Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products
Abstract
1. Introduction
2. Valorization of Agri-Food Wastes and By-Products
2.1. Management of Agri-Food Waste and By-Products
2.2. Wastes from Agriculture and Industrial Food Production
2.3. Bioactive Compounds from Agri-Food Waste and By-Products
2.3.1. Bioactive Compounds from Waste of Plant Origin
2.3.2. Bioactive Compounds from Waste of Animal Origin
2.4. Limitations and Possible Solutions in the Re-Use of Agri-Food Waste and By-Products
3. Plant-Derived Extracellular Vesicles as Natural Carriers of Bioactive Molecules
3.1. Extracellular Vesicles
3.2. Plant-Derived EVs
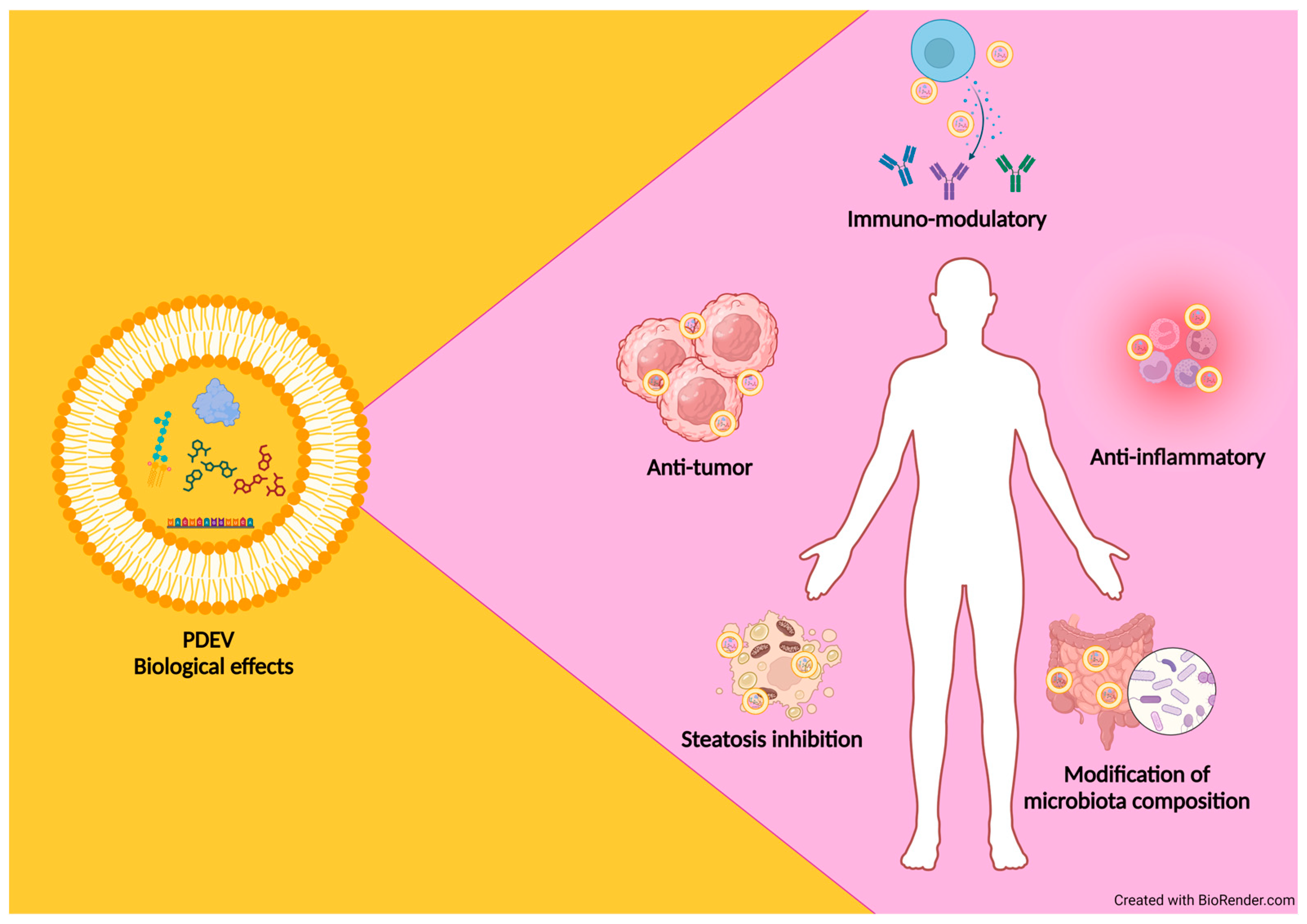
3.3. Functional Properties of PDEVs Isolated from Different Plant Sources
| Biological Properties | Source of Pdevs | References |
|---|---|---|
| Anticancer | Lemon | [136] |
| Citrus limon | [134] | |
| Grapefruit | [120] | |
| Ginseng | [133] | |
| Fruits and vegetables (blueberry, coconut, ginger, grapefruit, Hami melon, kiwifruit, orange, pea, pear, soybean, and tomato) | [118] | |
| Anti-Inflammatory | Broccoli | [140] |
| Cabbage | [123] | |
| Garlic | [139] | |
| Ginger rhizomes | [143] | |
| Grape | [109] | |
| Fruits and vegetables (blueberry, coconut, ginger, grapefruit, Hami melon, kiwifruit, orange, pea, pear, soybean, and tomato) | [118] | |
| Antioxidative | Strawberry | [132] |
- They are natural carriers of biomolecules involved in intercellular communication not only in plants but also in diverse kingdoms of life.
