Effect of Soy Protein Products on Growth and Metabolism of Bacillus subtilis, Streptococcus lactis, and Streptomyces clavuligerus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Strain Cultures and Fermentation
2.3. Analysis of Components of Five Kinds of Soy Protein Product
2.4. Analysis of Cell Growth
2.5. Measurement of Fermented Substrates in Metabolites
2.6. Data Analysis
3. Results and Discussion
3.1. Component Analysis of Five Kinds of Soy Protein Product
3.2. Effect of Five Kinds of Soy Protein Product on Cell Growth and Metabolism
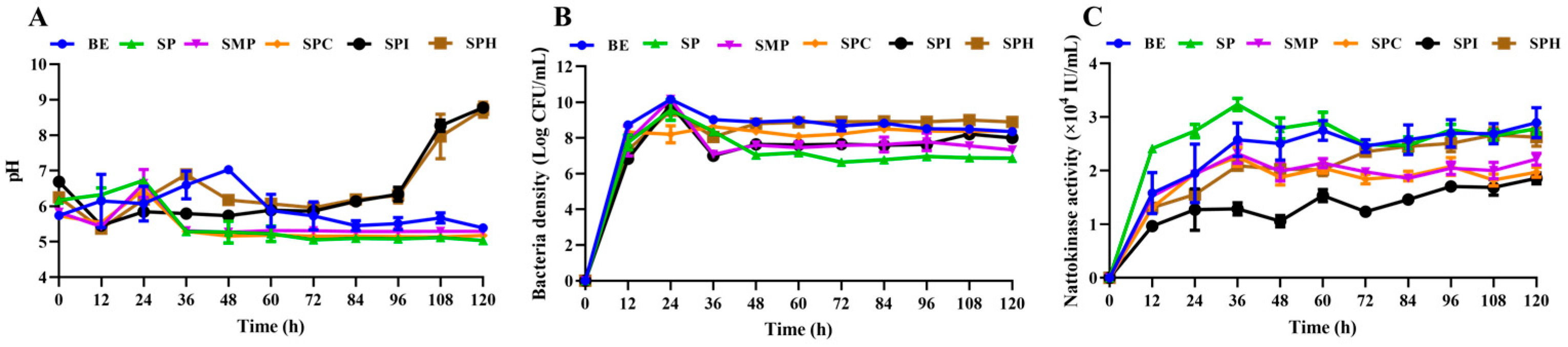
3.2.1. Effect of Five Kinds of Soy Protein Product on Cell Growth and NK Synthesis of BSNK-5
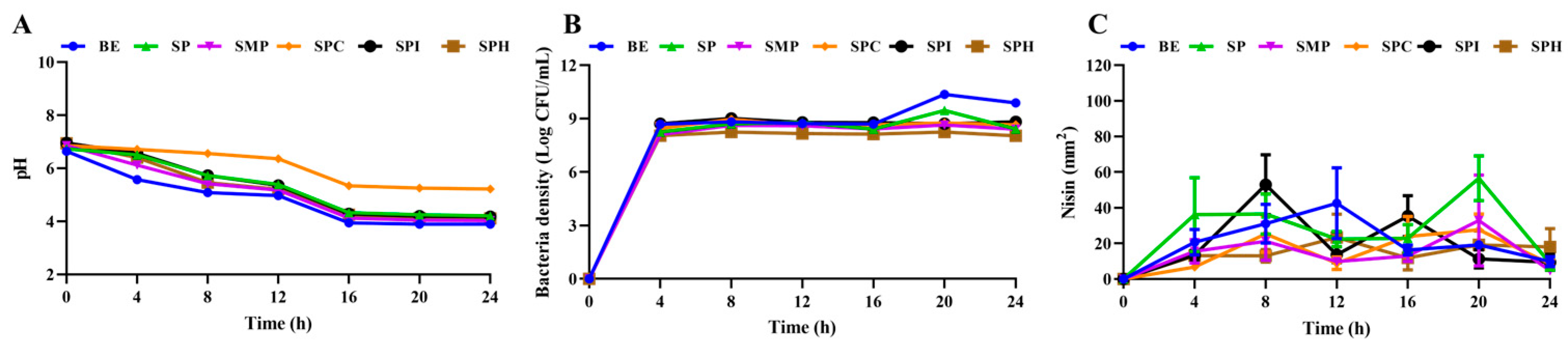
3.2.2. Effect of Five Kinds of Soy Protein Product on Cell Growth and Nisin Synthesis of S. lactis
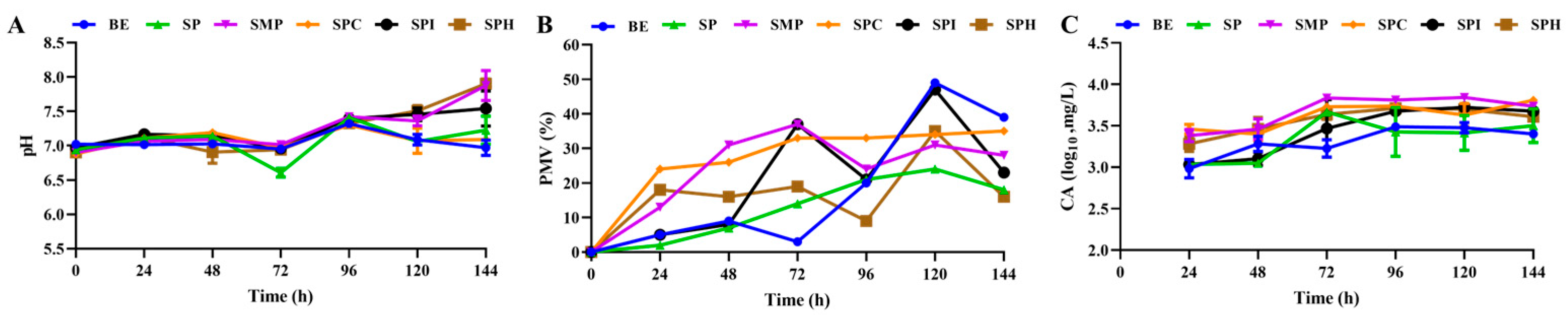
3.2.3. Effect of Five Kinds of Soy Protein Product on Cell Growth and CA Synthesis of S. clavuligerus
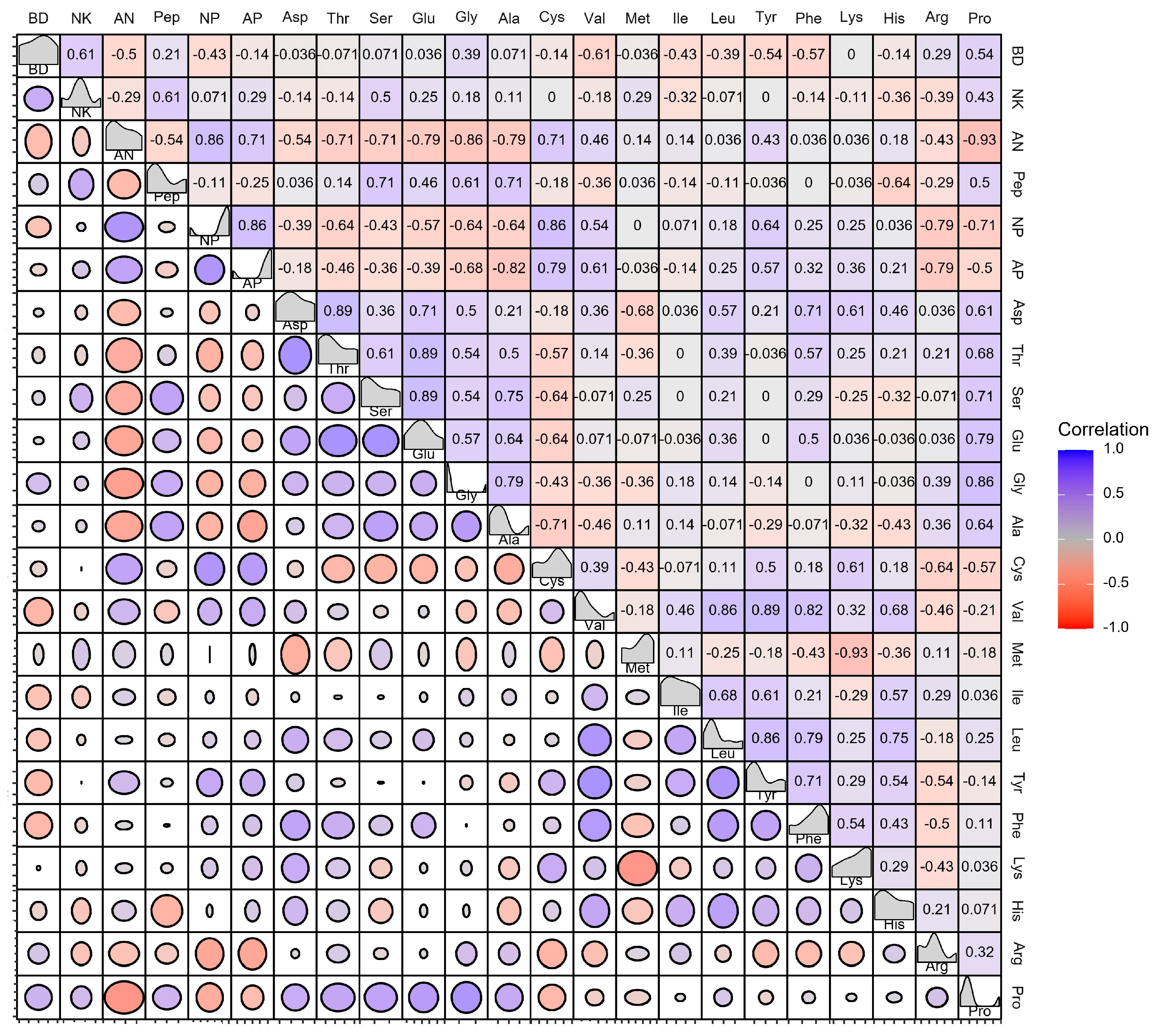
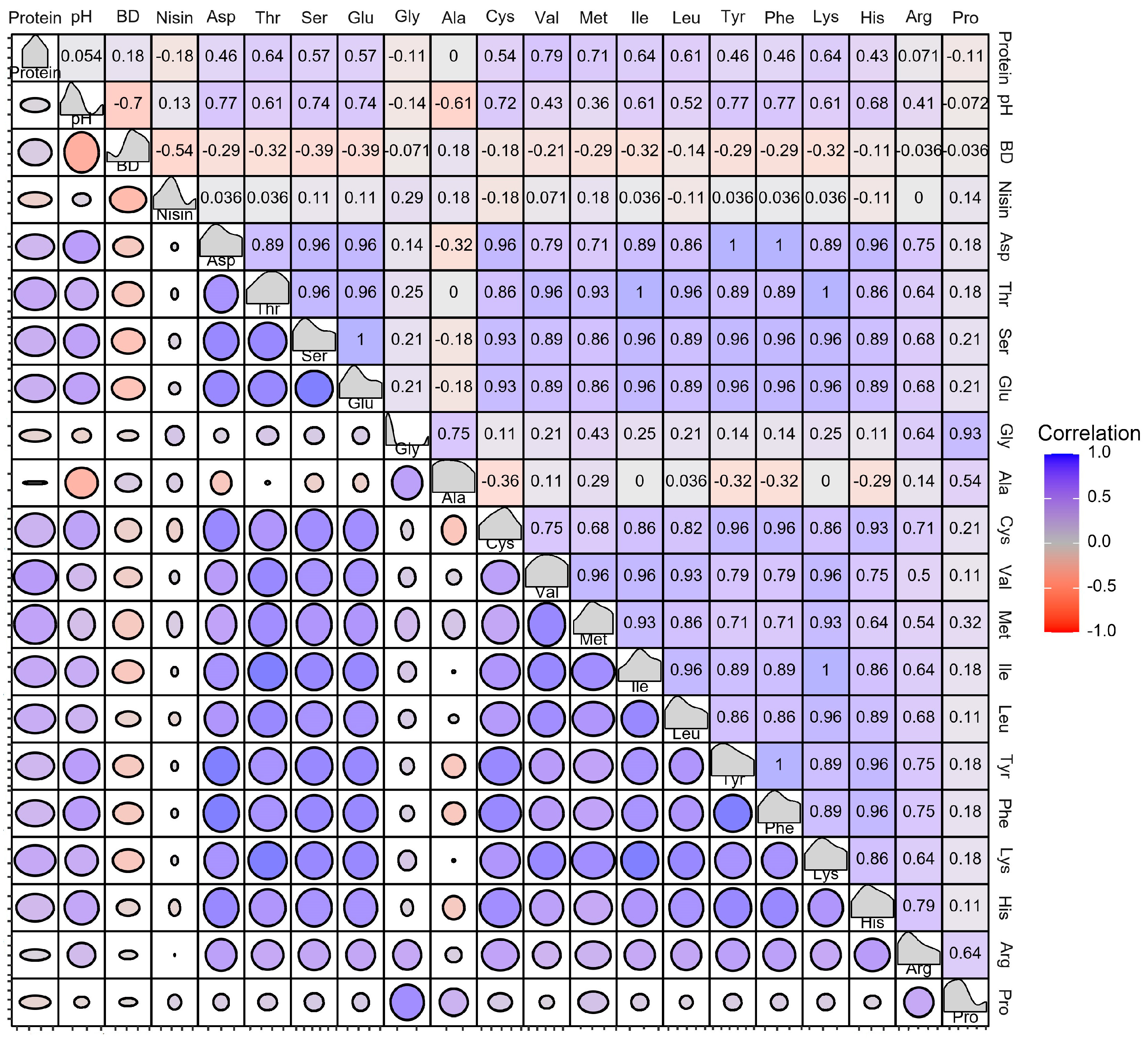
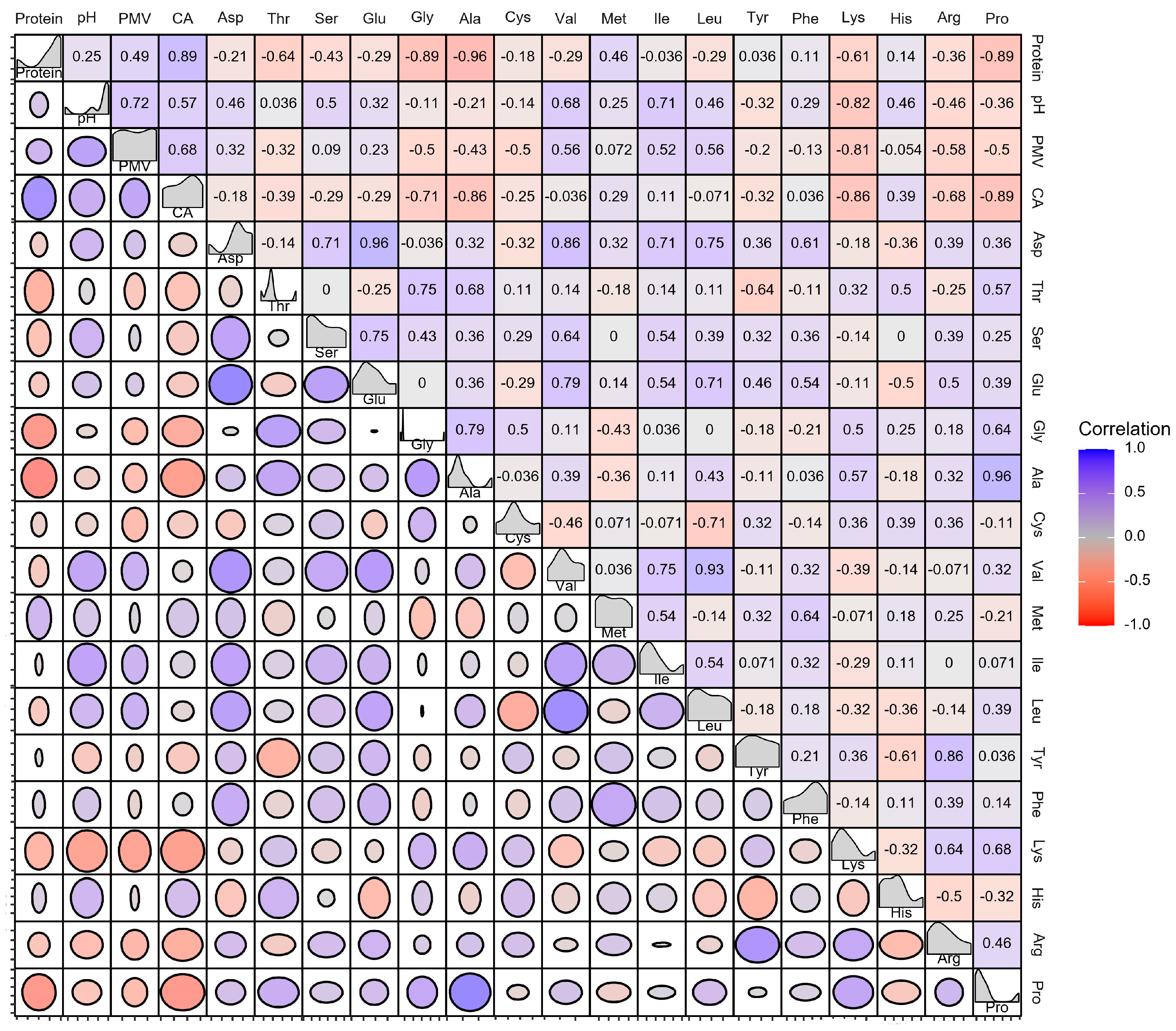
3.3. Dissecting the Correlation between the Soy Protein Products and Strains in Growth and Metabolites
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ser, H.L.; Law, J.W.F.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D.; Chan, K.G.; Goh, B.H.; Lee, L.H. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: A Systematic Review. Front. Microbiol. 2016, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Sella, S.R.B.; Bueno, T.; de Oliveira, A.A.B.; Karp, S.G.; Soccol, C.R. