Biotechnological Potential and Safety Evaluation of Dextran- and Riboflavin-Producing Weisella cibaria Strains for Gluten-Free Baking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
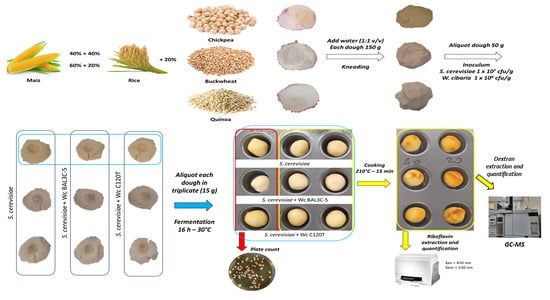
2.2. Breadmaking Assays at Laboratory Scale
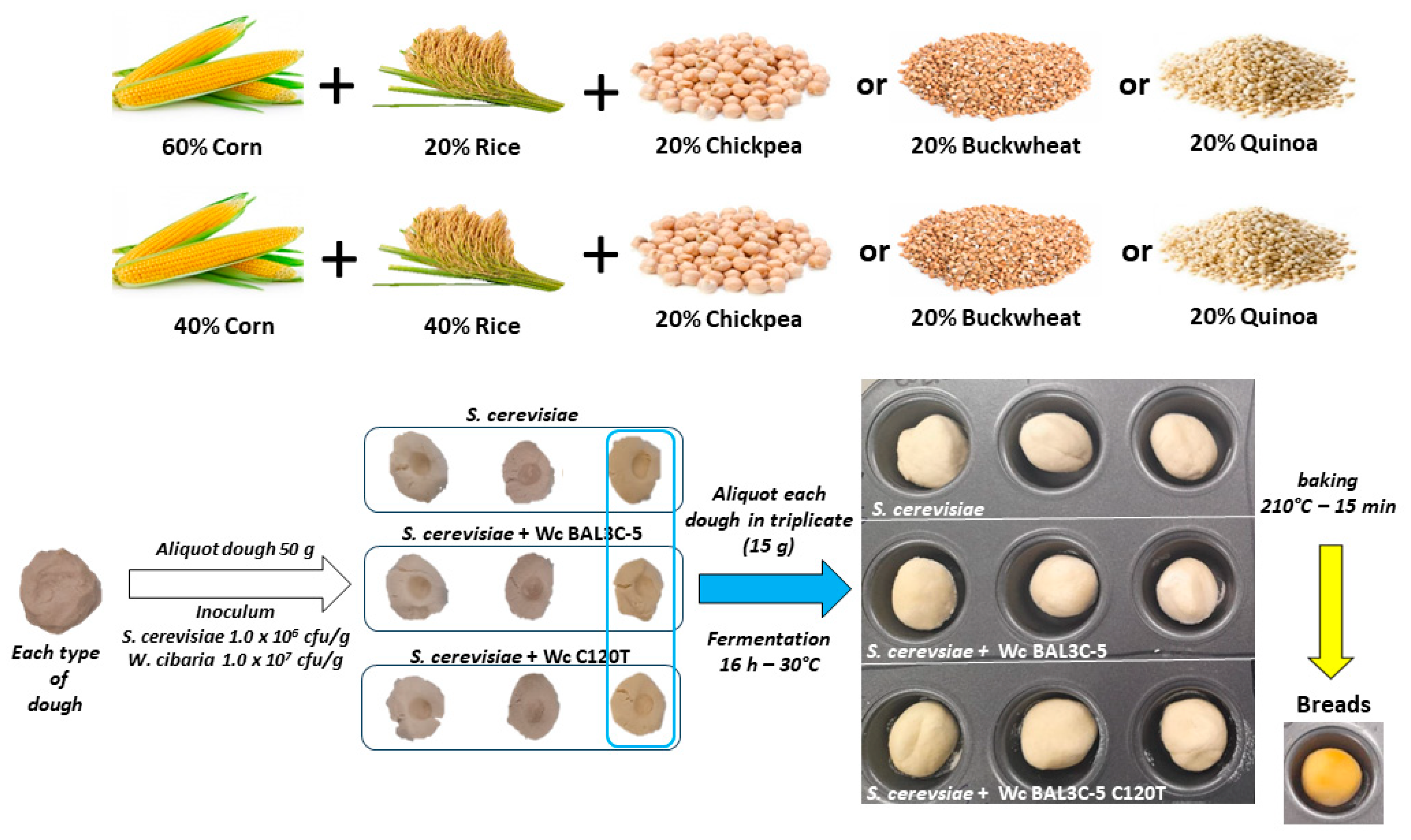
2.2.1. Preparation of the Dough
2.2.2. Inoculum of the Dough
2.3. Microbiological Analysis
2.4. Dextran Extraction and Quantification
2.5. Riboflavin Extraction and Quantification
2.6. Safety Evaluation
2.6.1. Antibiotic Resistance
2.6.2. Hemolysin Activity
2.6.3. Gelatinase Activity
2.7. Genome Analysis: Safety Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Safety Evaluation of the W. cibaria Strains
3.1.1. In Silico Analysis of Genetic Determinants for Biogenic Amine Biosynthesis
3.1.2. In Vivo and In Silico Analysis of Antibiotic Sensitivity
3.1.3. In Vivo and In Silico Analyses of Virulence Factors
3.2. Elaboration of GF Laboratory Bread
3.2.1. Selection of Flour for Preparation of the Dough
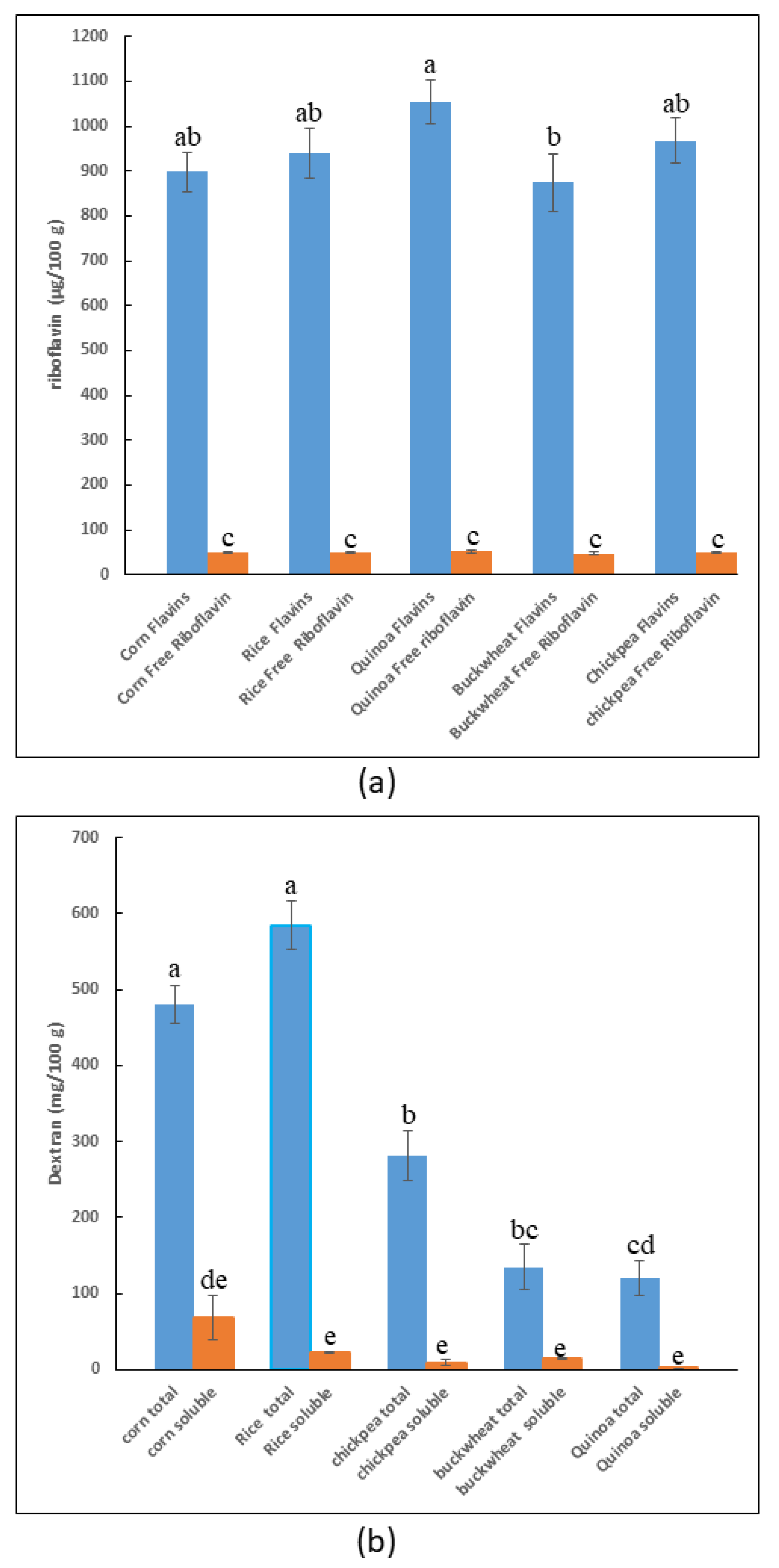
3.2.2. Analysis of the Dextran and Flavin Content of the Selected Flour
3.2.3. Experimental Design for Production of Laboratory Bread
3.2.4. Analysis of W. cibaria and Yeast Survival after Fermentation
3.3. Biofortification of the GF Bread
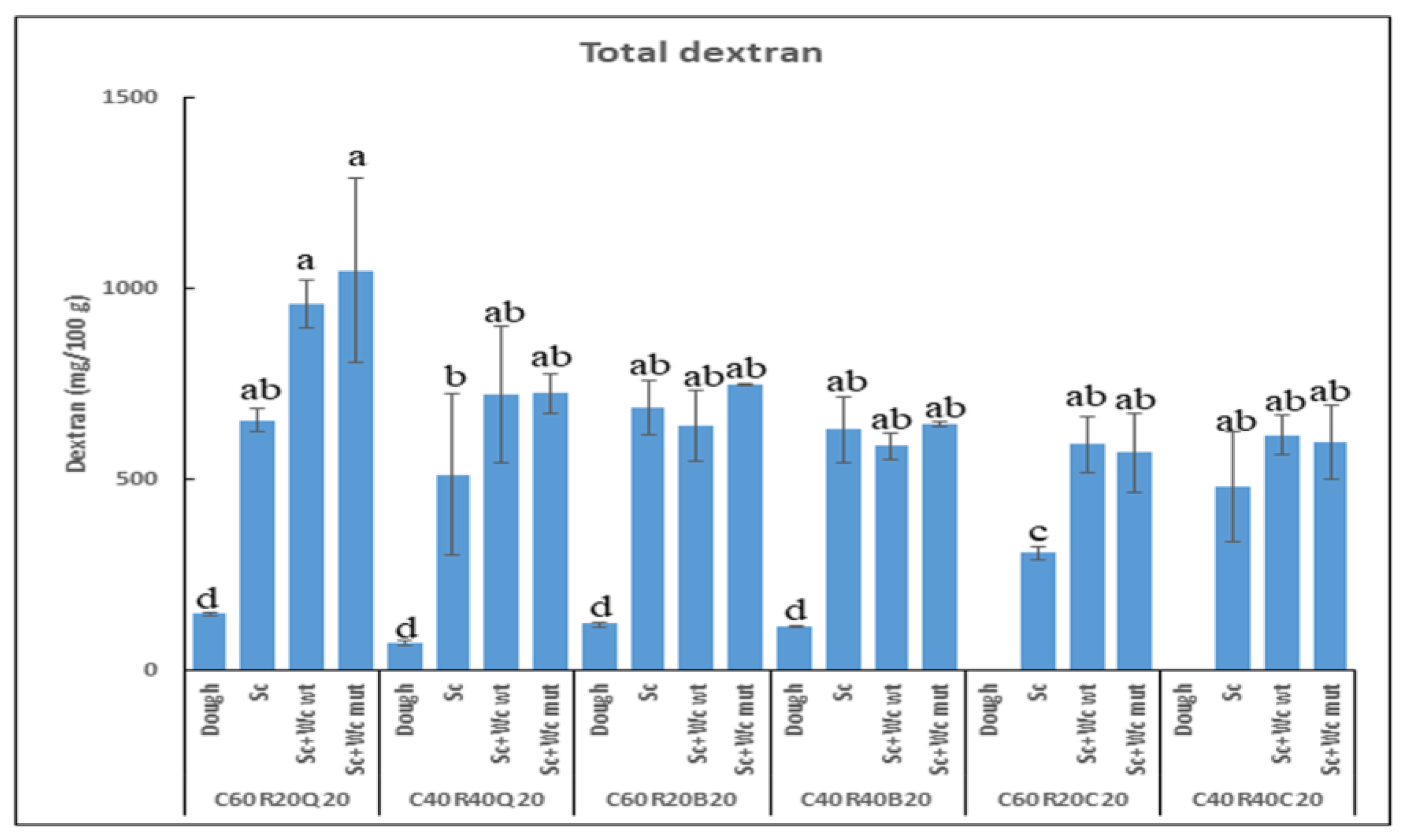
3.3.1. Detection of Total Dextran in Pilot Dough and Laboratory-Produced Bread
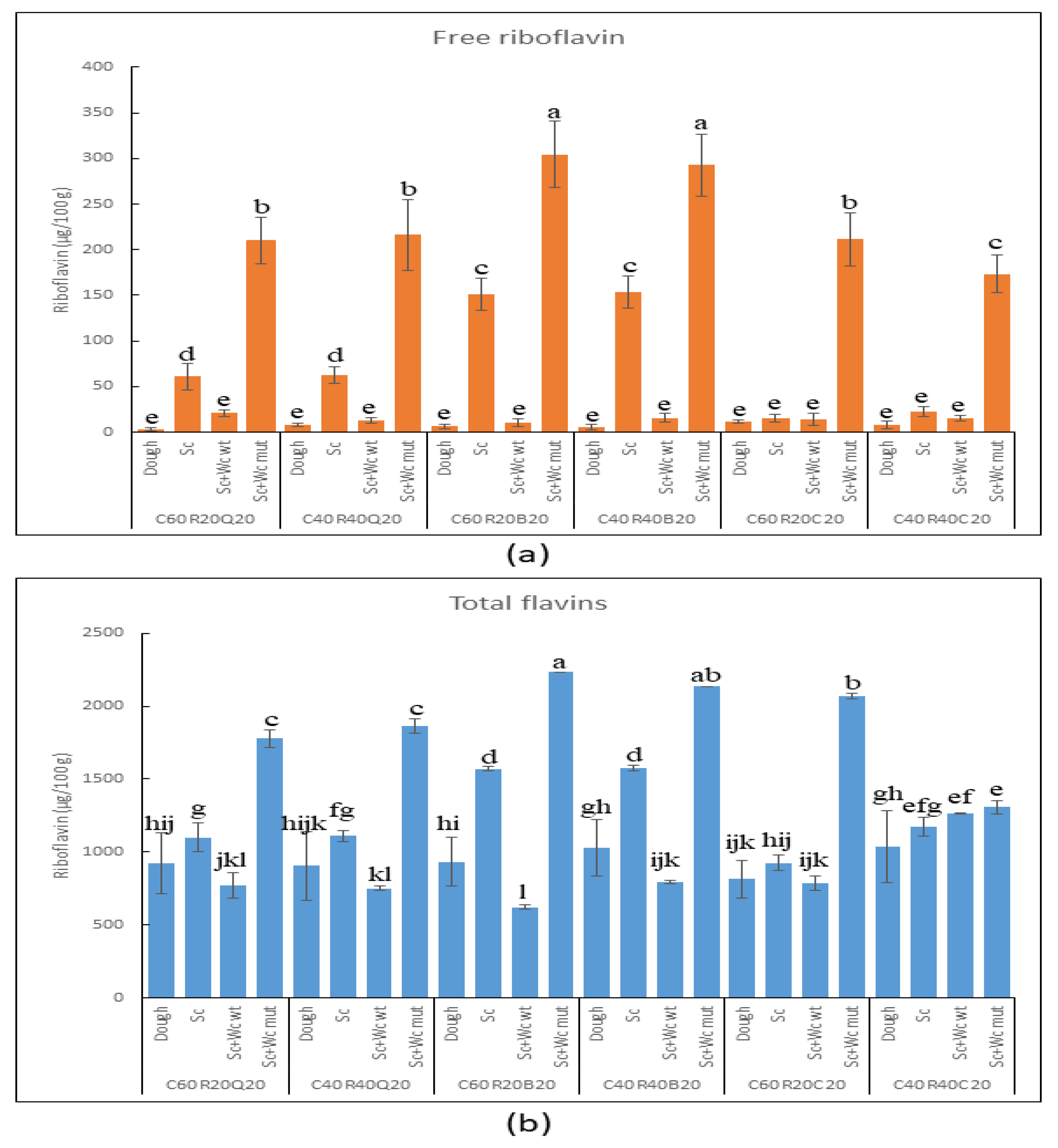
3.3.2. Detection of Riboflavin and Total Flavins in Pilot Dough and Laboratory Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Woomer, J.S.; Adedeji, A.A. Current Applications of Gluten-Free Grains—A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 14–24. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Palomares-Navarro, M.J.; Sánchez-Quezada, V.; Palomares-Navarro, J.J.; Ayala-Zavala, J.F.; Loarca-Piña, G. Nutritional and Nutraceutical Properties of Selected Pulses to Promote Gluten-Free Food Products. Plant Foods Hum. Nutr. 2023, 78, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, E.V.; Santos, F.G.; Krupa-Kozak, U.; Capriles, V.D. Nutritional Facts Regarding Commercially Available Gluten-Free Bread Worldwide: Recent Advances and Future Challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Orfila, C. The Availability and Nutritional Adequacy of Gluten-Free Bread and Pasta. Nutrients 2018, 10, 1370. [Google Scholar] [CrossRef]