- They are non-toxic and well tolerated by the mammalian immune system as they are currently present in foods.
- Their characteristic lipid membrane composition protects the internal cargo from external agents, which can deteriorate the bioactive compound carried by them.
- Their ability to pass through natural barriers (i.e., blood–brain barrier and the placenta) and reach specific target cells.
- Good yields from vegetable sources; this aspect makes them suitable for industrial applications.
- Possibility of isolating PDEVs at any time, as the source of isolation is represented by numerous plants with different growth periods [144].
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez García, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef] [PubMed]
- Valdés García, A.; Garrigós Selva, M.d.C. Microencapsulation of Natural Antioxidant Compounds Obtained from Biomass Wastes: A Review. In Proceedings of the Materials Science Forum, Salt Lake City, UT, USA, 23–27 October 2016; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2016; Volume 875, pp. 112–126. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small Extracellular Vesicles Isolation and Separation: Current Techniques, Pending Questions and Clinical Applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Lo, K.-J.; Wang, M.-H.; Ho, C.-T.; Pan, M.-H. Plant-Derived Extracellular Vesicles: A New Revolutionization of Modern Healthy Diets and Biomedical Applications. J. Agric. Food Chem. 2024, 72, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Thakur, A.; Liu, W.; Yan, Y. Plant-Derived Extracellular Vesicles (PDEVs) in Nanomedicine for Human Disease and Therapeutic Modalities. J. Nanobiotechnol. 2023, 21, 114. [Google Scholar] [CrossRef]
- Buratta, S.; Latella, R.; Chiaradia, E.; Salzano, A.M.; Tancini, B.; Pellegrino, R.M.; Urbanelli, L.; Cerrotti, G.; Calzoni, E.; Alabed, H.B.R.; et al. Characterization of Nanovesicles Isolated from Olive Vegetation Water. Foods 2024, 13, 835. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. In Moving Forwards and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Leong, B.F.; Chuah, W.C.; Chye, F.Y. Recent Advances and Emerging Trends in the Utilization of Dairy By-Products/Wastes. In Valorization of Agri-Food Wastes and By-Products: Recent Trends, Innovations and Sustainability Challenges; Bhat, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 18; pp. 371–389. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.T.; Wei, Y.H.; Chi-Wei Lan, J. Recent Advances on the Sustainable Approaches for Conversion and Reutilization of Food Wastes to Valuable Bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Food waste valorization. In Saving Food; Galanakis, C.M., Ed.; Academic Press: New York, NY, USA, 2019; Chapter 10; pp. 279–313. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-Products of Citrus Fruits: A Review. J. Food Process. Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds†. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus Waste Derived Nutra-/Pharmaceuticals for Health Benefits: Current Trends and Future Perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fiber and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Leccese, A.; Bartolini, S.; Viti, R. Antioxidant Properties of Peel and Flesh in ‘Goldrush’ and ‘Fiorina’ Scab-Resistant Apple (Malus domestica) Cultivars. N. Z. J. Crop. Hortic. Sci. 2009, 37, 71–78. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of Polyphenol-Rich Grape by-Products in Monogastric Nutrition. A Review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable Compound Extraction, Anaerobic Digestion, and Composting: A Leading Biorefinery Approach for Agricultural Wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis vinifera and Vitis labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1007, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, M.; Shi, T.; Guo, C.; Huang, Y.; Chen, Y.; Xie, M. Recovery of Dietary Fiber and Polyphenol from Grape Juice Pomace and Evaluation of Their Functional Properties and Polyphenol Compositions. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Paini, J.; Benedetti, V.; Ail, S.S.; Castaldi, M.J.; Baratieri, M.; Patuzzi, F. Valorization of Wastes from the Food Production Industry: A Review Towards an Integrated Agri-Food Processing Biorefinery. Waste Biomass Valorization 2022, 13, 31–50. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One Stage Olive Mill Waste Streams Valorisation via Hydrothermal Carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Akay, F.; Kazan, A.; Celiktas, M.S.; Yesil-Celiktas, O. A Holistic Engineering Approach for Utilization of Olive Pomace. J. Supercrit. Fluids 2015, 99, 1–7. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New Phenolic Compounds Hydrothermally Extracted from the Olive Oil Byproduct Alperujo and Their Antioxidative Activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Martos, S.; Lama-Muñoz, A.; Fernández-Bolaños, J.G.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and Identification of Minor SecoiriDOIds and Phenolic Components from Thermally Treated Olive Oil By-Products. Food Chem. 2015, 187, 166–173. [Google Scholar] [CrossRef]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and Flavonoid Profile and Antioxidant Activity in “Pomodorino Vesuviano” Tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- da Silva, A.C.; Jorge, N. Bioactive Compounds of Oils Extracted from Fruits Seeds Obtained from Agroindustrial Waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1600024. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Contreras-Esquivel, J.C.; Wicker, L.; Ochoa-Martínez, L.A.; Morales-Castro, J. Husk Tomato (Physalis Ixocarpa Brot.) Waste as a Promising Source of Pectin: Extraction and Physicochemical Characterization. J. Food Sci. 2017, 82, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, A.; Hashemi, M.; Safari, M. Valorization of Tomato Waste Proteins through Production of Antioxidant and Antibacterial Hydrolysates by Proteolytic Bacillus Subtilis: Optimization of Fermentation Conditions. J. Food Sci. Technol. 2016, 53, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese Whey Management: A Review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Bertolini, D.; Lopes, N.A.; Veras, F.F.; Gregory, G.; Brandelli, A. Characterization of Nanoliposomes Containing Bioactive Peptides Obtained from Sheep Whey Hydrolysates. LWT 2019, 101, 107–112. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited Review: Whey Proteins as Antioxidants and Promoters of Cellular Antioxidant Pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef]
- Toldrá, F.; Mora, L.; Reig, M. New Insights into Meat By-Product Utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Álvarez, C. Opportunities and Perspectives for Utilisation of Co-Products in the Meat Industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef]
- Thakur, J.; Borah, A. Microcapsules of Bioactive Compounds from Fruits and Vegetables Waste and Their Utilization: A Review. Pharma. Innov. 2021, 10, 151–157. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and Solutions of Extracting Value-Added Ingredients from Fruit and Vegetable by-Products: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Ciriminna, R.; Marina, A.; Fidalgo, A. Pectin Production and Global Market. Agro Food Ind. Hi Tech 2016, 27, 17–20. [Google Scholar]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in Biomedical and Drug Delivery Applications: A Review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Endress, H.-U. Pectins: Production, Properties and Applications. In Renewable Resources for Functional Polymers and Biomaterials: Poly-Saccharides, Proteins and Polyesters; Williams, P., Ed.; Special Collection; The Royal Society of Chemistry: London, UK, 2011; Chapter 8. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.N.; Xu, F.; He, Z.; Du, Y.; Lian, Y.; Wu, P.; Sun-Waterhouse, D. Recent Advances in Utilization of Pectins in Biomedical Applications: A Review Focusing on Molecular Structure-Directing Health-Promoting Properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 3386–3419. [Google Scholar] [CrossRef]