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, Y.; Wang, J.; Lyu, X.; Yang, R. Screening of a Bacillus subtilis strain producing both nattokinase and milk-clotting enzyme and its application in fermented milk with thrombolytic activity. J. Dairy Sci. 2021, 104, 9437–9449. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules produced by probiotics and intestinal microorganisms with immunomodulatory activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Abbasi, R.; Raza, Z.A.; Imran, M. Polyelectrolyte multicomponent colloidosomes loaded with nisin Z for enhanced antimicrobial activity against foodborne resistant pathogens. Front. Microbiol. 2018, 8, 2700. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Kızıldoğan, A.; Çelik, G.; Ünsaldı, E.; Özcan, S.; Ayaz-Güner, Ş.; Özcengiz, G. An integrative-omics analysis of an industrial clavulanic acid-overproducing Streptomyces clavuligerus. Appl. Microbiol. Biotechnol. 2022, 106, 6139–6156. [Google Scholar] [CrossRef] [PubMed]
- Du, L.H.; Hao, Y.N.; Chen, N.; Xu, Q.Y. Organic nitrogen source and their applications in microbial fermentation. Bull. Ferment. Sci. Technol. 2019, 48, 1–4. [Google Scholar]
- He, H.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Understanding and application of Bacillus nitrogen regulation: A synthetic biology perspective. J. Adv. Res. 2023, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Zhang, Y.X.; Li, H.; Zhang, C.; Zhang, J.; Uddin, J.; Liu, X.Q. Effect of soybean oligopeptide on the growth and metabolism of Lactobacillus acidophilus JCM 1132. RSC Adv. 2020, 10, 16737. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Xu, M.W.; Lu, J.; Cai, J.L. Simultaneous microbial fermentation and enzymolysis: A biotechnology strategy to improve the nutritional and functional quality of soybean meal. Food Rev. Int. 2023, 1–16. [Google Scholar] [CrossRef]