- Rai, S.; Kaur, A.; Chopra, C.S. Gluten-Free Products for Celiac Susceptible People. Front. Nutr. 2018, 5, 116. [Google Scholar] [CrossRef]
- Zannini, E.; Jones, J.M.; Renzetti, S.; Arendt, E.K. Functional Replacements for Gluten. Annu. Rev. Food Sci. Technol. 2012, 3, 227–245. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Rizzello, C.G. Design of a “Clean-Label” Gluten-Free Bread to Meet Consumers Demand. Foods 2021, 10, 462. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Health-Promoting Benefits to Stress Tolerance Mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic Acid Bacteria Biota and Aroma Profile of Italian Traditional Sourdoughs From the Irpinian Area in Italy. Front. Microbiol. 2019, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological Parameters Influencing Microbial Diversity and Stability of Traditional Sourdough. Int. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, M.; Zendo, T.; Nakayama, J. Diversity and Dynamics of Sourdough Lactic Acid Bacteriota Created by a Slow Food Fermentation System. J. Biosci. Bioeng. 2021, 131, 333–340. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide Producing Lactic Acid Bacteria: Their Techno-Functional Role and Potential Application in Gluten-Free Bread Products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Di Biase, M.; Conte, A.; Lonigro, S.L.; Caputo, L.; Cedola, A.; Del Nobile, M.A.; Logrieco, A.F.; Lavermicocca, P.; Valerio, F. Weissella cibaria Short-Fermented Liquid Sourdoughs Based on Quinoa or Amaranth Flours as Fat Replacer in Focaccia Bread Formulation. Int. J. Food Sci. Technol. 2021, 56, 3197–3208. [Google Scholar] [CrossRef]
- Montemurro, M.; Beccaccioli, M.; Perri, G.; Rizzello, C.G.; Reverberi, M.; Pontonio, E. A Chestnut-Hemp Type-II Sourdough to Improve Technological, Nutritional, and Sensory Properties of Gluten-Free Bread. Int. J. Food Microbiol. 2023, 404, 110322. [Google Scholar] [CrossRef]
