Di Rauso Simeone, G.; Di Matteo, A.; Rao, M.A.; Di Vaio, C. Variations of peel essential oils during fruit ripening in four lemon (Citrus limon (L.) Burm. F.) cultivars. J. Sci. Food Agric. 2020, 100, 193–200. [Google Scholar] [CrossRef]
- Marzocchi, S.; Baldi, E.; Crucitti, M.C.; Toselli, M.; Caboni, M.F. Effect of harvesting time on volatile compounds composition of bergamot (Citrus× Bergamia) essential oil. Flavour Fragr. J. 2019, 34, 426–435. [Google Scholar] [CrossRef]
- Ghani, A.; Taghvaeefard, N.; Hosseinifarahi, M.; Dakhlaoui, S.; Msaada, K. Essential oil composition and antioxidant activity of citron fruit (Citrus medica var. macrocarpa Risso.) peel as relation to ripening stages. Int. J. Environ. Health Res. 2022, 33, 1278–1288. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Al-Mariri, A.; Safi, M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 2014, 39, 36–43. [Google Scholar] [PubMed]
- Guo, J.J.; Gao, Z.P.; Xia, J.L.; Ritenour, M.A.; Li, G.Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Guo, J.; Hu, X.; Gao, Z.; Li, G.; Fu, F.; Shang, X.; Liang, Z.; Shan, Y. Global transcriptomic response of Listeria monocytogenes exposed to Fingered Citron (Citrus medica L. var. sarcodactylis Swingle) essential oil. Food Res. Int. 2021, 143, 110274–110284. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical composition and anti-biofilm activity of essential oil from Citrus medica L. var. sarcodactylis Swingle against Listeria monocytogenes. Ind. Crops Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z.; Nie, D.; Liu, S.; Li, Y.; Liu, M.; Zhang, Y.; Ou, N.; Li, Y. Selective antibacterial activity of Citrus Medica limonum essential oil against Escherichia coli K99 and Lactobacillus acidophilus and its antibacterial mechanism. LWT 2023, 186, 115215. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Contreras-Castillo, C.J.; Da Gloria, E.M. In vitro mechanism of antibacterial action of a citrus essential oil on an enterotoxigenic Escherichia coli and Lactobacillus rhamnosus. J. Appl. Microbiol. 2020, 129, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Safety 2021, 41, e12918. [Google Scholar] [CrossRef]
- Pedroso, R.D.S.; Balbino, B.L.; Andrade, G.; Dias, M.C.P.S.; Alvarenga, T.A.; Pedroso, R.C.N.; Pimenta, L.P.; Lucarini, R.; Pauletti, P.M.; Januário, A.H.; et al. In vitro and in vivo anti-Candida spp. activity of plant-derived products. Plants 2019, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; Bezerra, A.P.D.B.; Sousa, J.P.D.; Guerra, F.Q.S.; Lima, E.D.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.; Tao, N.; Zhang, M. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar] [CrossRef]
- de Araújo, A.C.J.; Freitas, P.R.; Barbosa, C.R.d.S.; Muniz, D.F.; Ribeiro-Filho, J.J.; Tintino, S.R.; Júnior, J.P.S.; Filho, J.M.B.; de Sousa, G.R.; Coutinho, H.D.M. Modulation of Drug Resistance by Limonene: Inhibition of Efflux Pumps in Staphylococcus aureus Strains RN-4220 and IS-58. Curr. Drug Metab. 2021, 22, 110–113. [Google Scholar] [CrossRef]