- Moras, B.; Rey, S.; Vilarem, G.; Pontalier, P. Pressurized water extraction of isoflavones by experimental design from soybean flour and soybean protein isolate. Food Chem. 2016, 214, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, V. Phosphorus flow in production of soy protein concentrate and isolate from defatted soybean flour. J. Am. Oil Chem. Soc. 2023, 100, 985–991. [Google Scholar] [CrossRef]
- Liang, Y.R.; Guo, Y.N.; Zheng, Y.X.; Liu, S.B.; Cheng, T.F.; Zhou, L.Y.; Guo, Z.W. Effects of high-pressure homogenization on physicochemical and functional properties of enzymatic hydrolyzed soybean protein concentrate. Front. Nutr. 2022, 9, 1054326. [Google Scholar] [CrossRef]
- Loman, A.A.; Islam, S.M.M.; Li, Q.; Ju, L.K. Soybean bio-refinery platform: Enzymatic process for production of soy protein concentrate, soy protein isolate and fermentable sugar syrup. Bioproc. Biosyst. Eng. 2016, 39, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Sun, N.; Cui, W.; Cheng, L.; Ren, K.; Wang, M.; Tong, X.; Jiang, L.; Wang, H. Effects of sucrase enzymatic hydrolysis combined with Maillard reaction on soy protein hydrolysates: Bitterness and functional properties. Int. J. Biol. Macromol. 2024, 56, 128344. [Google Scholar] [CrossRef] [PubMed]
