Abstract
‘Ruixue’ apples were used as the test material to study the effect of 10 μM methyl jasmonate (MeJA) on the quality and cell wall metabolism of apples after 18 d of storage. The results showed that MeJA significantly decreased the respiratory rate, reduced the titratable acid content and maintained a high soluble solids content. MeJA has been shown to suppress the activities and gene expressions of WSP, CSP, ISP, and cellulose in contrast to the control group, thereby maintaining a lower cell permeability and higher exocarp firmness. MeJA significantly decreased the expression of MdACS, MdACO, MdPL, Mdgal, and MdPG genes in the apple exocarp when compared to the control group. In addition, the overexpression of MdPL18 increased the content of cell wall polysaccharides such as WSP and CSP, enhanced cell wall-degrading enzyme activities, and accelerated fruit ripening and softening, whereas silencing MdPL18 did the opposite. Together, these results demonstrate that exogenous MeJA maintains the Ruixue apple fruit quality by regulating the metabolism of cell wall substances.
1. Introduction
Apples belong to the Rosaceae plant family and are the most widely grown common fruit in China [1]. In 2022, China’s apple production and cultivated area accounted for 56.4% and 42.7% of the global total, respectively. An apple is a typical respiratory climacteric fruit, and the fruit quality is best for fresh consumption when it reaches the peak of the respiratory climacteric. Climacteric fruits show a significant increase in ethylene release during the climacteric, and they respond to exogenous ethylene before the climacteric period, during the ripening process [2].
During the processes of harvest and storage, fruits undergo a series of physiological changes, such as changes in their color, texture, and volatile substance content [3]. These changes are influenced by various environmental factors, biological and abiotic stresses, as well as genetic regulation [4]. Ethylene can promote fruit ripening, regulate gender differentiation, and respond to biotic and abiotic stresses [5]. Studies have found that exogenous ethylene treatment can increase cell membrane permeability in papaya fruit and kiwifruit, accelerating fruit ripening and softening [6]. Ethylene treatment can also accelerate the conversion of nutrients in apple fruit, promoting its ripening [7]. From the above, it is clear that exogenous ethylene treatment can improve cell membrane permeability, promote fruit color change, and improve the flavor and conversion of nutrients in fruits.
Extensive research has shown that under the action of cell wall metabolism-related enzymes, the structure of the cell wall, which plays a supportive role, changes, the permeability of the cell is increased, the cellular fluid exudes, and the firmness of the fruit decreases, thus promoting fruit ripening [8]. Throughout the process of fruit softening, alterations occur in the structure of the cell walls, as well as the breakdown of various components within the cell walls [9]. Pectin, cellulose, and hemicellulose are randomly distributed in the cell wall polysaccharide network structure through hydrogen bonds, covalent bonds, and hydrophobic forces [10]. The distribution of pectin in fruits plays a crucial role in determining their texture. As fruits ripen, insoluble protopectin breaks down, while soluble pectin levels rise. This results in reduced intercellular adhesion and structural damage to the cells. These processes are often associated with the involvement of cell wall degradation-related enzymes, such as polygalacturonase (PG), pectin methylesterase (PME), and glycosidase (β-Gal) [11].
Jasmonates (JAs) include jasmonic acid (JA), its volatile ester derivative methyl jasmonate (methy-jasmonate, MeJA), and other amino acid derivatives [12]. They are a class of growth-regulating substances widely present in a variety of plants and can act as endogenous signaling molecules, inducing the expression of plant defense genes, regulating plant resistance, growth and development, and the synthesis of secondary metabolites [13]. They can also affect pollen development and fruit maturation and development [14]. Exogenous MeJA regulates ascorbic acid and glutathione metabolism, thereby improving the quality of loquat fruit [15]. Similarly, the content of ascorbic acid in carambola significantly increases after MeJA treatment. Moreover, it effectively hinders the function of PAL, POD, and PPO, ultimately preserving the ideal fruit condition during transport and preservation. [16].
The firmness index of the fruit is a crucial factor in assessing fruit ripeness. It was found that MeJA treatment could inhibit the decrease in firmness of peach and strawberry fruits [17,18]. Titratable acid is one of the important indicators for evaluating a fruit’s nutritional quality. The treatment of peach and blackberry fruits with MeJA resulted in a decrease in the titratable acids content [17]. Treatment with external MeJA can elevate the levels of volatile aromatic compounds in strawberries, thus enhancing their fragrance and boosting the strawberry quality. [18]. In addition, jasmonic acid also plays an important role in delaying the browning of fresh-cut fruits. MeJA treatment reduces the occurrence of browning in fresh-cut apples.
Pectin lyase is crucial in floral organ development and fruit ripening, serving various biological functions in plants [19,20,21]. Notably, it aids in fruit softening, while pectin-related lyase contributes to polysaccharide modification during ripening [22]. Previous research identified two distinct pectin lyase genes, PelⅠ and PelⅡ, in bananas and showed that their expression levels rise as the fruit matures [23]. In tomatoes, suppressing the pectin lyase gene enhances the fruit’s firmness, delaying softening without compromising the growth or quality [24]. Similarly, in non-climacteric strawberries, pectin lyase gene expression increases with fruit maturation [25]. Silencing this gene in strawberries inhibits endogenous pectin lyase gene expression, leading to pectin accumulation and a 30% increase in the fruit hardness compared to wild-type fruit [25]. These findings suggest pectin lyase as a potential candidate gene for regulating fruit texture.
‘Ruixue’ is a new apple variety with white flesh, a green surface, crispy and sweet taste, and rich flavor. In this research, the effects of exogenous MeJA on fruit quality indicators such as the firmness, SSC, TA, and ETH production of the ‘Ruixue’ apple fruit were studied. Furthermore, the changes in cell wall substances and related enzyme activities of apple fruit after MeJA treatment were further studied. The function of the transcription factor MdPL18 in the apple fruit was substantiated through transient expression. Overall, MeJA affects the postharvest quality of the ‘Ruixue’ apple through the metabolism of the cell wall.
