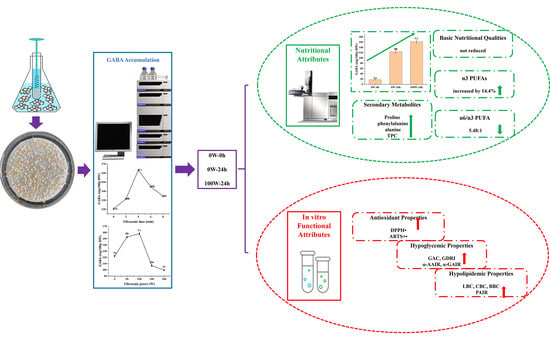
Enhancement of γ-Aminobutyric Acid and the Characteristics of Nutrition and Function in White Quinoa through Ultrasound Stress at the Pre-Germination Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Germination of Quinoa
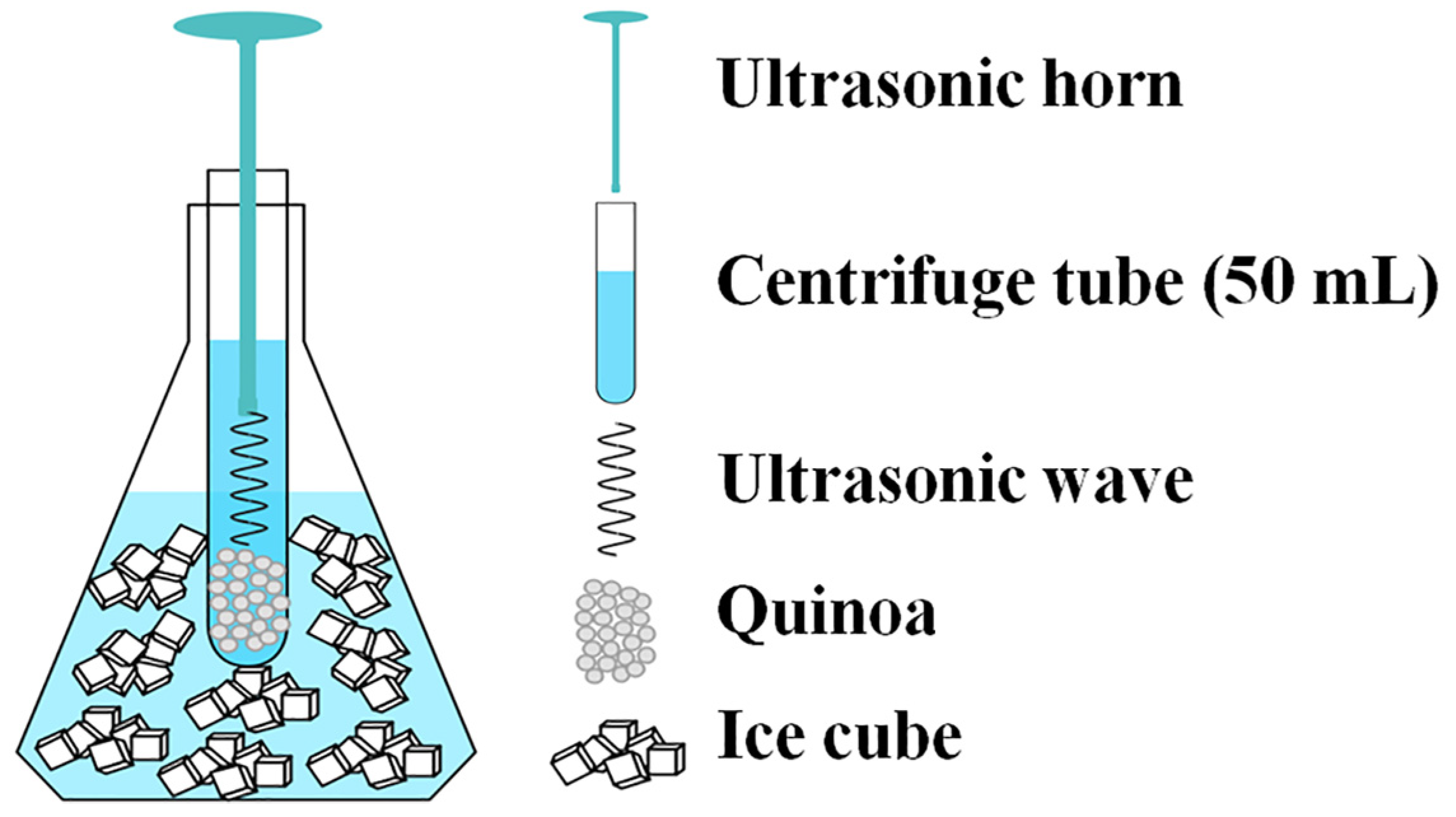
2.3. Ultrasonic Treatment
2.4. Determination of GABA Content, Moisture Absorption, Turbidity of Soaking Water, and Glutamate Decarboxylase (GAD) Activity
2.5. Analysis of Nutritional Quality
2.6. Analysis of Functional Characteristics
2.6.1. Determination of Antioxidant Properties
2.6.2. Determination of Hypoglycemic Activities