| Quality Attributes | Temperature (T) | Storage Time (S) | Interaction (T × S) |
|---|---|---|---|
| Visual quality | ns | *** | ns |
| Respiration rate (µmol CO2 kg−1 s−1) | **** | **** | *** |
| Citron Color Index (CCI) | **** | **** | * |
| Volatile Compounds | N. Peak | 0 d | 7 d | 14 d | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 5 °C | 10 °C | 20 °C | 5 °C | 10 °C | 20 °C | ||||||||||||||||
| α-Tujene | 1 | 1.04 | ± | 0.03 | 1.03 | ± | 0.01 | 0.91 | ± | 0.01 | 0.91 | ± | 0.01 | 1.03 | ± | 0.11 | 1.00 | ± | 0.08 | 1.05 | ± | 0.01 |
| α-Pinene | 2 | 2.21 | ± | 0.04 | 2.45 | ± | 0.03 | 2.16 | ± | 0.09 | 2.14 | ± | 0.06 | 2.43 | ± | 0.23 | 2.32 | ± | 0.08 | 2.40 | ± | 0.01 |
| Camphene | 3 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 |
| Sabinene | 4 | 0.19 | ± | 0.22 | 0.35 | ± | 0.01 | 0.35 | ± | 0.04 | 0.34 | ± | 0.01 | 0.37 | ± | 0.02 | 0.38 | ± | 0.06 | 0.37 | ± | 0.02 |
| β-Pinene | 5 | 2.11 | ± | 0.01 | 2.02 | ± | 0.08 | 1.86 | ± | 0.14 | 1.87 | ± | 0.04 | 2.04 | ± | 0.16 | 2.05 | ± | 0.25 | 2.08 | ± | 0.07 |
| Myrcene | 6 | 1.30 | ± | 0.01 | 1.81 | ± | 0.05 | 1.80 | ± | 0.02 | 1.69 | ± | 0.03 | 1.73 | ± | 0.06 | 1.66 | ± | 0.14 | 1.69 | ± | 0.00 |
| α-Phellandrene | 7 | 0.06 | ± | 0.00 | 0.08 | ± | 0.01 | 0.06 | ± | 0.01 | 0.07 | ± | 0.01 | 0.07 | ± | 0.00 | 0.08 | ± | 0.01 | 0.08 | ± | 0.01 |
| α-Terpinene | 8 | 0.59 | ± | 0.01 | 0.53 | ± | 0.05 | 0.47 | ± | 0.02 | 0.48 | ± | 0.00 | 0.52 | ± | 0.03 | 0.51 | ± | 0.04 | 0.55 | ± | 0.01 |
| p-Cimene | 9 | 0.10 | ± | 0.03 | 0.10 | ± | 0.04 | 0.07 | ± | 0.01 | 0.07 | ± | 0.00 | 0.09 | ± | 0.04 | 0.11 | ± | 0.02 | 0.08 | ± | 0.01 |
| Limonene | 10 | 47.60 | ± | 0.05 | 60.79 | ± | 2.79 | 63.58 | ± | 1.21 | 61.65 | ± | 0.43 | 59.59 | ± | 0.11 | 61.90 | ± | 0.13 | 57.28 | ± | 0.96 |
| cis-β-Ocimene | 11 | 1.19 | ± | 0.04 | 1.50 | ± | 0.01 | 1.49 | ± | 0.01 | 1.46 | ± | 0.03 | 1.73 | ± | 0.13 | 1.44 | ± | 0.04 | 1.77 | ± | 0.15 |
| trans-β-Ocimene | 12 | 1.62 | ± | 0.04 | 2.11 | ± | 0.01 | 2.14 | ± | 0.02 | 2.05 | ± | 0.04 | 2.43 | ± | 0.18 | 2.05 | ± | 0.05 | 2.48 | ± | 0.23 |
| γ-Terpinene | 13 | 28.03 | ± | 0.29 | 22.47 | ± | 2.10 | 19.99 | ± | 0.99 | 20.06 | ± | 0.10 | 22.09 | ± | 1.14 | 21.29 | ± | 0.01 | 22.99 | ± | 0.64 |
| trans-Sabinene hydrate | 14 | 0.05 | ± | 0.00 | 0.05 | ± | 0.02 | 0.06 | ± | 0.02 | 0.07 | ± | 0.01 | 0.03 | ± | 0.00 | 0.05 | ± | 0.00 | 0.06 | ± | 0.01 |
| Terpinolen | 15 | 1.04 | ± | 0.01 | 1.00 | ± | 0.09 | 0.91 | ± | 0.05 | 0.89 | ± | 0.01 | 0.98 | ± | 0.04 | 0.95 | ± | 0.01 | 1.02 | ± | 0.03 |
| Linalool | 16 | 0.26 | ± | 0.01 | 0.17 | ± | 0.06 | 0.21 | ± | 0.05 | 0.28 | ± | 0.06 | 0.13 | ± | 0.04 | 0.18 | ± | 0.03 | 0.20 | ± | 0.00 |
| Nonanal | 17 | 0.13 | ± | 0.00 | 0.06 | ± | 0.02 | 0.03 | ± | 0.01 | 0.06 | ± | 0.00 | 0.08 | ± | 0.01 | 0.06 | ± | 0.01 | 0.06 | ± | 0.00 |
| Citronellal | 18 | 0.25 | ± | 0.01 | 0.18 | ± | 0.07 | 0.23 | ± | 0.07 | 0.32 | ± | 0.06 | 0.12 | ± | 0.03 | 0.16 | ± | 0.08 | 0.12 | ± | 0.01 |
| Terpinen-4-ol | 19 | 0.03 | ± | 0.00 | 0.04 | ± | 0.01 | 0.03 | ± | 0.01 | 0.05 | ± | 0.01 | 0.09 | ± | 0.03 | 0.04 | ± | 0.00 | 0.05 | ± | 0.00 |