The objective of this study is to investigate the impact of MeJA on the taste quality, nutritional composition, and cell walls of apples throughout storage after harvesting. Through the examination of the enzyme activities related to cell walls, the effect of treating apple fruit with MeJA was studied. The expression patterns of fruit ripening-related genes were further determined to elucidate the effects of MeJA on the postharvest quality of ‘Ruixue’ apples.
2. Materials and Methods
2.1. Experimental Treatments and Plant Materials
The variety number of ‘Ruixue’ is CNA20151469.1. During our initial investigation, the ‘Ruixue’ apples underwent treatment with MeJA at concentrations of 10 μM, 100 μM, and 1000 μM, respectively (Figure S1). The results showed that the treatment with 10 μM MeJA had a significant effect on delaying the postharvest ripening of the apples, while the treatment with 100 μM MeJA did not significantly affect the quality of the apples, and the treatment with 1000 μM MeJA accelerated the ripening of the apples. In addition, according to the study by Lv et al. [26], the postharvest treatment of apple fruit with 10 μM MeJA alleviated the postharvest ripening. Hence, for this investigation, we selected a concentration of 10 μM MeJA (Figure 1A). Two groups were formed, with a total of 240 ‘Ruixue’ apples that were uniform and undamaged. The preparation of the MeJA solution involved dissolving 95% MeJA (Solarbio, Beijing, China) in deionized water with a 0.077% (v/v) triton x-100 solution. The first group was immersed in a solution of 10 μM MeJA for 10 min. The control group, on the other hand, was immersed in deionized water with a 0.077% (v/v) triton x-100 solution for 10 min. Following air drying, all the apples were stored at a temperature of 20 ± 0.5 °C, and samples were collected at intervals of 3 days (0, 3, 6, 9, 12, 15, and 18). During every sampling occasion, we randomly picked 15 apples from each group. Each treatment had three repeated experiments, with five apples for each replicate. The pericarp tissue (3–8 mm below the equator) of the control and MeJA-treated fruits were collected.
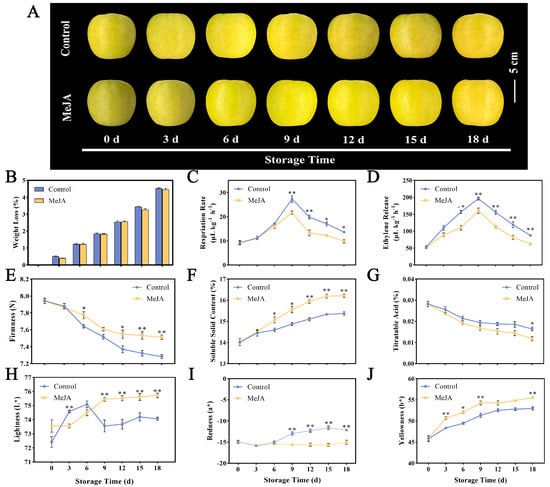
Figure 1.
Effects of MeJA on quality traits of ‘Ruixue’ apple fruit. (A) Apple fruit morphology, (B) weight loss, (C) respiratory rate, (D) ethylene release, (E) firmness, (F) SSC, (G) TA, (H) lightness, (I) redness, and (J) yellowness (*, p < 0.05; **, p < 0.01). Error bars show ±SE from three biological replicates.
2.2. Detection of Physiological Characteristics
During the period of 18 days, the percentage of weight loss for two sets of fruits was assessed every 3 days. The weight reduction was determined by utilizing the subsequent equation:
Weight loss (%) = (initial weight − final weight)/initial weight ∗ 100
The collection of ethylene was conducted using the drainage gas collection method. The ethylene release rate was analyzed using gas chromatography (GC-14A, Shimadzu, Kyoto, Japan). The respiration rate was measured using gas chromatography with a flame ionization detector (FID) (SP-9890, Lunan Ruihong, Tengzhou, China). The release of ethylene and the respiration rate were measured in terms of the amount of CO2 and ethylene generated per kilogram of fresh weight in an hour, respectively. Each experimental procedure was performed three times, and each replication consisted of 10 pieces of fruit. The hardness of the apple fruit was evaluated by taking measurements at two points equidistant around the circumference of the apples. A texture analyzer (GS-15, Ibbenbüren, Germany) was utilized for the analysis, employing a 10 mm diameter flat probe that could penetrate to a depth of 8 mm. SSC and TA were analyzed as described by yang et al. [27]. The sugar content of the fruit was measured using a digital refractometer. To detect titratable acids, 1.0 g of apple fruit was mixed with 3.0 mL of distilled water, ground, and transferred to a 50 mL volumetric flask. After filtering the solution for 10 min, 5 mL of the filtrate was placed in an Erlenmeyer flask. Then, 2 drops of 2% phenolphthalein reagent were added, followed by titration with NaOH. The calculation was performed using the given formula. In the formula, V is the total volume of the extracted sample liquid, mL; c is the NaOH titrant concentration, mol/L; ΔV is the difference between the volume of NaOH used to titrate the sample and the volume of NaOH used to titrate distilled water, mL; Vs is the volume of the filtrate used in the titration, mL; m is the sample weight, g; and the conversion coefficient f = 0.067.
TA(%) = (V × c × ΔV × ƒ)/(Vs × m) × 100
2.3. Determination of Cell Wall Component Content
The procedure to extract cell wall polysaccharides followed that of Melton et al. [28]. By utilizing varying solubility levels of cell wall components, the contents of apple fruit cell wall constituents were isolated and analyzed. A sequential extraction was conducted to obtain WSP, CSP, ISP, and hemicellulose. The pectin concentration was assessed using the carbazole–ethanol technique, the hemicellulose concentration was measured using the anthrone colorimetric approach, and the cellulose concentration was determined using the weight-based method.