2.6.3. Determination of Hypolipidemic Activities
2.7. Statistical Analysis
3. Results and Discussion
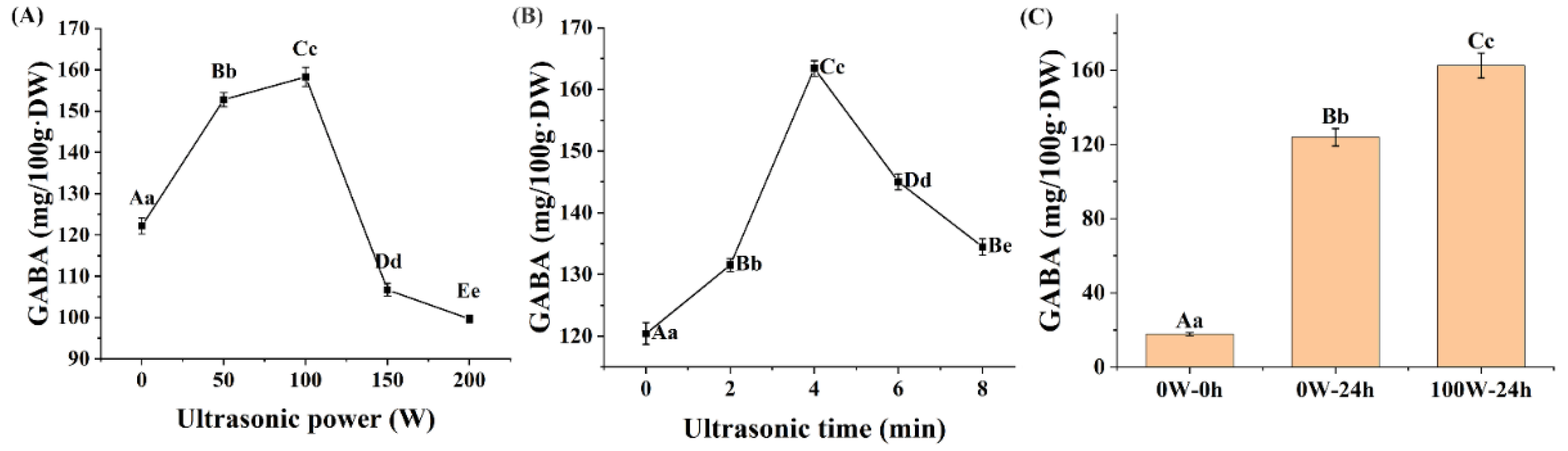
3.1. Effects of Ultrasonic Treatment on the GABA Accumulation of Quinoa during Germination
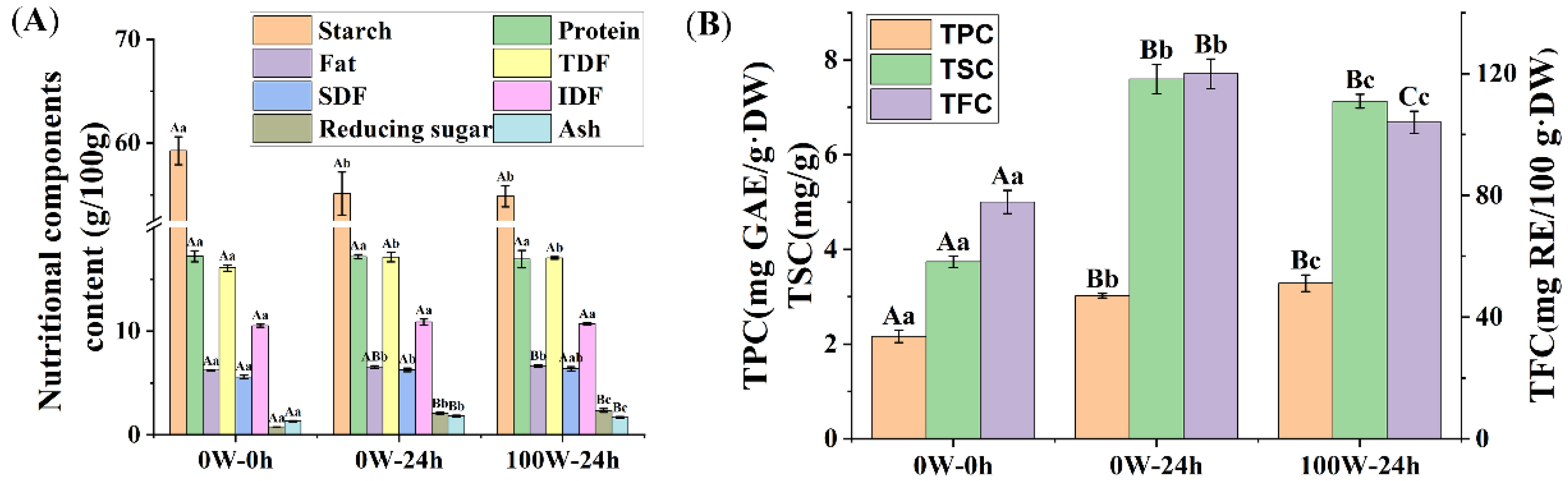
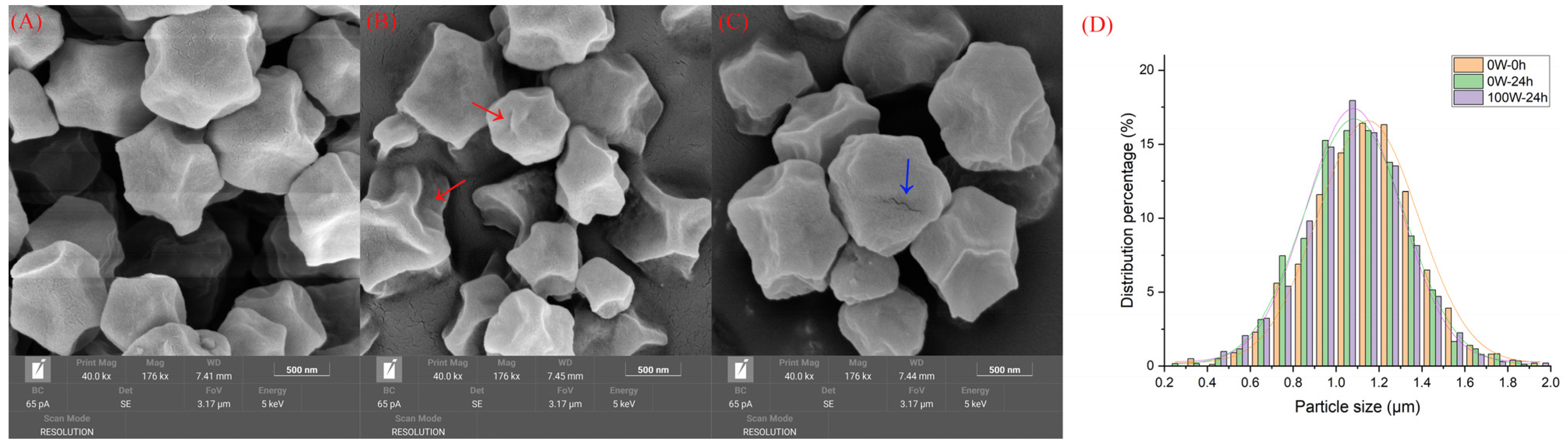
3.2. Effects of Ultrasonic Treatment on the Nutritional Quality of Quinoa during Germination
3.2.1. Effects of Ultrasonic Treatment on the Basic Nutritional Components in Quinoa during Germination
3.2.2. Effects of Ultrasonic Treatment on the Phytochemical Composition of Quinoa during Germination