| α-Terpineol | 20 | 0.24 | ± | 0.29 | 0.22 | ± | 0.11 | 0.51 | ± | 0.49 | 0.54 | ± | 0.09 | 0.13 | ± | 0.06 | 0.13 | ± | 0.06 | 0.23 | ± | 0.00 |
| Neral | 21 | 2.72 | ± | 0.01 | 0.63 | ± | 0.02 | 0.57 | ± | 0.30 | 1.21 | ± | 0.08 | 0.98 | ± | 0.39 | 0.93 | ± | 0.01 | 1.35 | ± | 0.04 |
| Geraniol | 22 | 0.47 | ± | 0.08 | 0.27 | ± | 0.16 | 0.59 | ± | 0.62 | 0.65 | ± | 0.18 | 0.13 | ± | 0.07 | 0.08 | ± | 0.04 | 0.27 | ± | 0.04 |
| Geranial | 23 | 4.80 | ± | 0.01 | 1.03 | ± | 0.02 | 0.93 | ± | 0.50 | 2.03 | ± | 0.10 | 1.69 | ± | 0.69 | 1.49 | ± | 0.01 | 2.30 | ± | 0.04 |
| Undecanal | 24 | 0.05 | ± | 0.05 | 0.03 | ± | 0.01 | 0.02 | ± | 0.00 | 0.03 | ± | 0.00 | 0.05 | ± | 0.01 | 0.03 | ± | 0.01 | 0.04 | ± | 0.01 |
| Citronellyl acetate | 25 | 0.44 | ± | 0.10 | 0.10 | ± | 0.01 | 0.14 | ± | 0.06 | 0.14 | ± | 0.01 | 0.27 | ± | 0.04 | 0.22 | ± | 0.05 | 0.21 | ± | 0.04 |
| Neryl acetate | 26 | 0.11 | ± | 0.01 | 0.11 | ± | 0.01 | 0.09 | ± | 0.03 | 0.07 | ± | 0.00 | 0.10 | ± | 0.03 | 0.10 | ± | 0.03 | 0.06 | ± | 0.00 |
| Geranyl acetate | 27 | 0.26 | ± | 0.01 | 0.25 | ± | 0.01 | 0.25 | ± | 0.03 | 0.23 | ± | 0.01 | 0.25 | ± | 0.04 | 0.20 | ± | 0.08 | 0.21 | ± | 0.02 |
| β-Bisabolene | 28 | 0.43 | ± | 0.04 | 0.32 | ± | 0.01 | 0.33 | ± | 0.04 | 0.32 | ± | 0.03 | 0.36 | ± | 0.04 | 0.27 | ± | 0.12 | 0.30 | ± | 0.03 |
| Germacrene B | 29 | 0.10 | ± | 0.01 | 0.03 | ± | 0.01 | 0.03 | ± | 0.01 | 0.03 | ± | 0.00 | 0.04 | ± | 0.01 | 0.03 | ± | 0.00 | 0.02 | ± | 0.00 |
| 0 d | 7 d | 14 d | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile Compounds | Fresh | 5 °C | 10 °C | 20 °C | 5 °C | 10 °C | 20 °C | p | |||||||
| Myrcene | 1.30 | b | 1.81 | a | 1.80 | a | 1.69 | a | 1.73 | a | 1.66 | a | 1.69 | a | ** |
| γ-Terpinene | 0.59 | a | 0.53 | abc | 0.47 | bc | 0.48 | c | 0.52 | bc | 0.51 | c | 0.55 | ab | * |
| Limonene | 47.60 | d | 60.79 | abc | 63.58 | a | 61.65 | ab | 59.59 | bc | 61.90 | ab | 57.28 | c | *** |
| cis-β-Ocimene | 1.19 | c | 1.50 | b | 1.49 | b | 1.46 | b | 1.73 | a | 1.44 | b | 1.77 | a | ** |
| trans-β-Ocimene | 1.62 | c | 2.11 | b | 2.14 | b | 2.05 | b | 2.43 | a | 2.05 | b | 2.48 | c | ** |
| A-Terpinolene | 28.03 | a | 22.47 | b | 19.99 | c | 20.06 | c | 22.09 | bc | 21.29 | bc | 22.99 | b | *** |
| Nonanal | 0.13 | a | 0.06 | b | 0.03 | c | 0.06 | b | 0.08 | b | 0.06 | b | 0.06 | b | *** |
| Terpinen-4-ol | 0.03 | b | 0.04 | b | 0.03 | b | 0.05 | b | 0.09 | a | 0.04 | b | 0.05 | b | * |
| Neral | 2.72 | a | 0.63 | c | 0.57 | c | 1.21 | b | 0.98 | bc | 0.93 | bc | 1.35 | b | **** |
| Geranial | 4.80 | a | 1.03 | d | 0.93 | d | 2.03 | bc | 1.69 | bcd | 1.49 | cd | 2.30 | b | **** |
| Citronellyl acetate | 0.44 | a | 0.10 | c | 0.14 | c | 0.14 | c | 0.27 | b | 0.22 | bc | 0.21 | bc | ** |
| Germacrene B | 0.10 | a | 0.03 | b | 0.03 | b | 0.03 | b | 0.04 | b | 0.03 | b | 0.02 | b | **** |
| Volatile Compounds | Temperature | Storage Time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 10 °C | 20 °C | p | 7 d | 14 d | p | ||||||