2.4. Determination of Cell Wall Degradation-Related Enzyme Activities
PG activities were determined using the DNS colorimetric method, which was slightly modified from our previous study [29]. The measurement of β-gal activity was performed through the hydrolysis of the nitrogalactose side chain and was quantified at a wavelength of 540 nm. The determination of PL activity was carried out following the protocol outlined by Payasi, with quantification performed at 550 nm [30]. The measurement of β-gal activity was conducted using the method described by Payasi, and quantification was performed at 550 nm [30].
2.5. Determination of Relative Gene Expression
The apple fruit samples were processed for total RNA extraction using the Quick RNA isolation kit (Tiangen, Beijing, China). The cDNA reverse transcription was carried out using the All-in-One First-Strand SuperMix with gDNA Eraser (TransGen Biotech, located in Beijing, China). The RT-qPCR analysis was performed with the ABI7500 System and SYBR Green Master Mix (Vazyme from Nanjing, China). All gene expression levels were normalized using Mdβ-Actin as the internal reference gene. The relative gene expression was calculated by 2−ΔΔCt. The RT-qPCR primers can be found in Table S1.
2.6. Transient Expression of MdPL18 in Apple Fruit
An overexpression vector, pCAMBIA 2300, was utilized to introduce the full-length coding DNA sequence (CDS) of MdPL18. As for the silencing vector, a 400 bp fragment of the MdPL18 CDS was introduced into TRV2 (virus-induced gene silencing). To augment the control, ‘Ruixue’ fruit was injected with A. tumefaciens (EHA105) co-cultured with OE-MdPL18 and TRV-MdPL18 using the empty vector (OD600 = 1, 100 µM acetosyringone, 100 mM MgCl2, and 100 mM MES). Following a 3-day infiltration period, qRT-PCR was performed on samples collected from the injection site. Three biological replicates were carried out, with each treatment involving 20 apples that were subjected to injection.
2.7. Stable Overexpression of MdPL18 in Apple Calli
The overexpression vector for MdPL18 CDS was incorporated into pCAMBIA2300 and utilized. ‘Orin’ apple calli (12 days old, OD600 = 0.8) were co-cultured with A. tumefaciens (EHA105) containing OE-MdPL18. These calli were then placed in MS medium (1 mg L−1 2–4D, 0.5 mg L−1 6-BA) and incubated in a dark chamber at 24 °C for 36 h. Following this, the incubated calli were transferred to the MS medium for transgenic selection. RT-PCR and qRT-PCR were used to detect transgenic expression in the obtained transgenic lineages.
2.8. Statistical Analysis
To identify statistically significant differences between samples, we employed the Student’s t-test (* p < 0.05, ** p < 0.01). In order to determine noteworthy variations across various sets of data, we employed Tukey’s single-factor analysis of variance (ANOVA) using SPSS 20 (IBM Corporation, Chicago, IL, USA). Graphs were generated using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). All reported values represent the mean ± standard error (SE) derived from three biological replicates.
3. Results
3.1. Changes in Quality Parameters of Apple Fruit Treated with Exogenous MeJA
The weight loss rate of fruit has a significant impact on the postharvest quality of apples and is a vital indicator of the fruit storage process. The quality loss in fruit soaked in MeJA and control solution increased with a longer storage time (Figure 1B). Compared with the control group’s 4.53% weight loss rate, there was no significant change in the weight loss rate of fruit treated with MeJA, which was 4.46%. As can be seen from Figure 1C, the peak respiration rates appeared simultaneously on day 9 in control and MeJA-treated fruit. The peak respiratory rate of the control group was 27.34 μL kg−1 h−1, which was 1.26 times that of the MeJA-treated group. MeJA significantly reduced the respiration rate of apples on days 9–18. The respiratory transition was accompanied by a large release of ethylene, so we tested the ethylene release between the two groups. The results showed that the MeJA treatment group also reached the maximum ethylene release of 160.69 μL kg−1 h−1 on the 9th day. The ethylene release amount in the control group was 196.23 μL kg−1 h−1 and then decreased (Figure 1D). The MeJA treatment reduced the ethylene release from the apples and effectively improved the quality of the apples. Firmness is one of the important indicators reflecting fruit maturity. During the 18-day storage period, the fruit firmness of both the control and treatment groups decreased steadily (Figure 1E). On the 6th day and 12th to 18th day after treatment, the firmness of the control group was significantly lower than that of the treatment group. Starting on the 6th day, the SSC of fruit treated with MeJA showed a significant increase compared to the control group (Figure 1F). The TA content showed a decreasing trend throughout the storage process (Figure 1G). Compared with the day of sampling (0th day), the TA content of MeJA-treated fruit decreased by 0.14% on the 18th day, while the TA content of the control fruit only decreased by 0.11% on the 18th day. Significant differences were observed in the L* value of fruit treated with MeJA on days 9–18 after harvest, reaching a maximum of 75.72 (Figure 1H). The increasing trend in the a* value of untreated fruit was more obvious. The b* value of both groups showed an increasing trend within 18 days of storage (Figure 1I,J).
3.2. Effects of Exogenous MeJA Treatment on Apple Fruit Cell Wall Components
The decrease in apple fruit tissue hardness is often associated with changes in cell wall polysaccharides. This study examined the effects of exogenous MeJA treatment on various apple fruit cell wall components, including WSP, CSP, ISP, HC, cellulose, and CWM. The WSP content of apple fruit generally increased during storage, but this increase was obviously hindered after MeJA treatment (Figure 2A). Starting on the 9th day, the level of WSP content notably rose in the control group, ultimately peaking at 1.84 mg g−1 on the 18th day. The CSP content of control group was 1.38 times that of the MeJA group on the 12th day (p < 0.05) (Figure 2B). The ISP content showed a steady increase during storage, with MeJA-treated fruit having lower levels compared to the control group (Figure 2C). Hemicellulose (HC) and cellulose contents decreased over time, with the control group showing a more pronounced decrease (Figure 2D–F). Overall, the MeJA treatment appeared to positively influence the fruit quality by affecting cell wall metabolism.