3.3. Effects of Ultrasonic Treatment on the Functional Characteristics of Quinoa during Germination
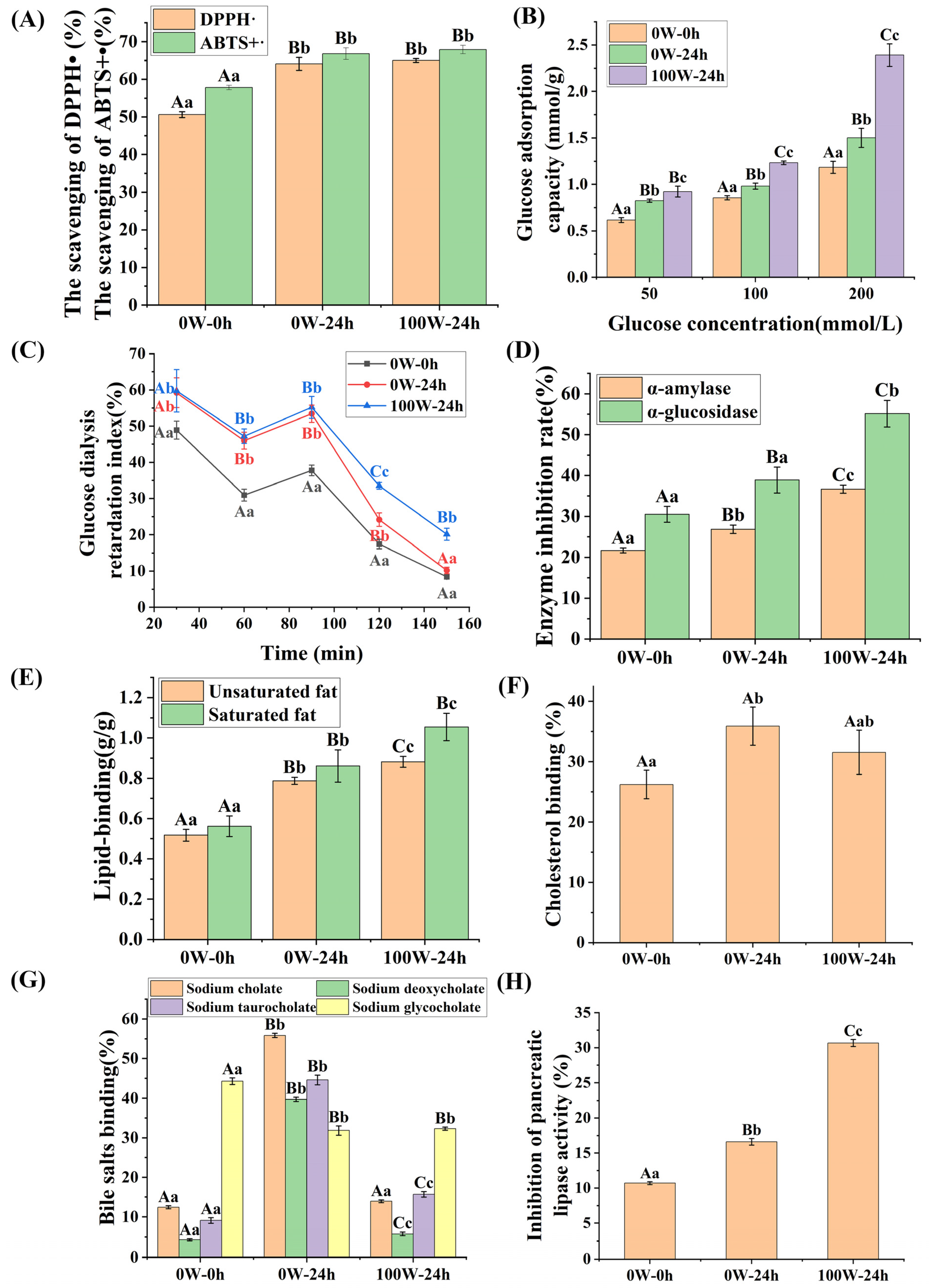
3.3.1. Effects of Ultrasonic Treatment on the Antioxidant Properties of Quinoa during Germination
3.3.2. Effects of Ultrasonic Treatment on the Hypoglycemic Activity of Quinoa during Germination
3.3.3. Effects of Ultrasonic Treatment on the Hypolipidemic Activity of Quinoa Plants during Germination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Quinoa Market Value 2022–2026. Available online: https://www.statista.com/statistics/1128506/global-quinoa-market-value-by-country/ (accessed on 10 January 2023).