- Wang, Y.; Maina, N.H.; Coda, R.; Katina, K. Challenges and Opportunities for Wheat Alternative Grains in Breadmaking: Ex-Situ- versus In-Situ-Produced Dextran. Trends Food Sci. Technol. 2021, 113, 232–244. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of Dextran-Producing Weissella cibaria on Baking Properties and Sensory Profile of Gluten-Free and Wheat Breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef]
- Ramos, L.; Alonso-Hernando, A.; Martínez-Castro, M.; Morán-Pérez, J.A.; Cabrero-Lobato, P.; Pascual-Maté, A.; Téllez-Jiménez, E.; Mujico, J.R. Sourdough Biotechnology Applied to Gluten-Free Baked Goods: Rescuing the Tradition. Foods 2021, 10, 1498. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Li, J.; Ju, X.; Wang, L. Impact of Exopolysaccharides-Producing Lactic Acid Bacteria on the Chemical, Rheological Properties of Buckwheat Sourdough and the Quality of Buckwheat Bread. Food Chem. 2023, 425, 136369. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Duan, M.; Zhu, S.; Zhou, X.; Zhou, Y. Effect of Tartary Buckwheat Sourdough Fermented by Different Exogenous Lactic Acid Bacteria on Antifreeze Property of Frozen Dough. Food Chem. Adv. 2023, 2, 100182. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Characterization of Growth and Exopolysaccharide Production of Selected Acetic Acid Bacteria in Buckwheat Sourdoughs. Int. J. Food Microbiol. 2016, 239, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Venturi, M.; Coda, R.; Maina, N.H.; Granchi, L. Isolation and Characterization of Indigenous Weissella confusa for In Situ Bacterial Exopolysaccharides (EPS) Production in Chickpea Sourdough. Food Res. Int. 2020, 138, 109785. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of Amaranth and Quinoa Flours on Exopolysaccharide Production and Protein Profile of Liquid Sourdough Fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Arriba, M.G.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Chiva, R.; Celador-Lera, L.; Velázquez, E.; Prieto, A.; Dueñas, M.T.; Tamame, M.; López, P. Lactic Acid Bacteria Isolated from Fermented Doughs in Spain Produce Dextrans and Riboflavin. Foods 2021, 10, 2004. [Google Scholar] [CrossRef]