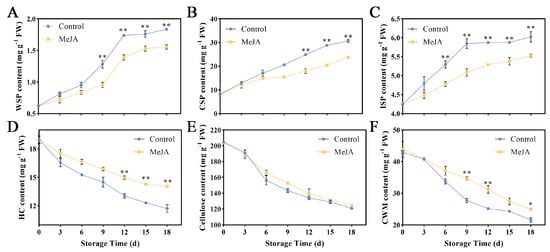
Figure 2.
Effect of MeJA on cell wall polysaccharides of ‘Ruixue’ apple fruit. Content of (A) WSP, (B) CSP, (C) ISP, (D) HC, (E) cellulose, and (F) CWM. An asterisk indicates significant differences between control and MeJA fruits (*, p < 0.05; **, p < 0.01). Error bars show ±SE from three biological replicates.
3.3. Effects of Exogenous MeJA Treatment on the Activities of Cell Wall-Degrading Enzymes in Apple Fruit
During storage, the PG activity of apple fruit showed a steadily increasing trend (Figure 3A). Starting from the 6th day after MeJA treatment, the PG activity was significantly lower than that of the control group, indicating that the MeJA treatment can effectively increase the PG activity. As can be seen from Figure 3B, on the 18th day, the PME activity of the control group was 387 U kg−1, which was significantly higher than the 367.5 U kg−1 of the MeJA group (p < 0.05). The Cx activity of the MeJA group had a certain upward trend, but it was significantly lower than the control, reaching 1.57 U kg−1 on the 18th day (Figure 3C). In addition, the β-gal activity was significantly higher in the MeJA group (Figure 3D).
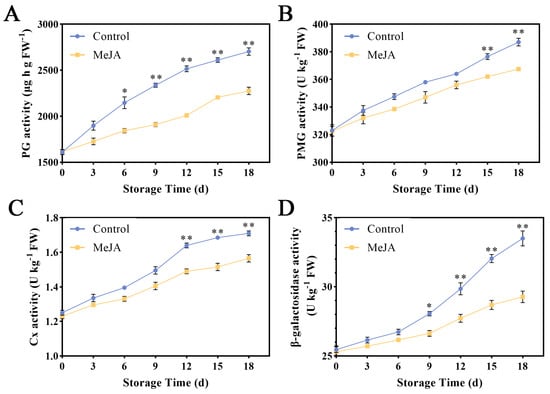
Figure 3.
Effect of MeJA on cell wall-degrading enzyme activities in ‘Ruixue’ apple fruit. (A) PG, (B) PMG, (C) Cx, and (D) β-gal. An asterisk indicates significant differences between control and MeJA fruits (*, p < 0.05; **, p < 0.01). Error bars show ±SE from three biological replicates.
3.4. Effects of Exogenous MeJA Treatment on the Cell Wall- and Ethylene-Related Enzyme Genes
Utilizing our prior transcriptomic data and in conjunction with previous studies, we examined genes related to cell wall hydrolase and ethylene. The relative expressions of nine candidate genes during storage were detected using qRT-PCR (Figure 4). During storage, all nine genes showed a steadily increasing expression pattern. ACC synthases (ACSs) and ACC oxidases (ACOs) are key enzymes for ethylene synthesis, so it is of great significance to detect the relative expressions of MdACSs and MdACOs. After MeJA treatment, the upward trends of MdACS1 and MdACS2 were more gradual than that of the control (p < 0.05 or p < 0.01). From the 9th to the 18th day, the relative expression levels of MdACO1 and MdACO2 were significantly lower in the MeJA group (p < 0.01). From the results, MeJA impacted the expression of MdACS and MdACO genes. Pectin lyase breaks down pectin, loosening the fruit tissue and promoting the softening of the fruit. As storage progressed, the expression of MdPL18 in the control group increased sharply. On the 18th day of storage, the expression level of the control group was even 3.02 times that of the treatment group. The expression of Mdgal1 in the MeJA group was significantly lower than that in the control group on the 6th day after treatment (p < 0.01), and the relative expression of Mdgal2 was significantly lower than that in the untreated group on the 12th day of storage (p < 0.01). The relative expression of MdPG1 began to show significant differences on the 9th day (p < 0.05). These results indicate that MeJA has an effect on the expression of cell wall- and ethylene-related enzyme genes.
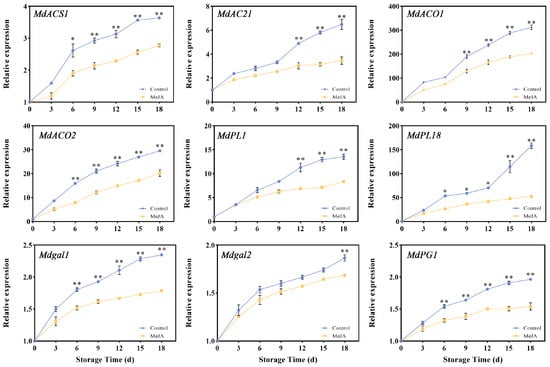
Figure 4.
Changes in relative expressions of MdACS1, MdACS2, MdACO1, MdACO2, MdPL1, MdPL18, Mdgal1, Mdgal2, and MdPG1 in the exocarp of apples after MeJA treatment. (*, p < 0.05; **, p < 0.01). Error bars show ±SE from three biological replicates.