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Ishaq, A.; Al-Ghamdi, A.A.; Hatamleh, A.A. Botany, Nutritional Value, Phytochemical Composition and Biological Activities of Quinoa. Plants 2021, 10, 2258. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Dong, L.; Huang, F.; Liu, L. Changes in saponins, phenolics and antioxidant activity of quinoa (Chenopodium quinoa willd) during milling process. LWT Food Sci. Technol. 2019, 114, 108381. [Google Scholar] [CrossRef]
- Vasques Farinazzi-Machado, F.M.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan Junior, O. Use of cereal bars with quinoa (Chenopodium quinoa W) to reduce risk factors related to cardiovascular diseases. Cienc. E Tecnol. De Aliment. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1, e001321. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Wang, Z.; Teimouri, S.; Pematilleke, N.; Brennan, C.S.; Farahnaky, A. Unlocking the Potential of Sprouted Cereals, Pseudocereals, and Pulses in Combating Malnutrition. Foods 2023, 12, 3901. [Google Scholar] [CrossRef]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Maria Paucar-Menacho, L.; Martinez-Villaluenga, C.; Duenas, M.; Frias, J.; Penas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Lazo-Velez, M.A.; Guajardo-Flores, D.; Mata-Ramirez, D.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Characterization and Quantitation of Triterpenoid Saponins in Raw and Sprouted Chenopodium berlandieri spp. (Huauzontle) Grains Subjected to Germination with or without Selenium Stress Conditions. J. Food Sci. 2016, 81, C19–C26. [Google Scholar] [CrossRef] [PubMed]
- Carlos Enciso-Roca, E.; Javier Aguilar-Felices, E.; Aldo Tinco-Jayo, J.; Luis Arroyo-Acevedo, J.; Herrera-Calderon, O. Biomolecules with Antioxidant Capacity from the Seeds and Sprouts of 20 Varieties of Chenopodium quinoa Willd. (Quinoa). Plants 2021, 10, 2417. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lue, G.; Gao, H. GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants. Metabolites 2023, 13, 347. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Bae, H. Use of Ultrasonication Technology for the Increased Production of Plant Secondary Metabolites. Molecules 2017, 22, 1046. [Google Scholar] [CrossRef]
- Ding, J.; Ulanov, A.V.; Dong, M.; Yang, T.; Nemzer, B.V.; Xiong, S.; Zhao, S.; Feng, H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018, 40, 791–797. [Google Scholar] [CrossRef]
- Ding, J.; Johnson, J.; Chu, Y.F.; Feng, H. Enhancement of gamma-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chem. 2019, 283, 239–247. [Google Scholar] [CrossRef]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.; Cao, J.; Liu, L.; Zhou, X.; Barba, F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Ampofo, J.O.; Ngadi, M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem. 2020, 64, 104974. [Google Scholar] [CrossRef]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Li, N.N.; Liu, Y.; Zhao, Y.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Simultaneous HPLC Determination of Amino Acids in Tea Infusion Coupled to Pre-column Derivatization with 2,4-Dinitrofluorobenzene. Food Anal. Methods 2016, 9, 1307–1314. [Google Scholar] [CrossRef]
- Chinese National Standardization Administration (GB). GB/T 5009.5-2016: Determination of Protein in Food; Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Chinese National Standardization Administration (GB). GB/T 5009.6-2016: Determination of Fat in Food; Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Chinese National Standardization Administration (GB). GB/T 5009.88-2014: Determination of Dietary Fiber in Food; Standards Press of China: Beijing, China, 2014. [Google Scholar]
- Chinese National Standardization Administration (GB). GB/T 5009.4-2016: Determination of Ash Content in Food; Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Lim, S.T.; Lee, J.H.; Shin, D.H.; Lim, H.S. Comparison of protein extraction solutions for rice starch isolation and effects of residual protein content on starch pasting properties. Starch-Starke 1999, 51, 120–125. [Google Scholar] [CrossRef]