- Bostancı, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. Potential of Pectin for Biomedical Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1866–1900. [Google Scholar] [CrossRef] [PubMed]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Application and Bioavailability of Phytochemicals Derived from Waste Streams. J. Agric. Food Chem. 2022, 70, 6884–6900. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of Phenolic Extraction from Averrhoa Carambola Pomace by Response Surface Methodology and Its Microencapsulation by Spray and Freeze Drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol Treatment as an Adjunct to Pharmacological Management in Type 2 Diabetes Mellitus-Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Zhang, P.Y. Polyphenols in Health and Disease. Cell Biochem. Biophys. 2015, 73, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Fei, P.; Xu, Y.; Zhao, S.; Gong, S.; Guo, L. Olive Oil Polyphenol Extract Inhibits Vegetative Cells of Bacillus Cereus Isolated from Raw Milk. J. Dairy Sci. 2019, 102, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, D.; Esposto, S.; Branciari, R.; Urbani, S.; Servili, M.; Perucci, S.; Ranucci, D. Effect of a Phenolic Extract from Olive Vegetation Water on Fresh Salmon Steak Quality during Storage. Ital. J. Food Saf. 2016, 5, 6167. [Google Scholar] [CrossRef]
- Miraglia, D.; Castrica, M.; Esposto, S.; Roila, R.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Sordini, B.; Veneziani, G.; Servili, M. Quality Evaluation of Shrimp (Parapenaeus longirostris) Treated with Phenolic Extract from Olive Vegetation Water during Shelf-Life, before and after Cooking. Foods 2021, 10, 2116. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Petrotos, K. Production of Novel Bioactive Yogurt Enriched with Olive Fruit Polyphenols. World Acad. Sci. Eng. Technol. 2012, 64, 867–872. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary Intake of Anthocyanins and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Netzel, M.; Fanning, K. Food-Based Anthocyanin Intake and Cognitive Outcomes in Human Intervention Trials: A Systematic Review. J. Hum. Nutr. Diet. 2017, 30, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.; Kumar, V.; Vyas, G.; Kaur, J. Optimization of Different Variable for Eco-Friendly Extraction of Betalains and Phytochemicals from Beetroot Pomace. Waste Biomass Valorization 2018, 9, 1485–1494. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Vidallon, M.L.P.; Mendoza, D.J.R.; Reyes, C.T. Health-Promoting Bioactivities of Betalains from Red Dragon Fruit (Hylocereus polyrhizus (Weber) Britton and Rose) Peels as Affected by Carbohydrate Encapsulation. J. Sci. Food Agric. 2016, 96, 4679–4689. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M. Ultrasound and Microwave Assisted Extraction of Opuntia Fruit Peels Biocompounds: Optimization and Comparison Using RSM-CCD. Molecules 2019, 24, 3618. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Mokrejs, P.; Langmaier, F.; Mladek, M.; Janacova, D.; Kolomaznik, K.; Vasek, V. Extraction of Collagen and Gelatine from Meat Industry By-Products for Food and Non Food Uses. Waste Manag. Res. 2009, 27, 31–37. [Google Scholar] [CrossRef]
- Lupu, M.; Gradisteanu Pircalabioru, G.; Chifiriuc, M.; Albulescu, R.; Tanase, C. Beneficial Effects of Food Supplements Based on Hydrolyzed Collagen for Skin Care (Review). Exp. Ther. Med. 2019, 20, 12–17. [Google Scholar] [CrossRef]
- Puigdellivol, J.; Comellas Berenger, C.; Pérez Fernández, M.Á.; Cowalinsky Millán, J.M.; Carreras Vidal, C.; Gil Gil, I.; Martínez Pagán, J.; Ruiz Nieto, B.; Jiménez Gómez, F.; Comas Figuerola, F.X.; et al. Effectiveness of a Dietary Supplement Containing Hydrolyzed Collagen, Chondroitin Sulfate, and Glucosamine in Pain Reduction and Functional Capacity in Osteoarthritis Patients. J. Diet. Suppl. 2019, 16, 379–389. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Gaspar, D.; Sorushanova, A.; Milcovich, G.; Spanoudes, K.; Mullen, A.M.; O’brien, T.; Pandit, A.; Zeugolis, D.I. Scaffold and Scaffold-Free Self-Assembled Systems in Regenerative Medicine; Scaffold and Scaffold-Free Self-Assembled Systems in Regenerative Medicine. Biotechnol. Bioeng. 2016, 113, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Reig, M.; Mora, L. Management of Meat By- and Co-Products for an Improved Meat Processing Sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef]
- Toldrà, M.; Lynch, S.A.; Couture, R.; Álvarez, C. Blood Proteins as Functional Ingredients. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 85–101. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Ferrara, G.; Lanni, M.; Emiliani, C. Exosome-Based Strategies for Diagnosis and Therapy. Recent Pat. CNS Drug Discov. 2015, 10, 10–27. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy Fights Disease through Cellular Self-Digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- D’acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles Are a Novel Population of Extracellular Vesicles of Mitochondrial Origin Altered in Down Syndrome. Sci. Adv. 2021, 7, eabe5085. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular Lipidomics of Exosomes Released by PC-3 Prostate Cancer Cells. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2013, 1831, 1302–1309. [Google Scholar] [CrossRef]