3.5. Correlation between the Candidate Gene MdPL18 (MD16G1070600) and Fruit Quality
During storage, we observed that the expression level of MdPL18 in the control group was significantly higher than that in the MeJA group, and MdPL18 showed a high correlation with various cell wall components (Figure S2). Therefore, we took MdPL18 as a candidate gene in this study. Firstly, the CDS of MdPL18 was cloned and isolated from the pericarp of a ‘Ruixue’ apple in this study, and then the amino acid sequence encoded by MdPL18 was analyzed with the corresponding sequences of other species using the method of constructing a phylogenetic gene tree. The results showed that the amino acid sequence encoded by MdPL18 had the highest homology with the PLs of Pyrus x bretschneideri, Malus sylvestris, and Prunus dulcis (Figure S3A). In addition, their protein structural domains were analyzed in this study using MEME [31] (Figure S3B). There were significant differences in the gene expression levels of the MdPL18 gene in different tissues (root, stem, leaf, flower, peel, and flesh) of the ‘Ruixue’ apple (Figure S4). Firstly, the highest expression level of the MdPL18 gene was found in the flesh, up to 34-fold, followed by the peel, stem, and leaf, while it was hardly expressed in the flower and root, compared with apple root.
3.6. Transient Overexpression of MdPL18 Promotes Ripening and Softening of ‘Ruixue’ Fruit
Transient overexpression experiments were conducted using ‘Ruixue’ fruit due to the difficulty of producing stably transformed perennial apple fruit. The fruit was harvested 2 weeks before commercial ripening (Figure 5A). The results showed that the MdPL18 gene expression level was significantly increased, nearly 2-fold, in fruit injected with MdPL18-pC2300 compared to the null control, while the MdPL18 gene expression level was significantly reduced in fruit injected with MdPL18-pTRV2 (Figure 5B). In addition, we measured cell wall-related substances in ‘Ruixue’ fruit that transiently overexpressed and transiently silenced the MdPL18 gene. Compared with the control, the WSP content of ‘Ruixue’ fruit with transient silencing of MdPL18 was significantly lower than that of the control (p < 0.01) (Figure 5C), and the CSP and ISP contents of the fruit showed the same expression trend (p < 0.01), with the CSP and ISP contents being only 7.26 and 3.59 mg g−1 (Figure 5D,E). In contrast, in the MdPL18-pC2300 group, the fruit WSP, CSP and ISP contents were 1.44-fold, 1.53-fold, and 1.48-fold higher than those in the control group, respectively. In addition, in the determination of the HC content, cellulose content, and CWM content of transiently transformed fruit, it was found that transient silencing of the MdPL18 gene increased the content of the above-three cell wall-related substances, and the transient overexpression of the MdPL18 gene decreased the content of the substances (p < 0.01) (Figure 5F–H).
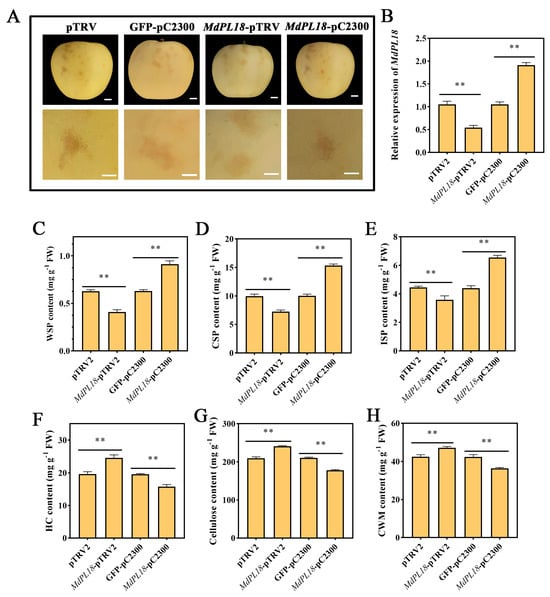
Figure 5.
Transient expression analysis and phenotype of MdPL18 in apple fruit. (A) Phenotypes of ‘Ruixue’ apples after transient overexpression and silencing of MdPL18, (B) gene expression levels of MdPL18, and the effects of transient overexpression and silencing of MdPL18 on (C) WSP, (D) CSP, (E) ISP, (F) HC, (G) fibrillar, and (H) CWM. An asterisk indicates significant differences between control and MeJA fruits (**, p < 0.01). Error bars show ±SE from three biological replicates.
3.7. Effect of Stable Overexpression of MdPL18 in Apple Calli on Cell Wall Material Content
To further determine the effect of MdPL18 on apples, apple callus lines stably overexpressing MdPL18 were formed in this study (Figure 6A). Firstly, the transgenic ‘Orin’ calli were characterized (Figure 6B), and the gene expression level of MdPL18 in apple calli overexpressing MdPL18 was found to be high in OE2, OE4, and OE7 compared to wild-type ‘Orin’ calli; these were significantly increased by 11.92-fold, 14.93-fold, and 14.99-fold, respectively (Figure 6C). Fruit texture changes are the manifestation of changes in its cell wall structure and components. The degradation of pectin, as a major component of the cell wall, is the key to fruit softening, and the hydrolysis of galactose on the side chain of pectin by β-galactosidase (β-gal) is one of the most important causes of fruit softening. Therefore, we examined the β-gal of apple calli, and we found that the β-gal in the overexpressed calli was significantly higher than in the WT, up to 18.09 μmol min−1 g−1 (Figure 6D). Overall, these results suggest that overexpression of MdPL18 promotes apple callus ripening.