- Krefft, D.; Prusinowski, M.; Maciszka, P.; Skokowska, A.; Zebrowska, J.; Skowron, P.M. T7-lac promoter vectors spontaneous derepression caused by plant-derived growth media may lead to serious expression problems: A systematic evaluation. Microb. Cell Fact. 2022, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Lapoin, W.; Young, M.; Nguyen, C.H. Changes in fermented soybean nutritional content generated under the different fermentation conditions by Bacillus Subtilis. Waste Biomass Valoriz. 2022, 13, 563–569. [Google Scholar] [CrossRef]
- Lee, S.D.; Park, S.W.; Oh, K.K.; Hong, S.I.; Kim, S.W. Improvement for the production of clavulanic acid by mutant Streptomyces clavuligerus. Lett. Appl. Microbiol. 2002, 34, 370–375. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, J.; Chu, J.; Wang, Y.H.; Zhuang, Y.P. Statistical optimizing of medium for clavulanic acid production by Streptomyces clavuligerus using response surface methodology. Appl. Biochem. Biotechnol. 2021, 193, 3936–3948. [Google Scholar] [CrossRef]
- Dong, M.Z.; An, J.Y.; Wang, L.T.; Fan, X.H.; Lv, M.J.; Zhu, Y.W.; Chang, Y.H.; Meng, D.; Yang, Q.; Fu, Y.J. Development of fermented chestnut with Bacillus natto: Functional and sensory properties. Food Res. Int. 2019, 130, 108941. [Google Scholar] [CrossRef]
- Liu, J.H. Construction of nisin High-Yield Lactococcus lactis Strain and Development of Novel Nisin Production System. Master’s Thesis, Tianjin University, Tianjin, China, 2018; pp. 40–41. [Google Scholar]
- Rodrigues, K.C.D.S.; Souza, A.T.D.; Badino, A.C.; Pedrolli, D.B.; Cerri, M.O. Screening of medium constituents for clavulanic acid production by Streptomyces clavuligerus. Braz. J. Microbiol. 2018, 49, 832–839. [Google Scholar] [CrossRef]