- Buratta, S.; Urbanelli, L.; Sagini, K.; Giovagnoli, S.; Caponi, S.; Fioretto, D.; Mitro, N.; Caruso, D.; Emiliani, C. Extracellular Vesicles Released by Fibroblasts Undergoing H-Ras Induced Senescence Show Changes in Lipid Profile. PLoS ONE 2017, 12, e0188840. [Google Scholar] [CrossRef] [PubMed]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and Comprehensive “Shotgun” Lipidome Profiling of Colorectal Cancer Cell Derived Exosomes. Methods 2016, 87, 83–95. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; De Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma Cells Release Exosomes Carrying MtDNA. J. Neural Transm. 2010, 117, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles as Key Mediators of Plant–Microbe Interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2022, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.C.; Buono, R.; Otegui, M.S. Plant Endosomal Trafficking Pathways. Curr. Opin. Plant Biol. 2011, 14, 666–673. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis in Arabidopsis and Tobacco Cells. Plant Cell. 2010, 22, 4009–4030. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, W.; He, Y.; Zhao, Q.; Wakazaki, M.; Zhuang, X.; Gao, J.; Zeng, Y.; Gao, C.; Ding, Y.; et al. A Whole-Cell Electron Tomography Model of Vacuole Biogenesis in Arabidopsis Root Cells. Nat. Plants 2019, 5, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Iwasaki, S.; Tamura, K.; Kondo, M.; Fuji, K.; Ogasawara, K.; Nishimura, M.; Hara-Nishimura, I. A Novel Membrane Fusion-Mediated Plant Immunity against Bacterial Pathogens. Genes Dev. 2009, 23, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. Correction to: The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. Aaps Pharmscitech 2021, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Rupert, D.L.M.; Claudio, V.; Lässer, C.; Bally, M. Methods for the Physical Characterization and Quantification of Extracellular Vesicles in Biological Samples. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3164–3179. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Sun, C.; Wang, L.; Guo, X.L. New Insight into Isolation, Identification Techniques and Medical Applications of Exosomes. J. Control. Release 2019, 308, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, S.; Carata, E.; Mariano, S.; Panzarini, E. Plant Extracellular Vesicles: Investigating Their Utilization as Beneficial Nutrients in Diet. Appl. Sci. 2023, 13, 6656. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current Understanding of Plant-Derived Exosome-like Nanoparticles in Regulating the Inflammatory Response and Immune System Microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, F.; Qiu, H.; Zhang, G.; Xu, H.; Xu, J. Coupling Factors and Exosomal Packaging MicroRNAs Involved in the Regulation of Bone Remodelling. Biol. Rev. 2018, 93, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived MicroRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, F.; Ouyang, D. A Review of the Pharmacology and Toxicology of Aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of Cabbage Exosome-like Nanovesicles and Investigation of Their Biological Activities in Human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic Exosome-like Vesicles: A New Way of Protein Secretion in Plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef] [PubMed]
- de la Canal, L.; Pinedo, M. Extracellular Vesicles: A Missing Component in Plant Cell Wall Remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current Knowledge on Exosome Biogenesis and Release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food. Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-Derived Nanoparticles Alter Macrophage Polarization to Inhibit Melanoma Growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Canup, B.S.B.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of Garlic-Derived Nanovesicles on Liver Cells Is Triggered by Interaction with CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, V.P.; Salazar, G.A.; Coronado-Arrázola, I.; Schultz, B.M.; Vallejos, O.P.; Berkowitz, L.; Álvarez-Lobos, M.M.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Heme Oxygenase-1 as a Modulator of Intestinal Inflammation Development and Progression. Front. Immunol. 2018, 9, 1956. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Son, S.; Oliveira, S.C.; Barber, G.N. STING-Dependent Signaling Underlies IL-10 Controlled Inflammatory Colitis. Cell. Rep. 2017, 21, 3873–3884. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef]