- Hernández-Alcántara, A.M.; Chiva, R.; Mohedano, M.L.; Russo, P.; Ruiz-Masó, J.Á.; del Solar, G.; Spano, G.; Tamame, M.; López, P. Weissella cibaria Riboflavin-Overproducing and Dextran-Producing Strains Useful for the Development of Functional Bread. Front. Nutr. 2022, 9, 978831. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Martín-Loarte, L.; Mohedano, M.L.; Tamame, M.; Ruiz-Masó, J.Á.; del Solar, G.; Dueñas, M.T.; López, P. A Methodology for the Selection and Characterization of Riboflavin-Overproducing Weissella cibaria Strains after Treatment with Roseoflavin. Front. Microbiol. 2023, 14, 1154130. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Loscher, C.; Brabazon, D.; Freeland, B. Bioactive Ingredients from Dairy-Based Lactic Acid Bacterial Fermentations for Functional Food Production and Their Health Effects. Nutrients 2023, 15, 4754. [Google Scholar] [CrossRef]
- Capozzi, V.; Menga, V.; Digesu, A.M.; De Vita, P.; van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological Production of Vitamin B2-Enriched Bread and Pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-Overproducing Strains of Lactobacillus fermentum for Riboflavin-Enriched Bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In Situ Riboflavin Fortification of Different Kefir-like Cereal-Based Beverages Using Selected Andean LAB Strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; Ahannach, S.; Breynaert, A.; Erreygers, I.; Wittouck, S.; Bron, P.A.; Van Beeck, W.; Eilers, T.; Alloul, A.; Blansaer, N.; et al. Spontaneous Riboflavin-Overproducing Limosilactobacillus reuteri for Biofortification of Fermented Foods. Front. Nutr. 2022, 9, 916607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Thakur, K.; Feng, J.-Y.; Cai, J.-S.; Zhang, J.-G.; Hu, F.; Russo, P.; Spano, G.; Wei, Z.-J. Riboflavin-Overproducing Lactobacilli for the Enrichment of Fermented Soymilk: Insights into Improved Nutritional and Functional Attributes. Appl. Microbiol. Biotechnol. 2020, 104, 5759–5772. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Carmo, L.M.; Valencia, G.A. The Influence of Clean Food Labels on Consumers’ Perception. Packag. Technol. Sci. 2023, 36, 757–766. [Google Scholar] [CrossRef]
- Asioli, D.; Jessica Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Sung, C.-C.; Tseng, J.-T. Willingness-to-Pay for Ready-to-Eat Clean Label Food Products at Convenient Stores. Future Foods 2023, 7, 100237. [Google Scholar] [CrossRef]