- Gao, Y.X.; Hu, M.; Meng, W.M.; Wen, W.; Zhang, P.F.; Fan, B.; Wang, F.Z.; Li, S.Y. Study on the quality of soybean proteins fermented by Bacillus subtilis BSNK-5: Insights into nutritional, functional, safety, and flavor properties. Food Chem. 2024, 443, 138523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N. Optimization of Cutlure Conditions and Probiotic Potential Evaluation of Bacillus Subtilis JB286. Master’s Thesis, Shandong Agricultural University, Tai’an, China, May 2023. [Google Scholar]
- Nguyen, T.; Nguyen, C.H. Determination of factors affecting the protease content generated in fermented soybean by Bacillus subtilis 1423. Energy Rep. 2020, 6, 831–836. [Google Scholar] [CrossRef]
- Qian, Z. Mutation Breeding and Fermentation Process Optimization of the Strain Producting High Nattokinase Activity. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2018; pp. 45–46. [Google Scholar]
- Khelissa, S.; Chihib, N.E.; Gharsallaoui, A. Conditions of nisin production by Lactococcus lactis subsp. lactis and its main uses as a food preservative. Arch. Microbiol. 2021, 203, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, B.; Qiao, J.; Xiong, H.; Chen, X.; Li, P.; Li, K.; Tang, G.; Wang, Z. Improvement of nisin biosynthesis efficiency by optimizations of medium composition and segmentation fermentation. China Brew. 2022, 41, 135–140. [Google Scholar]
- Özel, B.; Şimşek, Ö.; Akçelik, M.; Saris, P.E.J. Innovative approaches to nisin production. Appl. Microbiol. Biot. 2018, 102, 6299–6307. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The efficient clade: Lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017, 35, 756. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Rang, J.; Xia, L. The strategy of genetic engineering Lactococcus lactis to produce nisin. J. Natl. Sci. Hunan Norm. Univ. 2021, 44, 102–107. [Google Scholar]