| Sabinene | 0.36 | ns | 0.36 | ns | 0.35 | ns | ns | 0.34 | b | 0.37 | a | * |
| β-Pinene | 2.03 | ns | 1.95 | ns | 1.98 | ns | ns | 1.92 | b | 2.05 | a | * |
| Myrcene | 1.77 | a | 1.73 | ab | 1.69 | b | * | 1.76 | a | 1.69 | b | ** |
| trans-Sabinene hydrate | 0.04 | b | 0.05 | a | 0.06 | a | ** | 0.06 | a | 0.05 | b | * |
| Linalool | 0.15 | c | 0.19 | b | 0.24 | a | *** | 0.22 | a | 0.17 | b | ** |
| α-Terpineol | 0.18 | b | 0.32 | ab | 0.38 | a | ns | 0.42 | a | 0.16 | b | ** |
| Neral | 0.80 | b | 0.75 | b | 1.28 | a | **** | 0.80 | b | 1.08 | a | *** |
| Geraniol | 0.20 | b | 0.33 | ab | 0.46 | a | ns | 0.50 | a | 0.16 | b | ** |
| Geranial | 1.36 | b | 1.21 | b | 2.16 | a | **** | 1.33 | b | 1.82 | a | *** |
| Undecanal | 0.04 | a | 0.02 | b | 0.03 | a | ** | 0.03 | b | 0.04 | a | ** |
| Neryl acetate | 0.10 | a | 0.10 | a | 0.07 | b | ** | 0.09 | ns | 0.09 | ns | ns |
| Strains | Halos (mm) * | ||||||
|---|---|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | |||||
| Fresh | 5 °C | 10 °C | 20 °C | 5 °C | 10 °C | 20 °C | |
| Yersinia enterocolitica subsp. enterocolitica DSM4780 | n.d ** | n.d. | n.d | n.d. | n.d. | n.d. | n.d. |
| Staphylococcus aureus ATCC 6538P | n.d. | n.d. | 9 ± 1 | n.d. | n.d. | 9 ± 1 | 11 ± 2 |
| Listeria monocytogenes LMG 23193 | n.d. | n.d. | 7 ± 1 | n.d. | 10 ± 2 | 7 ± 2 | 11 ± 1 |
| Candida albicans DSM1386 | n.d. | n.d. | 8 ± 2 | n.d. | n.d. | n.d. | 12 ± 2 |
| Escherichia coli K12 | n.d. | n.d. | 8 ± 2 | n.d. | 7 ± 2 | n.d. | 11 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintieri, L.; Palumbo, M.; Ricci, I.; Pace, B.; Caputo, L.; Adduci, A.; Luparelli, A.; Cefola, M.; Siano, F.; Cozzolino, R. Postharvest Quality of Citrus medica L. (cv Liscia-Diamante) Fruit Stored at Different Temperatures: Volatile Profile and Antimicrobial Activity of Essential Oils. Foods 2024, 13, 1596. https://doi.org/10.3390/foods13111596
Quintieri L, Palumbo M, Ricci I, Pace B, Caputo L, Adduci A, Luparelli A, Cefola M, Siano F, Cozzolino R. Postharvest Quality of Citrus medica L. (cv Liscia-Diamante) Fruit Stored at Different Temperatures: Volatile Profile and Antimicrobial Activity of Essential Oils. Foods. 2024; 13(11):1596. https://doi.org/10.3390/foods13111596
Chicago/Turabian StyleQuintieri, Laura, Michela Palumbo, Ilde Ricci, Bernardo Pace, Leonardo Caputo, Angelo Adduci, Anna Luparelli, Maria Cefola, Francesco Siano, and Rosaria Cozzolino. 2024. "Postharvest Quality of Citrus medica L. (cv Liscia-Diamante) Fruit Stored at Different Temperatures: Volatile Profile and Antimicrobial Activity of Essential Oils" Foods 13, no. 11: 1596. https://doi.org/10.3390/foods13111596
APA StyleQuintieri, L., Palumbo, M., Ricci, I., Pace, B., Caputo, L., Adduci, A., Luparelli, A., Cefola, M., Siano, F., & Cozzolino, R. (2024). Postharvest Quality of Citrus medica L. (cv Liscia-Diamante) Fruit Stored at Different Temperatures: Volatile Profile and Antimicrobial Activity of Essential Oils. Foods, 13(11), 1596. https://doi.org/10.3390/foods13111596