- Pinuel, L.; Boeri, P.; Zubillaga, F.; Alejandro Barrio, D.; Torreta, J.; Cruz, A.; Vasquez, G.; Pinto, A.; Carrillo, W. Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation. Plants 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, R.; Rajput, Y.S.; Mann, B.; Gandhi, K. Distinction between glycomacropeptide and beta-lactoglobulin with ‘stains all’ dye on tricine SDS-PAGE gels. Food Chem. 2021, 340, 127923. [Google Scholar] [CrossRef]
- Pham Van, H.; Nguyen Thi Hoang, Y.; Nguyen Thi Lan, P.; Nguyen Phung Ha, T.; Nguyen Thi Thu, T. Nutritional composition, enzyme activities and bioactive compounds of mung bean (Vigna radiata L.) germinated under dark and light conditions. LWT Food Sci. Technol. 2020, 133, 110100. [Google Scholar] [CrossRef]
- Miao, J.; Lin, H.; Zhang, S.; Huo, J.; Deng, S. Effect on amino acid and mineral content of the loach (Misgurnus anguillicaudatus) by adding Fe (II) chelating hairtail protein hydrolysates (Fe (II)-HPH) to the feed. Food Sci. Nutr. 2020, 8, 1575–1582. [Google Scholar] [CrossRef]
- Chinese National Standardization Administration (GB). GB/T 5009.168-2016: Determination of Fatty Acids in Food; Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Hassan Mekky, R.; Thabet, M.M.; Rodriguez-Perez, C.; Elnaggar, D.M.Y.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Comparative metabolite profiling and antioxidant potentials of seeds and sprouts of three Egyptian cultivars of Vicia faba L. Food Res. Int. 2020, 136, 109537. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Din, M.G.S.; Selim, M.A.-F.; Owis, A.I.; Abouzid, S.F. Mass spectrometry-based metabolites profiling of nutrients and anti-nutrients in major legume sprouts. Food Biosci. 2021, 39, 100800. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Xie, C.; Yang, H.; Dias, G.; Kong, L.; Bekhit, A.E.-D. Optimization of extraction parameters of antioxidant activity of extracts from New Zealand and Chinese Asparagus officinalis L. root cultivars. Ind. Crops Prod. 2018, 119, 191–200. [Google Scholar] [CrossRef]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH Radical Scavenging Activity of Tricin and Its Conjugates Isolated from “Njavara” Rice Bran: A Density Functional Theory Study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Seczyk, L.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves—The influence of protein-phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Tang, C.D.; Wu, L.R.; Zhang, F.S.; Kan, J.Q.; Zheng, J. Comparison of different extraction methods on the physicochemical, structural properties, and in vitro hypoglycemic activity of bamboo shoot dietary fibers. Food Chem. 2022, 386, 132642. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Li, J.; Wang, Y.; Wendi, L.V.; Zhang, S.; Peng, G.; Lu, Y.; Sun, H.; Dong, Y. Physicochemical properties, antioxidant activities and hypoglycemic effects of soluble dietary fibers purified from Lentinula edodes. Food Sci. Technol. 2023, 43, e131122. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chu, J.X.; Lu, Z.X.; Lv, F.X.; Bie, X.M.; Zhang, C.; Zhao, H.Z. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, K.; Zhang, X.; Li, L.F.; Zhang, H.X.; Zhou, L.; Liang, J.; Li, X. In vitro binding capacities, physicochemical properties and structural characteristics of polysaccharides fractionated from Passiflora edulis peel. Food Biosci. 2022, 50, 102016. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Ai, C.; Wen, C.; Liu, Z.; Wang, L.; Jiang, L.; Shen, P.; Zhang, G.; Song, S. Galactofucan from Laminaria japonica is not degraded by the human digestive system but inhibits pancreatic lipase and modifies the intestinal microbiota. Int. J. Biol. Macromol. 2021, 166, 611–620. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, N.; Li, W.; Zhang, B.; Shi, T.; Xie, M.; Yu, M. Effect of Ultrasonic Induction on the Main Physiological and Biochemical Indicators and γ–Aminobutyric Acid Content of Maize during Germination. Foods 2022, 11, 1358. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Karagic, D.; Liu, X.; Cui, J.; Gui, J.; Gu, M.; Gao, W. Effects of ultrasonication on increased germination and improved seedling growth of aged grass seeds of tall fescue and Russian wildrye. Sci. Rep. 2016, 6, 22403. [Google Scholar] [CrossRef]