- Simsek, Ö.; Saris, P.E.J. Cycle changing the medium results in increased nisin productivity per cell in Lactococcus lactis. Biotechnol. Lett. 2009, 31, 415–421. [Google Scholar] [CrossRef]
- Ákos, J.; Gábor, N. The stability of amoxicillin trihydrate and potassium clavulanate combination in aqueous solutions. Acta Vet. Hung. 2009, 57, 485–493. [Google Scholar]
- Feng, T.; Miao, R.; Li, X.; Wang, Y.; Yang, B.; Cao, B.; Liu, X.; Wang, Y.; Chu, J. Optimization in fermentation medium for clavulanic acid production by Streptomyces clavuligerus. Chem. Bio Eng. 2019, 36, 12–15, 25. [Google Scholar]
- Zhang, Q.F.; Lan, G.Q.; Tian, X.Y.; He, L.P.; Li, C.Q.; Tao, H.; Zeng, X.F.; Wang, X. Effect of adding Bifidobacterium animalis BZ25 on the flavor, functional components and biogenic amines of natto by Bacillus subtilis GUTU09. Foods 2022, 11, 2674. [Google Scholar] [CrossRef]
- Liu, D.D.; Han, Z.X.; Hu, Z.W.; Yu, C.R.; Wang, Y.; Tong, J.; Fang, X.; Yue, W.J.; Nie, G.J. Comparative analysis of the transcriptome of Bacillus subtilis natto incubated in different substrates for nattokinase production. Process Biochem. 2023, 129, 30–43. [Google Scholar] [CrossRef]
- Li, T. Study on Fermentation Technology of Nisin. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2007; p. 2. [Google Scholar]
- Liu, W.W. High Production of Nisin Strain’s Selection and Culture Medium Optimization. Master’s Thesis, Tianjin University, Tianjin, China, 2010; p. 4. [Google Scholar]