- Gliguem, H.; Khamassi, F.; Hanafi, A.; Hajji, W.; Esseghaier, M.H.; Bellagha, S. Legume Flour Enrichment of Cereal Products: Effects on Consumer Perception and Physical Quality. Acta Sci. Nutr. Health 2022, 6, 03–14. [Google Scholar] [CrossRef]
- Russo, P.; De Simone, N.; Capozzi, V.; Mohedano, M.L.; Ruiz-Masó, J.Á.; Del Solar, G.; López, P.; Spano, G. Selection of Riboflavin Overproducing Strains of Lactic Acid Bacteria and Riboflavin Direct Quantification by Fluorescence. Flavins Flavoproteins Methods Protoc. 2021, 2280, 3–14. [Google Scholar] [CrossRef]
- ISO 10932:2010; Milk and Milk Products Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB). ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/46434.html (accessed on 20 December 2023).
- Filardi, R.; Gargari, G.; Mora, D.; Arioli, S. Characterization of Antibiotic-Resistance Traits in Akkermansia muciniphila Strains of Human Origin. Sci. Rep. 2022, 12, 19426. [Google Scholar] [CrossRef]
- ISO 19344:2015; Milk and Milk Products Starter Cultures, Probiotics and Fermented Products Quantification of Lactic Acid Bacteria by Flow Cytometry. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/64658.html (accessed on 20 December 2023).
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T.; et al. In Vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Daliri, E.B.-M.; Balnionytė, T.; Stankevičiūtė, J.; Lastauskienė, E.; Burokas, A. In Vitro Screening and Characterization of Lactic Acid Bacteria from Lithuanian Fermented Food with Potential Probiotic Properties. Front. Microbiol. 2023, 14, 1213370. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. Card 2020: Antibiotic Resistome Surveillance With The Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative Pathogenomic Platform With An Interactive Web Interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A Web Tool to Identify Clustered Regularly Interspaced Short Palindromic Repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Jang, S.H.; Hwang, M.H.; Chang, H.-I. Complete Genome Sequence of ΦMH1, a Leuconostoc Temperate Phage. Arch. Virol. 2010, 155, 1883–1885. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The Viral Proteomic Tree Server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 18 December 2023).