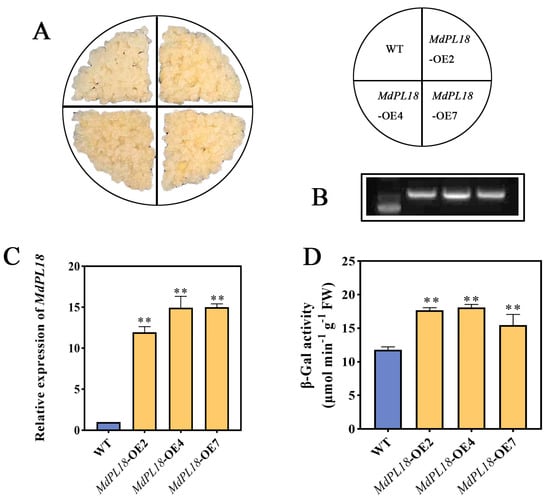
Figure 6.
Overexpression of MdPL18 in apple calli (Malus domestica cv. ‘Orin’). (A) Phenotypes of apple callus in WT (wild type) and OE- MdPL18, (B) RT-PCR detection of MdPL18 expression levels in WT and overexpressing apple calli, (C) qRT-PCR to detect the expression level of MdPL18 in WT and overexpressed apple healing tissues, (D) β-galactosidase activity in WT and overexpressed apple calli. An asterisk indicates significant differences between control and MeJA calli (**, p < 0.01). Error bars show ±SE from three biological replicates.
4. Discussion
MeJA treatment can increase the endogenous ethylene production in the fruit and promote the coloring and ripening of the fruit [26]. In our investigation, we observed that the MeJA application expedited the rate of respiration in the ‘Ruixue’ fruit. Research on strawberries has shown that treating them with MeJA can inhibit the growth and maturation of the fruit [18]. The application of MeJA resulted in the elevation of gene expression in those related to pigment metabolism, sugar metabolism, fruit softening, and hormone metabolism. Additionally, MeJA application increased the levels of JA, anthocyanin, and the sugar content [32]. In plums, MeJA significantly increased ethylene production and the respiration rate. MeJA treatment significantly reduced the L* and hue angle of the fruit [33]. MeJA significantly increased the total phenolic content but decreased the titratable acidity [34]. Studies on tomatoes showed that MeJA accelerated the production of endogenous ethylene in the fruit and resulted in early tomato fruit coloring periods [35]. In apples, MeJA treatment at low concentrations increased ethylene production in the fruit, while higher concentrations decreased ethylene production. Kondo found that MeJA treatment of ‘Golden Crown’ apples accelerated the production of ethylene [36]. During plum storage, MeJA treatment resulted in significant differences in the L*, color and hue angle values of fruit from the control group [34]. In this study, the L* values of fruit treated with MeJA were significantly higher than those of the untreated group on days 9–18 after harvest, up to 75.72. Our research shows that exogenous MeJA treatment could alleviate the softening of the apple fruit and improve the fruit quality. This result is consistent with studies on strawberry and peach fruits [17,37]. SSC and TA have a critical effect on the fruit flavor during apple fruit development and are important indicators used as a means of evaluating fruit storage quality. In this study, we found that postharvest MeJA treatment significantly increased the SSC content of ‘Ruixue’ apples and maintained a better fruit quality. MeJA treatment was found to significantly increase the SSC content of fruits in apricot and blueberry studies [38]. With an overall decreasing trend throughout the postharvest apple ripening stage, MeJA treatment significantly contributed to the decrease in TA content, which is the same as the findings in pear fruits [39].
The results showed that there was a trend towards a significant decrease in the WSP content, CSP content, and ISP content of the fruit after exogenous MeJA treatment for 6 d. PG activity is an important factor in the breakdown and conversion of pectin. It is responsible for the depolymerization and solubilization of pectin. However, for PG to effectively break down pectin, the pectin must first undergo demethylation by pectin methyl esterase. This process is essential for the efficient depolymerization of pectin. The inhibition of β-gal activity during the early stage of ripening has been found to have a significant impact on fruit softening. This indicates that the removal of pectin galactose side chains plays a crucial role in the cell wall changes that result in the loss of firmness during ripening [40]. In this study, we found that PG, β-Gal, and other related enzyme activities in exogenous MeJA-treated apple fruit were consistently lower than in the control throughout the postharvest apple ripening stage, indicating that MeJA-treated apple fruit restrained the production rate of cell wall-related enzyme activities throughout the storage period.
The silencing of MdACS1 in the apple resulted in a decrease in ethylene production in the fruit [41]. Previous studies have shown that MdACS is responsible for system Ⅱ ethylene biosynthesis during fruit ripening [41]. In this study, the expressions of MdACS1 and MdACS2 were significantly up-regulated in ‘Ruixue’ fruit during postharvest storage, and the expression of the treated group was significantly lower than that of the control group starting on the 12th d after MeJA treatment (p < 0.01). According to reports, system II might utilize MdACO1 and MdACO2 as pivotal elements in the synthesis of ethylene [42]. In the present study, it was found that the expressions of MdACO1 and MdACO2 were significantly down-regulated following MeJA treatment compared with control. The expression levels of MdPL1 and MdPL18 increased over time during storage. Following MeJA treatment, MdPL18 expression remained relatively stable in contrast to the sharp increase observed in the control group. This suggests a significant positive correlation between MdPL18 and pectin degradation. Furthermore, the relative expression levels of the two β-gal candidate genes were also influenced by MeJA, showing a gradual increase.
An association between the expression of pectin lyases and fruit ripening softening has been reported in many fruits, including bananas, tomatoes, and mangoes. However, there is limited research on the functional role of pectin lyase in apple fruit softening. This study focused on MdPL18, which showed significant differences in its expression between the control group and the MeJA group and that it was highly correlated with cell wall components. MdPL18 was selected as a candidate gene for further investigation. The transient overexpression and silencing of the MdPL18 gene in ‘Ruixue’ apple fruit revealed that overexpression accelerated the degradation of apple fruit cell wall materials and increased the enzyme activities, indicating its involvement in apple fruit softening. Moreover, the overexpression of MdPL18 in apple fruit calli notably boosted β-Gal activity, further supporting its role in cell wall metabolism and fruit softening.