- López-Agudelo, V.A.; Gómez-Ríos, D.; Ramirez-Malule, H. Clavulanic acid production by Streptomyces clavuligerus: Insights from systems biology, strain engineering, and downstream processing. Antibiotics 2021, 10, 84. [Google Scholar] [CrossRef]
- Wang, M.L. Studies on Clavulanic Acid-Producing Strain. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2003; p. 54. [Google Scholar]
- Liu, Y.Q. Mutagenesis and fermentation process optimization of Streptomyces clavulans F613-1. China Brew. 2020, 39, 95–99. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Hu, M.; Gao, Y.; Zhang, P.; Meng, W.; Zhang, F.; Fan, B.; Wang, F.; Li, S. Effect of Soy Protein Products on Growth and Metabolism of Bacillus subtilis, Streptococcus lactis, and Streptomyces clavuligerus. Foods 2024, 13, 1525. https://doi.org/10.3390/foods13101525
Wen W, Hu M, Gao Y, Zhang P, Meng W, Zhang F, Fan B, Wang F, Li S. Effect of Soy Protein Products on Growth and Metabolism of Bacillus subtilis, Streptococcus lactis, and Streptomyces clavuligerus. Foods. 2024; 13(10):1525. https://doi.org/10.3390/foods13101525
Chicago/Turabian StyleWen, Wei, Miao Hu, Yaxin Gao, Pengfei Zhang, Weimin Meng, Fengxia Zhang, Bei Fan, Fengzhong Wang, and Shuying Li. 2024. "Effect of Soy Protein Products on Growth and Metabolism of Bacillus subtilis, Streptococcus lactis, and Streptomyces clavuligerus" Foods 13, no. 10: 1525. https://doi.org/10.3390/foods13101525
APA StyleWen, W., Hu, M., Gao, Y., Zhang, P., Meng, W., Zhang, F., Fan, B., Wang, F., & Li, S. (2024). Effect of Soy Protein Products on Growth and Metabolism of Bacillus subtilis, Streptococcus lactis, and Streptomyces clavuligerus. Foods, 13(10), 1525. https://doi.org/10.3390/foods13101525