- Panel, E.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Updated List of QPS-Recommended Microorganisms for Safety Risk Assessments Carried out by EFSA 2023; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- Fessard, A.; Remize, F. Why Are Weissella Spp. Not Used as Commercial Starter Cultures for Food Fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]
- Abriouel, H.; Lerma, L.L.; Casado Muñoz, M.D.C.; Montoro, B.P.; Kabisch, J.; Pichner, R.; Cho, G.-S.; Neve, H.; Fusco, V.; Franz, C.M.A.P.; et al. The Controversial Nature of the Weissella Genus: Technological and Functional Aspects versus Whole Genome Analysis-Based Pathogenic Potential for Their Application in Food and Health. Front. Microbiol. 2015, 6, 1197. [Google Scholar] [CrossRef]
- Kamboj, K.; Vasquez, A.; Balada-Llasat, J.-M. Identification and Significance of Weissella Species Infections. Front. Microbiol. 2015, 6, 1204. [Google Scholar] [CrossRef] [PubMed]
- Fairfax, M.R.; Lephart, P.R.; Salimnia, H. Weissella confusa: Problems with Identification of an Opportunistic Pathogen That Has Been Found in Fermented Foods and Proposed as a Probiotic. Front. Microbiol. 2014, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-A.; Tirupathi Pichiah, P.B.; Yu, J.-J.; Oh, S.-H.; Daily, J.W., III; Cha, Y.-S. Anti-Obesity Effect of Kimchi Fermented with Weissella koreensis OK1-6 as Starter in High-Fat Diet-Induced Obese C57BL/6J Mice. J. Appl. Microbiol. 2012, 113, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Lim, H.-S.; Kim, S.-M.; Lee, H.-C.; Oh, J.-S. Effect of Weissella cibaria on Fusobacterium nucleatum-Induced Interleukin-6 and Interleukin-8 Production in KB Cells. J. Bacteriol. Virol. 2011, 41, 9. [Google Scholar] [CrossRef]
- Fhoula, I.; Rehaiem, A.; Najjari, A.; Usai, D.; Boudabous, A.; Sechi, L.A.; Hadda-Imene, O. Functional Probiotic Assessment and In Vivo Cholesterol-Lowering Efficacy of Weissella Sp. Associated with Arid Lands Living-Hosts. BioMed Res. Int. 2018, 2018, 1654151. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Lee, N.-K.; Choi, A.-J.; Choe, J.-S.; Bae, C.H.; Paik, H.-D. Antagonistic and Antioxidant Effect of Probiotic Weissella cibaria JW15. Food Sci. Biotechnol. 2018, 28, 851–855. [Google Scholar] [CrossRef]
- Oh, E.Y.; Lee, S.-M. Natural Killer Cell Activation by Weissella cibaria JW15 Isolated from Kimchi. J.Bacteriol. Virol. 2021, 51, 62–73. [Google Scholar] [CrossRef]
- Fanelli, F.; Montemurro, M.; Verni, M.; Garbetta, A.; Bavaro, A.R.; Chieffi, D.; Cho, G.-S.; Franz, C.M.A.P.; Rizzello, C.G.; Fusco, V. Probiotic Potential and Safety Assessment of Type Strains of Weissella and Periweissella Species. Microbiol. Spectr. 2023, 11, e0304722. [Google Scholar] [CrossRef]
- Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2393 (accessed on 20 December 2023).
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The Biogenic Amines Putrescine and Cadaverine Show in Vitro Cytotoxicity at Concentrations That Can Be Found in Foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the Characterisation of Microorganisms Used as Feed Additives or as Production Organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef] [PubMed]
- Fhoula, I.; Boumaiza, M.; Tayh, G.; Rehaiem, A.; Klibi, N.; Ouzari, I.-H. Antimicrobial Activity and Safety Features Assessment of Weissella Spp. from Environmental Sources. Food Sci. Nutr. 2022, 10, 2896–2910. [Google Scholar] [CrossRef]
- Suhonen, A. Antibiotic Susceptibility of Lactic Acid Bacteria. Master Thesis, University of Helsinki, Helsinki, Finland, 2019. Available online: https://helda.helsinki.fi/server/api/core/bitstreams/244c659b-de61-40ec-ab2f-baa965f18160/content (accessed on 20 December 2023).
- Quattrini, M.; Korcari, D.; Ricci, G.; Fortina, M.G. A Polyphasic Approach to Characterize Weissella cibaria and Weissella confusa Strains. J. Appl. Microbiol. 2020, 128, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Bourdichon, F.; Patrone, V.; Fontana, A.; Milani, G.; Morelli, L. Safety Demonstration of a Microbial Species for Use in the Food Chain: Weissella confusa. Int. J. Food Microbiol. 2021, 339, 109028. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-J.; Gwon, H.-M.; Jeong, W.-S.; Yeo, S.-H.; Kim, S.-Y. Safety Evaluation of Weissella cibaria JW15 by Phenotypic and Genotypic Property Analysis. Microorganisms 2021, 9, 2450. [Google Scholar] [CrossRef]
- Sturino, J.M. Literature-Based Safety Assessment of an Agriculture- and Animal-Associated Microorganism: Weissella confusa. Regul. Toxicol. Pharmacol. RTP 2018, 95, 142–152. [Google Scholar] [CrossRef]
- Akpınar Kankaya, D.; Tuncer, Y. Antibiotic Resistance in Vancomycin-Resistant Lactic Acid Bacteria (VRLAB) Isolated from Foods of Animal Origin. J. Food Process. Preserv. 2020, 44, e14468. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.-M.; Kim, H.-Y. Weissella and the Two Janus Faces of the Genus. Appl. Microbiol. Biotechnol. 2023, 107, 1119–1127. [Google Scholar] [CrossRef]
- Nunziata, L.; Brasca, M.; Morandi, S.; Silvetti, T. Antibiotic Resistance in Wild and Commercial Non-Enterococcal Lactic Acid Bacteria and Bifidobacteria Strains of Dairy Origin: An Update. Food Microbiol. 2022, 104, 103999. [Google Scholar] [CrossRef]
- Yadav, M.; Sunita; Shukla, P. Probiotic Potential of Weissella paramesenteroides MYPS5.1 Isolated from Customary Dairy Products and Its Therapeutic Application. 3 Biotech 2022, 12, 9. [Google Scholar] [CrossRef]
- Arellano, K.; Vazquez, J.; Park, H.; Lim, J.; Ji, Y.; Kang, H.-J.; Cho, D.; Jeong, H.W.; Holzapfel, W.H. Safety Evaluation and Whole-Genome Annotation of Lactobacillus plantarum Strains from Different Sources with Special Focus on Isolates from Green Tea. Probiotics Antimicrob. Proteins 2020, 12, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Cizeikiene, D.; Jagelaviciute, J. Investigation of Antibacterial Activity and Probiotic Properties of Strains Belonging to Lactobacillus and Bifidobacterium Genera for Their Potential Application in Functional Food and Feed Products. Probiotics Antimicrob. Proteins 2021, 13, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Barbosa, J.; Pereira, S.I.A.; Rodríguez, A.; Córdoba, J.J.; Teixeira, P. Study of Lactic Acid Bacteria Isolated from Traditional Ripened Foods and Partial Characterization of Their Bacteriocins. LWT 2023, 173, 114300. [Google Scholar] [CrossRef]
- Burešová, I.; Tokár, M.; Mareček, J.; Hřivna, L.; Faměra, O.; Šottníková, V. The Comparison of the Effect of Added Amaranth, Buckwheat, Chickpea, Corn, Millet and Quinoa Flour on Rice Dough Rheological Characteristics, Textural and Sensory Quality of Bread. J. Cereal Sci. 2017, 75, 158–164. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of Product Quality, Sensory Profile and Ultrastructure of Breads Made from a Range of Commercial Gluten-Free Flours Compared to Their Wheat Counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- WHO Team. Guideline: Fortification of Maize Flour and Corn Meal with Vitamins and Minerals; World Health Organization: Geneva, Switzerland, 2016.