| Agri-Food Industry | Main Waste Origin | Waste Type | Waste Management | References |
|---|---|---|---|---|
| Beverage industry | Citrus | Pomace and peel | Animal feed | [15] |
| Bioactive compound extraction | [17,18,19,20] | |||
| Apple | Pomace | Animal feed; fertilizer | [15] | |
| Bioactive compound extraction | [21,22,23,26] | |||
| Grape | Stalks and pomace | Soil conditioner and energy | [24] | |
| Animal feed | [25] | |||
| Bioactive compound extraction | [26,27,28,29] | |||
| Olive oil production | Olive | Pomace | Bioactive compound extraction | [30,31,32,33,34] |
| Tomato industrial processing | Tomato | Peel, seeds, and pulp | Animal feed; fertilizer | [26] |
| Bioactive compound extraction | [35,36,37,38] | |||
| Dairy industry | Milk | Whey and wastewater | Food derivative preparation | [15] |
| Bioactive derivatives | [40,41] | |||
| Biotechnological applications | [11] | |||
| Meat processing | Meat | Skin, bones, cartilage, blood, viscera, etc. | Human foods and animal feed | [43,44] |
| Biomedical applications | [43,44] | |||
| Technological applications | [42] |
| Bioactive Compounds from Waste of Plant Origin | |||
| Bioactive Compound Class | Main Waste Source | Properties/Bioactivity | References |
| Pectin | Apple pomace and citrus peel | Gellifier, thickener, and stabilizer | [48] |
| Hypocholesterolemia and hypoglycemic effects | [49,50] | ||
| Immunomodulatory and anti-inflammatory | [48,49] | ||
| Antidote against harmful agents | [51] | ||
| Anticancer | [49] | ||
| Phenolic compounds | Citrus peel, and apple, grape, and olive pomace | Antioxidant | [46,56,57,58,62,63,64] |
| Antimicrobial | [46,56,57,58,62,64] | ||
| Anti-inflammatory | [19,46,62] | ||
| Anticancer | [15,60,61,62] | ||
| Anthocyanins | Berry residues, apple peel, and grape pomace | Natural colorants | [69,77] |
| Antioxidant and anti-inflammatory | [70,71,72,73] | ||
| Natural pigments (betalains, carotenoids, and chlorophylls) | Beetroot pomace, red dragon fruit peel, tomato peel; spinach, lettuce, and broccoli wastes; microalgae residues | Coloring pigments | [69,74] |
| Antioxidant, anti-inflammatory, anticancer, antimicrobial, cardio- and neuro-protective activities | [69,77] | ||
| Bioactive Compounds from Waste of Animal Origin | |||
| Bioactive Compound Class | Main Waste Source | Properties/Bioactivity | References |
| Whey proteins | Whey | Nutritional value | [11,42] |
| Anti-hypertensive, antioxidant, anti-obesity, and anticancer | [78] | ||
| Meat processing proteins (gelatin, collagen, bioactive peptides, and albumin) | Skin, cartilage, bones, and blood | Emulsifier and gellifier; anti-aging; regenerative activities; nutritional; anti-hypertensive; antioxidant; antithrombotic; antimicrobial | [79,80,81,82,83,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latella, R.; Calzoni, E.; Urbanelli, L.; Cerrotti, G.; Porcellati, S.; Emiliani, C.; Buratta, S.; Tancini, B. Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods 2024, 13, 1492. https://doi.org/10.3390/foods13101492
Latella R, Calzoni E, Urbanelli L, Cerrotti G, Porcellati S, Emiliani C, Buratta S, Tancini B. Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods. 2024; 13(10):1492. https://doi.org/10.3390/foods13101492
Chicago/Turabian StyleLatella, Raffaella, Eleonora Calzoni, Lorena Urbanelli, Giada Cerrotti, Serena Porcellati, Carla Emiliani, Sandra Buratta, and Brunella Tancini. 2024. "Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products" Foods 13, no. 10: 1492. https://doi.org/10.3390/foods13101492
APA StyleLatella, R., Calzoni, E., Urbanelli, L., Cerrotti, G., Porcellati, S., Emiliani, C., Buratta, S., & Tancini, B. (2024). Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods, 13(10), 1492. https://doi.org/10.3390/foods13101492