- Lopez-Ribera, I.; Vicient, C.M. Use of ultrasonication to increase germination rates of Arabidopsis seeds. Plant Methods 2017, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, H.; Liu, J.; Liu, D.; Wang, C. The γ-Aminobutyric Acid (GABA) Synthesis Gene Regulates the Resistance to Water Core-Induced Hypoxia Stress for Pear Fruits. Agronomy 2023, 13, 1062. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Shewale, S.D.; Pandit, A.B. Enzymatic production of glucose from different qualities of grain sorghum and application of ultrasound to enhance the yield. Carbohydr. Res. 2009, 344, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Zeng, W.-C.; Zhang, W.-H.; Liao, X.-P.; Shi, B. Effect of ultrasound on the activity and conformation of a-amylase, papain and pepsin. Ultrason. Sonochem. 2014, 21, 930–936. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Guo, Y.; Fu, J.; Jiang, W.; Du, S. Physicochemical and structural properties of starches isolated from quinoa varieties. Food Hydrocoll. 2020, 101, 105515. [Google Scholar] [CrossRef]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocoll. 2021, 115, 106604. [Google Scholar] [CrossRef]
- Aparicio-Garcia, N.; Martinez-Villaluenga, C.; Frias, J.; Penas, E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef]
- Rani, M.; Singh, G.; Siddiqi, R.A.; Gill, B.S.; Sogi, D.S.; Bhat, M.A. Comparative Quality Evaluation of Physicochemical, Technological, and Protein Profiling of Wheat, Rye, and Barley Cereals. Front. Nutr. 2021, 8, 694679. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Subash-Babu, P. Effects of increasing ratios of dietary omega-6/omega-3 fatty acids on human monocyte immunomodulation linked with atherosclerosis. J. Funct. Foods 2018, 41, 258–267. [Google Scholar] [CrossRef]
- Matias, J.; Rodriguez, M.J.; Granado-Rodriguez, S.; Cruz, V.; Calvo, P.; Reguera, M. Changes in Quinoa Seed Fatty Acid Profile Under Heat Stress Field Conditions. Front. Nutr. 2022, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sicairos, E.S.; Dominguez-Rodriguez, M.; Montoya-Rodriguez, A.; Milan-Noris, A.K.; Reyes-Moreno, C.; Milan-Carrillo, J. Phytochemical Compounds and Antioxidant Activity Modified by Germination and Hydrolysis in Mexican Amaranth. Plant Foods Hum. Nutr. 2020, 75, 192–199. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, S.K.C. Macronutrients, Phytochemicals, and Antioxidant Activity of Soybean Sprout Germinated with or without Light Exposure. J. Food Sci. 2015, 80, S1391–S1398. [Google Scholar] [CrossRef]
- Suarez-Estrella, D.; Borgonovo, G.; Buratti, S.; Ferranti, P.; Accardo, F.; Pagani, M.A.; Marti, A. Sprouting of quinoa (Chenopodium quinoa Willd.): Effect on saponin content and relation to the taste and astringency assessed by electronic tongue. LWT Food Sci. Technol. 2021, 144, 7. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and MetabolomicProfiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Ehsen, S.; Abideen, Z.; Rizvi, R.F.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M.A.; Ansari, R. Ecophysiological adaptations and anti-nutritive status of sustainable cattle feed Haloxylon stocksii under saline conditions. Flora 2019, 257, 151425. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, Z.; Meenu, M.; Xu, B. Impact of Germination Time on Resveratrol, Phenolic Acids, and Antioxidant Capacities of Different Varieties of Peanut (Arachis hypogaea L.) from China. Antioxidants 2021, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Wang, J.; Guo, R.; Wang, C.Z.; Yan, X.B.; Xu, B.; Zhang, D.Q. Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets. Livest. Sci. 2014, 167, 257–262. [Google Scholar] [CrossRef]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159. [Google Scholar] [CrossRef]