5. Conclusions
Our study demonstrates that treating ‘Ruixue’ apple fruit with appropriate concentrations of exogenous MeJA can effectively reduce fruit softening, mitigate the rapid increase in the respiration rate and ethylene release, and effectively preserve the fruit’s quality. The analysis of cell wall components and their associated degradative enzymes further supports the significant ameliorating impact of MeJA on postharvest apple fruit ripening. Moreover, the discovery of the MdPL18 gene in ‘Ruixue’ apples is noteworthy. The transient overexpression and silencing of MdPL18 in ‘Ruixue’ apple fruit indicate that this gene contributes to apple fruit softening by facilitating the breakdown of cell wall materials. These findings offer a solid theoretical foundation for improving the postharvest storage practices for apples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13111594/s1.
Author Contributions
X.D.: conceptualization, software, writing—original draft preparation, and writing—review and editing. B.W.: methodology, resources, and writing—review and editing. Y.G.: validation and data curation. X.Y.: formal analysis, visualization, supervision. X.C.: validation. Y.Z.: investigation. Z.Z.: conceptualization, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Shaanxi Engineering Research Center of Apple, China (2020zdzx03-01-03), Weinan Experimental Demonstration Station Construction Project of Northwest A&F University (2023WNXNZX-1) and the Earmarked Fund for China Agriculture Research System (CARS-27).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Abbreviation | Definition |
| MeJA | methyl jasmonate |
| WSP | water-soluble pectin |
| CSP | chelator-soluble pectin |
| ISP | ion-soluble pectin |
| PME | pectin methylesterase |
| PG | polygalacturonase |
| β-Gal | glycosidase |
| PAL | phenylalanine ammonia-lyase |
| POD | peroxidase |
| PPO | polyphenol oxidase |
| ETH | ethylene |
| SSC | soluble solids content |
| TA | titratable acidity |
| HC | hemicellulose |
| CWM | cell wall material |
| PMG | polymethylgalacturonase |
| Cx | cellulase |
References
- Zhao, J.; Quan, P.; Liu, H.; Li, L.; Xing, L. Transcriptomic and Metabolic Analyses Provide New Insights into the Apple Fruit Quality Decline during Long-Term Cold Storage. J. Agric. Food Chem. 2020, 68, 4699–4716. [Google Scholar] [CrossRef]
- Chang, L.Y.; Brecht, J.K. Responses of 1-methylcyclopropene (1-MCP)-treated banana fruit to pre- and post-treatment ethylene exposure. Sci. Hortic. 2023, 309, 111636. [Google Scholar] [CrossRef]
- Fang, H.; Zuo, J.; Ma, Q.; Zhang, X.; Xu, Y.; Ding, S.; Wang, J.; Luo, Q.; Li, Y.; Wu, C.; et al. Phytosulfokine promotes fruit ripening and quality via phosphorylation of transcription factor DREB2F in tomato. Plant Physiol. 2024, kiae012. [Google Scholar] [CrossRef] [PubMed]
- Favre, L.; Hunter, D.A.; O’donoghue, E.M.; Erridge, Z.A.; Napier, N.J.; Somerfield, S.D.; Hunt, M.; McGhie, T.K.; Cooney, J.M.; Saei, A.; et al. Integrated multi-omic analysis of fruit maturity identifies biomarkers with drastic abundance shifts spanning the harvest period in ‘Royal Gala’ apple. Postharvest Biol. Technol. 2022, 193, 925–945. [Google Scholar] [CrossRef]
- Cornelius, S.; BarryJames, J. Giovannoni. Ethylene and Fruit Ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Lim, S.; Lee, J.G.; Lee, E.J. Comparison of fruit quality and GC-MS-based metabolite profiling of kiwifruit ‘Jecy green’: Natural and exogenous ethylene-induced ripening. Food Chem. 2017, 234, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tatsuki, M.; Endo, A.; Ohkawa, H. Influence of time from harvest to 1-MCP treatment on apple fruit quality and expression of genes for ethylene biosynthesis enzymes and ethylene receptors. Postharvest Biol. Technol. 2007, 43, 28–35. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Su, X.; Chen, L.; Zhu, Z. Transcriptome analysis reveals key metabolic pathways and gene expression involving in cell wall polysaccharides-disassembling and postharvest fruit softening in custard apple (Annona squamosa L.). Int. J. Biol. Sci. 2023, 240, 141–158. [Google Scholar] [CrossRef]
- Bank, A.D. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- Liu, M.P.; Wang, R.; Sun, W.W.; Han, W.J.; Li, G.; Zong, W.; Fu, J.M. Effects of postharvest calcium treatmenttreatment on the firmness of persimmon (Diospyros kaki) fruit based on a decline in WSP. Sci. Hortic. 2023, 307, 111490. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and Action of Jasmonates in Plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D. Jasmonates: Key players in plant stress tolerance. In Emerging Plant Growth Regulators in Agriculture; Academic Press: Cambridge, MA, USA, 2022; Chapter 5. [Google Scholar] [CrossRef]
- Dobritzsch, S.; Weyhe, M.; Schubert, R.; Dindas, J.; Hause, G.; Kopka, J.; Hause, B. Dissection of jasmonate functions in tomato stamen development by transcriptome and metabolome analyses. BMC Biol. 2015, 13, 28. [Google Scholar] [CrossRef]