- Schoenlechner, R.; Siebenhandl, S.; Berghofer, E. Pseudocereals. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Dal Bello, F., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2008; pp. 149–VI. ISBN 978-0-12-373739-7. [Google Scholar]
- Ikeda, S.; Yamashita, Y.; Tomura, K.; Kreft, I. Nutritional Comparison in Mineral Characteristics between Buckwheat and Cereals. Fagopyrum 2006, 23, 61–65. [Google Scholar]
- Sciarini, L.S.; Steffolani, M.E.; Fernández, A.; Paesani, C.; Pérez, G.T. Gluten-Free Breadmaking Affected by the Particle Size and Chemical Composition of Quinoa and Buckwheat Flour Fractions. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2020, 26, 321–332. [Google Scholar] [CrossRef]
- Yegrem, L. Nutritional Composition, Antinutritional Factors, and Utilization Trends of Ethiopian Chickpea (Cicer arietinum L.). Int. J. Food Sci. 2021, 2021, 5570753. [Google Scholar] [CrossRef]
- Culetu, A.; Susman, I.E.; Duta, D.E.; Belc, N. Nutritional and Functional Properties of Gluten-Free Flours. Appl. Sci. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Vogelmann, S.A.; Seitter, M.; Singer, U.; Brandt, M.J.; Hertel, C. Adaptability of Lactic Acid Bacteria and Yeasts to Sourdoughs Prepared from Cereals, Pseudocereals and Cassava and Use of Competitive Strains as Starters. Int. J. Food Microbiol. 2009, 130, 205–212. [Google Scholar] [CrossRef]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually Stimulating Interactions between Lactic Acid Bacteria and Saccharomyces cerevisiae in Sourdough Fermentation. LWT 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Moussa, T.; Khalil, N.M. Solid-State Fermentation for The Production of Dextran from Saccharomyces cerevisiae and Its Cytotoxic Effects. Life Sci. J. 2012, 9, 2210–2218. [Google Scholar]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough Fermentation of Whole and Sprouted Lentil Flours: In Situ Formation of Dextran and Effects on the Nutritional, Texture and Sensory Characteristics of White Bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Venturi, M.; Cardone, G.; Pini, N.; Marti, A.; Granchi, L. In Situ Dextran Synthesis by Weissella confusa Ck15 and Leuconostoc pseudomesenteroides DSM 20193 and Their Effect on Chickpea Sourdough Bread. Int. J. Food Sci. Technol. 2021, 56, 5277–5285. [Google Scholar] [CrossRef]
- Niro, S.; D’Agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-Free Alternative Grains: Nutritional Evaluation and Bioactive Compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Afzaal, M.; Ikram, A.; Imran, A.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Hussain, M. Exploring the Amino Acid Composition and Vitamin-B Profile of Buckwheat Varieties. J. Food Process. Preserv. 2021, 45, e15743. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Rochette, I.; Guyot, J.-P.; Humblot, C. Influence of Fermentation and Other Processing Steps on the Folate Content of a Traditional African Cereal-Based Fermented Food. Int. J. Food Microbiol. 2018, 266, 79–86. [Google Scholar] [CrossRef]
- Hjortmo, S.; Patring, J.; Jastrebova, J.; Andlid, T. Biofortification of Folates in White Wheat Bread by Selection of Yeast Strain and Process. Int. J. Food Microbiol. 2008, 127, 32–36. [Google Scholar] [CrossRef]
- Kariluoto, S.; Vahteristo, L.; Salovaara, H.; Katina, K.; Liukkonen, K.-H.; Piironen, V. Effect of Baking Method and Fermentation on Folate Content of Rye and Wheat Breads. Cereal Chem. 2004, 81, 134–139. [Google Scholar] [CrossRef]
- Batifoulier, F.; Verny, M.-A.; Chanliaud, E.; Rémésy, C.; Demigné, C. Effect of Different Breadmaking Methods on Thiamine, Riboflavin and Pyridoxine Contents of Wheat Bread. J. Cereal Sci. 2005, 42, 101–108. [Google Scholar] [CrossRef]
- Schlösser, T.; Wiesenburg, A.; Gätgens, C.; Funke, A.; Viets, U.; Vijayalakshmi, S.; Nieland, S.; Stahmann, K.-P. Growth Stress Triggers Riboflavin Overproduction in Ashbya gossypii. Appl. Microbiol. Biotechnol. 2007, 76, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Abbas, C.A.; Sibirny, A.A. Genetic Control of Biosynthesis and Transport of Riboflavin and Flavin Nucleotides and Construction of Robust Biotechnological Producers. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 321–360. [Google Scholar] [CrossRef] [PubMed]