- Zilic, S.; Basic, Z.; Sukalovic, V.H.-T.; Maksimovic, V.; Jankovic, M.; Filipovic, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Ha, K.S.; Jo, S.H.; Mannam, V.; Kwon, Y.I.; Apostolidis, E. Stimulation of Phenolics, Antioxidant and alpha-Glucosidase Inhibitory Activities During Barley (Hordeum vulgare L.) Seed Germination. Plant Foods Hum. Nutr. 2016, 71, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, F.; Caceres, P.J.; Martinez-Villaluenga, C.; Rosell, C.M.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015, 173, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic Changes in Phenolic Compounds and Antioxidant Activity in Oats (Avena nuda L.) during Steeping and Germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Xu, B.F.; Shi, P.Q.; Tian, H.L.; Li, Y.; Wang, X.Y.; Wu, S.; Liang, P.F. The influences of acetylation, hydroxypropylation, enzymatic hydrolysis and crosslinking on improved adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Chem. 2022, 368, 130883. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Gu, M.D.; Fang, H.C.; Gao, Y.H.; Su, T.; Niu, Y.G.; Yu, L.L. Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Aalim, H.; Luo, Z.S. Insight into rice (Oryza sativa L.) cooking: Phenolic composition, inhibition of alpha-amylase and alpha-glucosidase, and starch physicochemical and functional properties. Food Biosci. 2021, 40, 100917. [Google Scholar] [CrossRef]
- Dong, Y.F.; Li, Q.; Zhao, Y.H.; Cao, J.X. Effects of ultrasonic assisted high-temperature cooking method on the physicochemical structure characteristics and in vitro antioxidant capacities of dietary fiber from Dendrocalamus brandisii Munro shoots. Ultrason. Sonochem. 2023, 97, 106462. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Xie, J.; Xue, B.; Li, X.H.; Gan, J.H.; Zhu, T.; Sun, T. Effect of acid and oxidative degradation on the structural, rheological, and physiological properties of oat beta-glucan. Food Hydrocoll. 2021, 112, 106284. [Google Scholar] [CrossRef]
- Luo, L.; Yang, Z.; Wang, H.; Ashokkumar, M.; Hemar, Y. Impacts of sonication and high hydrostatic pressure on the structural and physicochemical properties of quinoa protein isolate dispersions at acidic, neutral and alkaline pHs. Ultrason. Sonochem. 2022, 91, 106232. [Google Scholar] [CrossRef]
- Herrera, T.; del Hierro, J.N.; Fornari, T.; Reglero, G.; Martin, D. Inhibitory effect of quinoa and fenugreek extracts on pancreatic lipase and alpha-amylase under in vitro traditional conditions or intestinal simulated conditions. Food Chem. 2019, 270, 509–517. [Google Scholar] [CrossRef]
- Ding, Q.Z.; Li, Z.K.; Wu, W.; Su, Y.Y.; Sun, N.Z.; Luo, L.; Ma, H.L.; He, R.H. Physicochemical and functional properties of dietary fiber from Nannochloropsis oceanica: A comparison of alkaline and ultrasonic-assisted alkaline extractions. LWT Food Sci. Technol. 2020, 133, 110080. [Google Scholar] [CrossRef]

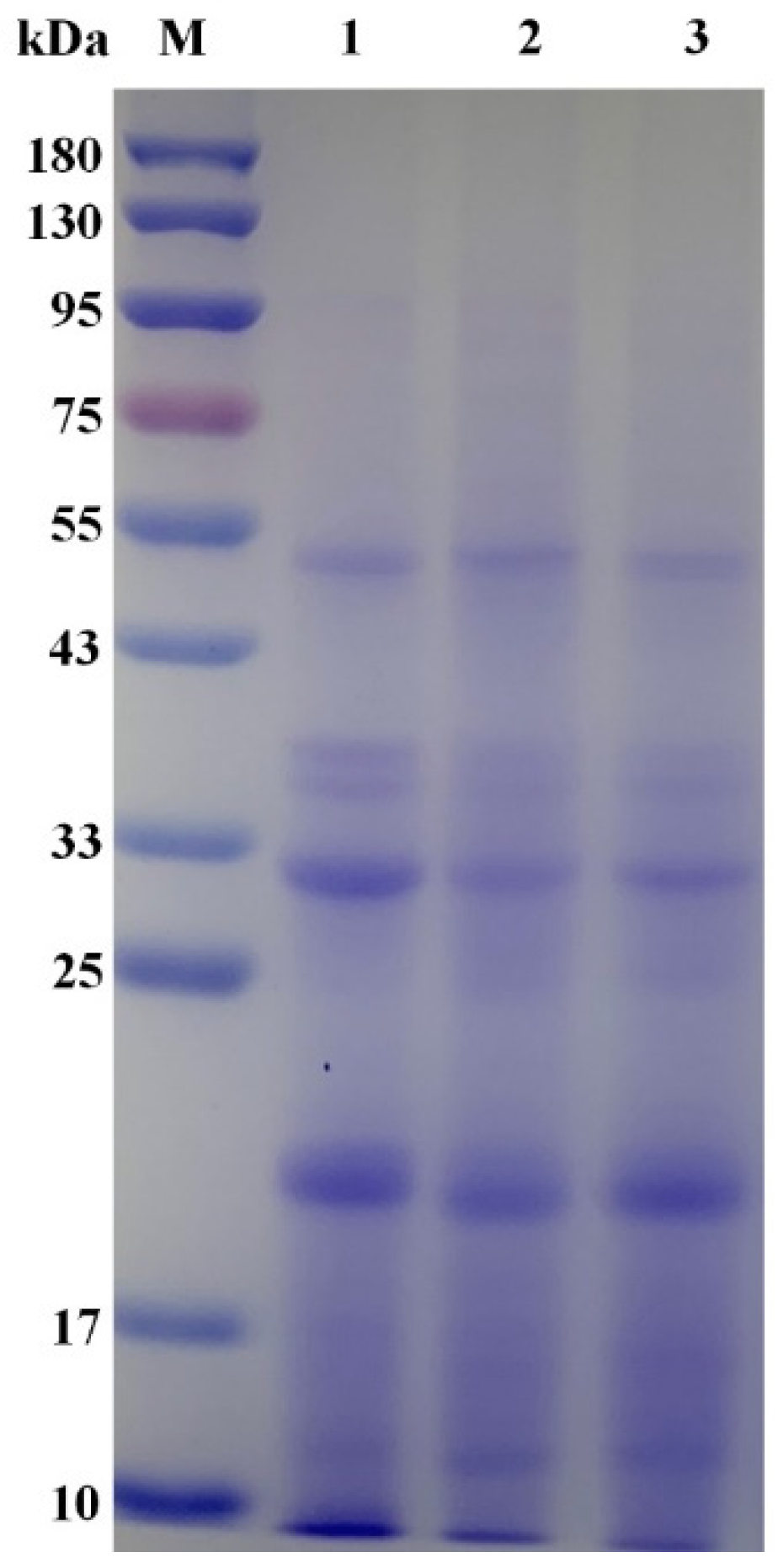
| Amino Acid (g/100g Protein) | 0 W–0 h | 0 W–24 h | 100 W–24 h |
|---|---|---|---|
| Threonine | 2.93 ± 0.02 Aa | 2.92 ± 0.01 Aa | 2.91 ± 0.04 Aa |
| Valine | 5.22 ± 0.02 Aa | 6.29 ± 0.04 Bb | 5.54 ± 0.02 Cc |
| Methionine | 6.38 ± 0.01 Aa | 7.50 ± 0.03 Bb | 7.42 ± 0.03 Cc |
| Isoleucine | 7.64 ± 0.02 Aa | 7.18 ± 0.04 Bb | 6.81 ± 0.02 Cc |
| Leucine | 9.36 ± 0.04 Aa | 8.80 ± 0.02 Bb | 8.75 ± 0.05 Bb |
| Phenylalanine | 2.81 ± 0.02 Aa | 2.84 ± 0.01 Aa | 2.97 ± 0.02 Bb |
| Lysine | 4.19 ± 0.01 Aa | 4.43 ± 0.02 Bb | 4.34 ± 0.03 Cc |
| Histidine | 2.36 ± 0.01 Aa | 2.32 ± 0.01 Bb | 2.36 ± 0.02 Aa |
| Arginine | 5.23 ± 0.01 Aa | 5.43 ± 0.02 Bb | 5.36 ± 0.03 Bc |
| Aspartate | 6.11 ± 0.01 Aa | 6.48 ± 0.03 Bb | 6.69 ± 0.03 Cc |
| Serine | 2.91 ± 0.01 Aa | 3.15 ± 0.04 Bb | 3.10 ± 0.03 Bb |
| Glutamate | 8.06 ± 0.06 Aa | 8.31 ± 0.03 Bb | 8.24 ± 0.04 Bb |
| Glycine | 3.36 ± 0.01 Aa | 3.38 ± 0.01 Aa | 3.47 ± 0.02 Bb |
| Alanine | 2.61 ± 0.01 Aa | 3.03 ± 0.02 Bb | 3.38 ± 0.03 Cc |
| Cysteine | 0.88 ± 0.01 Aa | 0.87 ± 0.01 Aa | 0.87 ± 0.02 Aa |
| Tyrosine | 3.07 ± 0.02 Aa | 3.12 ± 0.02 Ab | 2.94 ± 0.01 Bc |
| Proline | 4.77 ± 0.01 Aa | 5.72 ± 0.09 Bb | 6.26 ± 0.07 Cc |
| Total amino acids | 77.89 ± 0.06 Aa | 81.78 ± 0.17 Bb | 81.40 ± 0.04 Cc |
| Total hydrophobic amino acids | 38.80 ± 0.04 Aa | 41.37 ± 0.13 Bb | 41.13 ± 0.05 Cc |
| Total hydrophilic amino acids | 39.10 ± 0.04 Aa | 40.41 ± 0.04 Bb | 40.27 ± 0.07 Bc |
| Total acid amino acids | 14.16 ± 0.05 Aa | 14.79 ± 0.02 Bb | 14.92 ± 0.01 Cc |
| Total basic amino acids | 34.32 ± 2.87 Aa | 35.56 ± 3.11 Aa | 35.19 ± 2.98 Aa |