- Cao, S.F.; Cai, Y.T.; Yang, Z.F.; Joyce, D.C.; Zheng, Y.H. Effect of MeJA treatment on polyamine, energy status and anthracnose rot of loquat fruit. Food Chem. 2014, 145, 86–89. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Ali, A.; Seymour, G.; Tucker, G. Enhancing the antioxidant content of carambola (Averrhoa carambola) during cold storage and methyl jasmonate treatments. Postharvest Biol. Technol. 2016, 118, 79–86. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Chang, L.L.; Zhang, Y.T.; Wang, G.X.; Dong, J.; Zhong, C.F.; Wang, L.N.; Li, T.H. The effects of exogenous methyl jasmonate on FaNES1 gene expression and the biosynthesis of volatile terpenes in strawberry (Fragaria × ananassa Duch.) fruit. J. Hortic. Sci. Biotech. 2013, 88, 393–398. [Google Scholar] [CrossRef]
- Jiang, J.J.; Yao, L.N.; Yu, Y.J.; Lv, M.L.; Miao, Y.; Cao, J.S. PECTATE LYASE-LIKE10 is associated with pollen wall development in Brassica campestris. J. Integr. Plant Biol. 2014, 56, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Wieczorek, K.; Elashry, A.; Quentin, M.; Grundler, F.M.; Favery, B.; Seifert, G.J.; Bohlmann, H. A distinct role of pectate lyases in the formation of feeding structures induced by cyst and root-knot nematodes. Sci. Rep. 2014, 27, 901–912. [Google Scholar] [CrossRef]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-wide identification and analysis of polygalacturonase genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef] [PubMed]
- Marín-Rodríguez, M.C.; Orchard, J.; Seymour, G.B. Pectate lyases cell wall degradation and fruit softening. J. Exp. Bot. 2002, 53, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.; Sheldon, J.; Stiegelmeyer, S.; Perez, L. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Doménech, N.; Jiménez-Bemudez, S.; Matas, A.J.; Rose, J.K.; Munoz-Blanco, J.; Mercado, J.A.; Quesada, M.A. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J. Exp. Bot. 2008, 59, 2769–2779. [Google Scholar] [CrossRef]
- Lv, J.Y.; Zhang, M.Y.; Zhang, J.H.; Ge, Y.H.; Li, C.Y.; Meng, K.; Li, J.R. Effects of methyl jasmonate on expression of genes involved in ethylene biosynthesis and signaling pathway during postharvest ripening of apple fruit. Sci. Hortic. 2018, 229, 157–166. [Google Scholar] [CrossRef]
- Yang, X.T.; Song, J.; Du, L.N.; Charles, F.; Leslie, C.P.; Sherry, F.; Zhang, Z.Q. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.D.; Smith, B.G. Isolation of Plant Cell Walls and Fractionation of Cell Wall Polysaccharides. Curr. Protoc. Food Anal. Chem. 2001, E3.1.1–E3.1.23. [Google Scholar] [CrossRef]
- Li, X.L.; Su, Q.F.; Jia, R.J.; Wang, Z.D.; Fu, J.H.; Guo, J.H.; Zhao, Z.Y. Comparison of cell wall changes of two different types of apple cultivars during fruit development and ripening. J. Inter. Agric. 2023, 22, 2705–2718. [Google Scholar] [CrossRef]
- Payasi, A.; Misra, P.C.; Sanwal, G.G. Effect of phytohormones on pectate lyase activity in ripening Musa acuminata—ScienceDirect. Plant Physiol. Biochem. 2004, 42, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://meme-suite.org/meme/tools/meme (accessed on 1 May 2024).
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of Anthocyanin and Associated Gene Expression in Radish Sprouts Exposed to Light and Methyl Jasmonate. Chem. Rev. 2013, 61, 4127–4132. [Google Scholar] [CrossRef]
- Alejandra, M.E.; Pedro, J.Z.; Salvador, C.; Fabian, G.; Domingo, M.R.; Daniel, V.; Maria, S. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 1. Improvement of fruit growth and quality attributes at harvest. Postharvest Biol. Technol. 2014, 98, 98–105. [Google Scholar] [CrossRef]
- Kucuker, E.; Ozturk, B.; Celik, S.M.; Aksit, H. Pre-harvest spray application of methyl jasmonate plays an important role in fruit ripening, fruit quality and bioactive compounds of Japanese plums. Sci. Hortic. 2014, 176, 162–169. [Google Scholar] [CrossRef]
- Li, Z.; Min, D.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X. The roles of SlMYC2 in regulating ascorbate-glutathione cycle mediated by methyl jasmonate in postharvest tomato fruits under cold stress. Sci. Hortic. 2021, 288, 110406. [Google Scholar] [CrossRef]
- Kondo, S.; Setha, S.; Rudell, D.R.; Buchanan, D.A.; Mattheis, J.P. Aroma volatile biosynthesis in apples affected by 1-MCP and methyl jasmonate. Postharvest Biol. Technol. 2005, 36, 61–68. [Google Scholar] [CrossRef]
- Han, Y.L.; Chen, C.; Yan, Z.M.; Li, J.; Wang, Y.H. The methyl jasmonate accelerates the strawberry fruits ripening process. Sci. Hortic. 2019, 249, 250–256. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Fan, C.; Li, G.J.; Dong, T.T. Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]
- Jing, S.Y.; Liu, Y.; Liu, H.F.; Li, N.; Li, L. Methyl jasmonate regulates protective enzyme activities to improve resistance to Venturia nashicola in pear (Pyrus bretschneideri Rehd.). Eur. J. Plant Pathol. 2020, 158, 789–797. [Google Scholar] [CrossRef]
- Gupta, R.; Mehta, G.; Deswal, D.; Sharma, S.; Singh, A. Cellulases and Their Biotechnological Applications. In Biotechnology for Environmental Management and Resource Recovery; Springer: New Delhi, India, 2013; pp. 89–106. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Z.Y.; Zhang, L.C.; Tan, D.M.; Wei, Y.; Yuan, H.; Wang, A.D. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016, 88, 735–748. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.-H.; Nain, B.; Cohen, D.; et al. A Genomics Approach Reveals That Aroma Production in Apple Is Controlled by Ethylene Predominantly at the Final Step in Each Biosynthetic Pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).