- Kariluoto, S.; Aittamaa, M.; Korhola, M.; Salovaara, H.; Vahteristo, L.; Piironen, V. Effects of Yeasts and Bacteria on the Levels of Folates in Rye Sourdoughs. Int. J. Food Microbiol. 2006, 106, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Capozzi, V.; López, P.; Fiocco, D.; Spano, G. Probiotic Abilities of Riboflavin-Overproducing Lactobacillus Strains: A Novel Promising Application of Probiotics. Appl. Microbiol. Biotechnol. 2014, 98, 7569–7581. [Google Scholar] [CrossRef]
- Ripa, I.; Ruiz-Masó, J.Á.; De Simone, N.; Russo, P.; Spano, G.; Del Solar, G. A Single Change in the Aptamer of the Lactiplantibacillus plantarum Rib Operon Riboswitch Severely Impairs Its Regulatory Activity and Leads to a Vitamin B2—Overproducing Phenotype. Microb. Biotechnol. 2022, 15, 1253–1269. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Jonnalagadda, S.S.; Liu, S.; Marquart, L.; McKeown, N.; Reicks, M.; Riccardi, G.; Seal, C.; Slavin, J.; Thielecke, F.; et al. Developing a Standard Definition of Whole-Grain Foods for Dietary Recommendations: Summary Report of a Multidisciplinary Expert Roundtable Discussion. Adv. Nutr. 2014, 5, 164–176. [Google Scholar] [CrossRef]

| Bacteria | Ampicillin | Vancomycin | Gentamicin | Kanamycin | Streptomycin | Erythromycin | Clindamycin | Tetracycline | Chloramphenicol |
|---|---|---|---|---|---|---|---|---|---|
| Leuconostoc spp. | 2 | n.r. | 16 | 16 | 64 | 1 | 1 | 8 | 4 |
| BAL3C-5 | 1 | >128 | 4 | 32 | 32 | <0.25 | <0.25 | 32 | 4 |
| BAL3C-5 C120T | 1 | >128 | 2 | 32 | 16 | <0.25 | <0.25 | 32 | 4 |
| Flour | Inocula | S. cerevisiae (CFU/g) | W. cibaria (CFU/g) | Flour | S. cerevisiae (CFU/g) | W. cibaria (CFU/g) | |
|---|---|---|---|---|---|---|---|
| Corn (40%)—Rice (40%) | Chickpea (20%) | Sc | 2.09 × 108 | <1.00 × 104 LAB * | Corn (60%)—Rice (20%) | 1.89 × 108 | <1.00 × 104 LAB |
| Sc+Wc wt | 7.60 × 108 | 1.80 × 109 | 7.80 × 107 | 1.90 × 109 | |||
| Sc+Wc mut | 7.70 × 107 | 1.58 × 109 | 6.10 × 107 | 1.40 × 109 | |||
| Quinoa (20%) | Sc | 1.38 × 108 | <1.00 × 104 LAB | 1.11 × 108 | <1.00 × 104 LAB | ||
| Sc+Wc wt | 1.02 × 108 | 1.03 × 109 | 9.00 × 107 | 5.80 × 108 | |||
| Sc+Wc mut | 6.80 × 107 | 1.90 × 108 | 7.80 × 107 | 2.5 × 108 | |||
| Buckwheat (20%) | Sc | 8.10 × 107 | <1.00 × 104 LAB | 1.30 × 108 | <1.00 × 104 LAB | ||
| Sc+Wc wt | 4.40 × 107 | 1.36 × 109 | 5.00 × 107 | 1.35 × 109 | |||
| Sc+Wc mut | 3.80 × 107 | 1.28 × 109 | 6.90 × 107 | 1.13 × 109 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Diez-Ozaeta, I.; Mangieri, N.; Tamame, M.; Spano, G.; Dueñas, M.T.; López, P.; Mohedano, M.L. Biotechnological Potential and Safety Evaluation of Dextran- and Riboflavin-Producing Weisella cibaria Strains for Gluten-Free Baking. Foods 2024, 13, 69. https://doi.org/10.3390/foods13010069
Russo P, Diez-Ozaeta I, Mangieri N, Tamame M, Spano G, Dueñas MT, López P, Mohedano ML. Biotechnological Potential and Safety Evaluation of Dextran- and Riboflavin-Producing Weisella cibaria Strains for Gluten-Free Baking. Foods. 2024; 13(1):69. https://doi.org/10.3390/foods13010069
Chicago/Turabian StyleRusso, Pasquale, Iñaki Diez-Ozaeta, Nicola Mangieri, Mercedes Tamame, Giuseppe Spano, Maria Teresa Dueñas, Paloma López, and Mari Luz Mohedano. 2024. "Biotechnological Potential and Safety Evaluation of Dextran- and Riboflavin-Producing Weisella cibaria Strains for Gluten-Free Baking" Foods 13, no. 1: 69. https://doi.org/10.3390/foods13010069
APA StyleRusso, P., Diez-Ozaeta, I., Mangieri, N., Tamame, M., Spano, G., Dueñas, M. T., López, P., & Mohedano, M. L. (2024). Biotechnological Potential and Safety Evaluation of Dextran- and Riboflavin-Producing Weisella cibaria Strains for Gluten-Free Baking. Foods, 13(1), 69. https://doi.org/10.3390/foods13010069