| Total aromatic amino acids | 5.88 ± 0.04 Aa | 5.96 ± 0.02 Bb | 5.91 ± 0.01 ABac |
| Total essential amino acids | 38.53 ± 0.06 Aa | 39.97 ± 0.11 Bb | 38.74 ± 0.03 Cc |
| Total half essential amino acids | 7.59 ± 0.01 Aa | 7.75 ± 0.03 Bb | 7.72 ± 0.05 Bb |
| Evaluation Criterion | Amino Acid | 0 W–0 h | 0 W–24 h | 100 W–24 h |
|---|---|---|---|---|
| AAS | Isoleucine | 2.73 | 2.56 | 2.43 |
| Leucine | 1.42 | 1.33 | 1.32 | |
| Lysine | 0.72 | 0.76 | 0.75 | |
| Methionine + Cysteine | 2.90 | 3.35 | 3.31 | |
| Phenylalanine + Tyrosine | 0.93 | 0.95 | 0.93 | |
| Valine | 1.54 | 1.85 | 1.63 | |
| Threonine | 0.84 | 0.83 | 0.83 | |
| CS | Isoleucine | 1.41 | 1.33 | 1.26 |
| Leucine | 1.09 | 1.02 | 1.02 | |
| Lysine | 0.60 | 0.63 | 0.62 | |
| Methionine + Cysteine | 1.27 | 1.47 | 1.45 | |
| Phenylalanine + Tyrosine | 0.63 | 0.64 | 0.64 | |
| Valine | 0.79 | 0.95 | 0.84 | |
| Threonine | 0.62 | 0.62 | 0.62 | |
| EAAI | 86.64 | 90.05 | 87.16 |
| Fatty Acid | Abbreviation | 0 W–0 h | 0 W–24 h | 100 W–24 h |
|---|---|---|---|---|
| SFAs (g/100 g) | ||||
| Myristate | C14:0 | 0.0109 ± 0.0001 Aa | 0.0121 ± 0.0001 Bb | 0.0126 ± 0.0000 Cc |
| Pentadecanoate | C15:0 | 0.0037 ± 0.0000 Aa | 0.0044 ± 0.0000 Bb | 0.0044 ± 0.0000 Bb |
| Palmitate | C16:0 | 0.5545 ± 0.0035 Aa | 0.5959 ± 0.0013 Bb | 0.6032 ± 0.0010 Cc |
| Stearate | C18:0 | 0.0276 ± 0.0011 Aa | 0.0336 ± 0.0002 Bb | 0.0327 ± 0.0003 Bb |
| Arachidate | C20:0 | 0.0263 ± 0.0003 Aa | 0.0279 ± 0.0003 Bb | 0.0285 ± 0.0000 Bc |
| Behenate | C22:0 | 0.0358 ± 0.0001 Aa | 0.0412 ± 0.0003 Bb | 0.0427 ± 0.0002 Cc |
| Tricosanoate | C23:0 | 0.1294 ± 0.0007 Aa | 0.1078 ± 0.0015 Bb | 0.0959 ± 0.0001 Cc |
| Lignocerate | C24:0 | 0.0205 ± 0.0004 Aa | 0.0258 ± 0.0004 Bb | 0.0277 ± 0.0007 Cc |
| MUFAs (g/100 g) | ||||
| Oleate | C18:1N9C | 1.2299 ± 0.0073 Aa | 1.2057 ± 0.0092 Bb | 1.2309 ± 0.0024 Aa |
| 11-eicosenoate | C20:1 | 0.0772 ± 0.0007 Aa | 0.0789 ± 0.0010 ABb | 0.0799 ± 0.0007 Bb |
| Nervonoate | C24:1 | 0.0100 ± 0.0001 Aa | 0.0108 ± 0.0001 Bb | 0.0109 ± 0.0002 Bb |
| PUFAs (g/100 g) | ||||
| Linoleate | C18:2N6 | 3.0319 ± 0.0200 Aa | 3.2308 ± 0.0287 Bb | 3.2291 ± 0.0056 Bb |
| Alpha linolenate | C18:3N3 | 0.5012 ± 0.0033 Aa | 0.5610 ± 0.0065 Bb | 0.5605 ± 0.0019 Bb |
| Arachidonate | C20:4N6 | 0.0722 ± 0.0005 Aa | 0.0732 ± 0.0007 Ab | 0.0750 ± 0.0001 Bc |
| TFAs (g/100 g) | 5.7311 ± 0.0378 Aa | 6.0094 ± 0.0498 Bb | 6.0341 ± 0.0107 Bb | |
| SFAs (% of TFAs) | 14.11 ± 0.02 Aab | 14.13 ± 0.05 Aa | 14.05 ± 0.01 Ab | |
| MUFAs (% of TFAs) | 22.98 ± 0.01 Aa | 21.56 ± 0.01 Bb | 21.90 ± 0.01 Cc | |
| PUFAs (% of TFAs) | 62.90 ± 0.01 Aa | 64.32 ± 0.06 Bb | 64.05 ± 0.02 Cc | |
| UFAs (% of TFAs) | 85.89 ± 0.02 Aab | 85.88 ± 0.06 Aa | 85.95 ± 0.02 Ab | |
| n3 PUFAs (% of TFAs) | 8.74 ± 0.00 Aa | 9.34 ± 0.03 Bb | 10.00 ± 0.00 Cc | |
| n6 PUFAs (% of TFAs) | 54.16 ± 0.01 Aa | 54.98 ± 0.03 Bb | 54.76 ± 0.01 Cc | |
| n6/n3 PUFAs | 6.19 ± 0.00 Aa | 5.89 ± 0.01 Bb | 5.48 ± 0.00 Cc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Zhou, Q.; Zhou, L.; Wang, J.; Ren, T.; Zheng, Y.; Lv, W.; Zhao, W. Enhancement of γ-Aminobutyric Acid and the Characteristics of Nutrition and Function in White Quinoa through Ultrasound Stress at the Pre-Germination Stage. Foods 2024, 13, 57. https://doi.org/10.3390/foods13010057
Wu M, Zhou Q, Zhou L, Wang J, Ren T, Zheng Y, Lv W, Zhao W. Enhancement of γ-Aminobutyric Acid and the Characteristics of Nutrition and Function in White Quinoa through Ultrasound Stress at the Pre-Germination Stage. Foods. 2024; 13(1):57. https://doi.org/10.3390/foods13010057
Chicago/Turabian StyleWu, Mengying, Qian Zhou, Liangfu Zhou, Jie Wang, Ting Ren, Yu Zheng, Wei Lv, and Wen Zhao. 2024. "Enhancement of γ-Aminobutyric Acid and the Characteristics of Nutrition and Function in White Quinoa through Ultrasound Stress at the Pre-Germination Stage" Foods 13, no. 1: 57. https://doi.org/10.3390/foods13010057
APA StyleWu, M., Zhou, Q., Zhou, L., Wang, J., Ren, T., Zheng, Y., Lv, W., & Zhao, W. (2024). Enhancement of γ-Aminobutyric Acid and the Characteristics of Nutrition and Function in White Quinoa through Ultrasound Stress at the Pre-Germination Stage. Foods, 13(1), 57. https://doi.org/10.3390/foods13010057